糖皮质激素联合丙种球蛋白治疗丙种球蛋白无反应型川崎病的临床疗效
2021-11-30张慎荣周芳邵启民
张慎荣 周芳 邵启民


[關键词] 川崎病;静脉丙种球蛋白无反应;糖皮质激素;冠状动脉损害
[中图分类号] R5 [文献标识码] B [文章编号] 1673-9701(2021)18-0057-04
Clinical study of combination treatment of glucocorticoid and intravenous immunoglobulin (IVIG) in IVIG unresponsive Kawasaki disease
ZHANG Shenrong ZHOU Fang SHAO Qimin
Department of Rheumatology & Immunology, Nephrology, Hangzhou Children′s Hospital, Hangzhou 310014, China
[Abstract] Objective To investigate the combination treatment of glucocorticoid and intravenous immunoglobulin (IVIG) in IVIG unresponsive Kawasaki disease(KD). Methods Date of 68 cases with initial IVIG unresponsive KD in Hangzhou Children's Hospital were collected from June 2016 to June 2020 and were divided into group A (IVIG) and group B (glucocorticoid combined with IVIG) according to re-treatment methods. 42 cases were in group A and 26 cases were in group B. The whole fever time,duration of fever after re-treatment,coronary arteries lesion,laboratory examination results of WBC, PLT, hsCRP,ESR, IL-6 before and one week after re-treatment were analyzed in the two groups. Results The whole fever time,duration of fever after re-treatment of group B were shorter than that of group A(P<0.05).The incidence of coronary artery dilatation and coronary artery aneurysm in group B were slightly higher than those in group A, but there were no significant differenc (P>0.05). The WBC of group B after re-treatment was higher than that of group A, and the difference of group B before and re-treatment was lower than that of group A.The difference of hsCRP before and after re-treatment in group B was higher than that in group A. After re-treatment, IL-6 in group B was lower than that in group A, and the difference of IL-6 in group B before and after re-treatment was higher than that in group A, with statistical significance (P<0.05). Conclusion Both schemes were effective in the treatment of IVIG unresponsive KD.There was no significant difference in the incidence of coronary artery lesion between the two groups. Glucocorticoid combined with IVIG did not increase the risk of coronary artery lesion. Different treatment regimens had effects on the levels of WBC, hsCRP and IL-6 in the two groups before and after re-treatment. Compared with IVIG alone, glucocorticoid combined with IVIG in the acute stage of KD could better control the inflammatory indexes of IVIG unresponsive KD children and shorten the time of fever.
[Key words] Kawasaki disease; No response to intravenous gamma globulin; Glucocorticoids; Coronary arteries lesion
川崎病(Kawasaki disease,KD)是一种以全身血管炎为主要病变的急性发热性疾病,好发于5岁以下婴幼儿[1],冠状动脉损害(coronary arteries lesion,CAL)是其嚴重并发症。静脉注射丙种球蛋白(intravenous immunoglobulin,IVIG)的应用大大降低了KD 患儿冠脉损害的发生率,但仍有10%~20%的川崎病患儿接受首剂IVIG治疗后失败,这部分患儿称之为IVIG无反应型KD,其发生CAL的风险增高[2,3]。本文通过对IVIG无反应型KD患儿进行病例回顾性分析,比较糖皮质激素联合IVIG与仅应用IVIG治疗IVIG无反应型KD的临床效果,寻找更有利于IVIG无反应型KD患儿的治疗方案。
1 资料与方法
1.1 一般资料
选取2016年6月至2020年6月于我院住院治疗的确诊为IVIG无反应型KD患儿68例作为研究对象,入选标准:诊断符合2004年及2017年美国心脏协会发布的《川崎病的诊断、治疗及远期管理》声明中IVIG无反应型KD定义:在首剂IVIG治疗36 h后仍持续发热或再次出现发热[2];在首剂IVIG治疗完成后至少36 h仍持续发热或再次出现发热[3]。排除合并严重心肝肾疾病及精神疾病者,两组患者均对本研究知情同意且签署知情同意书,并经伦理委员会审批通过检查。根据再次治疗方案不同分为A组和B组,两组患者的性别、年龄、病程等一般资料比较,差异无统计学意义(P>0.05),具有可比性。
1.2 治疗方法
根据再次治疗方案不同分为A组和B组,A组为仅应用第2剂IVIG(2 g/kg)治疗,共42例;B组为激素联合第2剂IVIG治疗(起始静脉滴注甲泼尼龙2 mg/(kg·d),后根据退热情况改泼尼松(华中药业股份有限公司,国药准字H42021394)1~2 mg/(kg·d)分次口服,2~3周逐渐减停),共26例,两组均口服阿司匹林。
1.3 观察指标
①总热程(发热开始至治疗后体温稳定48 h的总天数);②热退时间(再次治疗当天至体温稳定48 h所需时间);③实验室检测结果:治疗前(再次治疗前最近1次检查结果)、治疗后(再次治疗后最接近1周时)白细胞(WBC)、血小板(PLT)、超敏C反应蛋白(hs-CRP)、血沉、IL-6,并计算治疗前后差值;④治疗后1周以内超声心动图评价CAL情况。
1.4 CAL诊断及分类标准[8]
根据超声心动图检查结果分类。①超声心动图正常指冠状动脉壁光滑,回声细薄,无任何部位扩张。冠状动脉内径:0~3岁<2.5 mm,~9岁<3.0 mm,~14 岁<3.5 mm。②冠状动脉扩张(CAD)指冠状动脉内径超过上述标准但<4.0 mm,冠状动脉内径/主动脉根部内径(CA/AO)<0.3。③冠状动脉瘤(CAA)指不同形状的冠状动脉扩张,冠状动脉内径为4~7 mm,CA/AO>0.3,或冠状动脉呈瘤状扩张。④巨大冠状动脉瘤:冠状动脉内径≥8.0 mm,CA/AO≥0.6。
1.5 统计学处理
采用SPSS 22.0软件对数据进行分析和处理,正态分布计量资料采用均数±标准差表示,组间比较采用t检验。非正态分布计量资料以中位数(P25~P75)表示,采用Mann-whitney U检验。计数资料用[n(%)]表示,组间比较分别应用Pearson χ2检验和Fishers确切概率法。P<0.05表示差异有统计学意义。
2 结果
2.1 两组患儿的临床时间比较
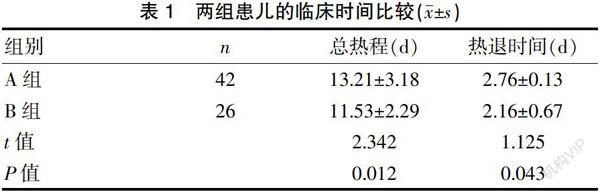
IVIG无反应型KD患儿68例,其中男54例,女14例,男女性别比为3.86∶1;月龄最小3个月,最大10岁11个月,中位数24.7个月。两组患儿在性别、年龄方面比较,差异无统计学意义(P>0.05)。B组患儿总热程、热退时间均短于A组患儿,差异有统计学意义(P均<0.05)。见表1。
2.2 两组患儿冠状动脉损害情况
两种方案治疗IVIG耐药型KD均有效,B组冠状动脉扩张及冠脉瘤发生率均略高于A组,但差异无统计学意义(P>0.05);两组冠状动脉损害发生率差异无统计学意义(P>0.05)。见表2。
2.3 两组患儿治疗前后实验室指标比较
不同治疗方案对两组患儿治疗前后WBC、hs-CRP、IL-6水平存在影响,B组治疗后WBC高于A组,B组WBC治疗前后差值低于A组;B组治疗前hs-CRP及治疗前后hs-CRP差值均高于A组;B组治疗后IL-6低于A组,B组IL-6治疗前后差值高于A组,差异均有统计学意义(P<0.05)。见表3。
3 讨论
KD患儿发病率和患病数持续上升,现已成为发达国家儿童获得性心脏病最常见的病因[1,4]。IVIG的应用大大降低了KD 患儿CAL发生率,但仍有10%~20%的患儿对IVIG耐药,且这部分患儿发生冠状动脉扩张(coronary artery dilatation,CAD)及冠状动脉瘤(coronary artery aneurysm,CAA)风险增高[5],远期出现缺血性心脏病、动脉粥样硬化等发生的风险增大[6,7],是成年后发生严重心血管事件的危险因素之一[8,9]。因此为这部分患儿寻找更有利的治疗方案非常重要。目前治疗包括第二剂IVIG、第二剂IVIG+糖皮质激素、英夫利昔单抗单次静脉注射、环孢霉素静脉注射或口服、阿那白滞素皮下注射及环磷酰胺静脉注射和血浆置换等[3,10-12],但选择上尚存在争议。
糖皮質激素可抑制免疫反应,具有强大的抗议效果,是临床治疗多种血管炎的一线药物[13-15]。随着对其在KD临床疗效及冠脉病变的长期及深入研究,发现糖皮质激素不但不会诱发CAL,还能迅速改善炎性反应[2,3,16,17]。2017年美国心脏病协会修订了KD诊断、治疗和长期随访指南中明确提出对于IVIG无反应型KD患儿可使用IVIG+糖皮质激素,并推荐2种激素使用剂量;对于首次治疗直接使用糖皮质激素存在争议,但如预测KD患儿有IVIG耐药高风险,及时加用糖皮质激素治疗安全有效[3]。
Zhu等[18]及 Yang等[19]均通过Mate分析发现糖皮质激素不增加KD冠脉扩张发生率,且可明显缩短热程。本研究中B组热退时间及总热程均短于A组,表明较之单纯使用第2剂IVIG治疗IVIG无反应型KD,糖皮质激素联合IVIG的治疗方案退热快并可缩短总热程。与上述学者的研究结论一致。
本研究中B组患儿出现冠状动脉扩张及冠脉瘤发生率均略高于A组,但无统计学差异,提示应用糖皮质激素并没有增加急性期IVIG无反应型KD患儿冠状动脉损害的发生风险。国内研究表明在IVIG无反应型KD患儿的远期随访中等也未发现糖皮质激素应用后存在冠脉损害风险增加[20]。
在炎症控制方面,通过比较本研究两种治疗方案的实验室指标发现,B组治疗前hsCRP及治疗前后hsCRP差值均高于A组,B组治疗后IL-6低于A组,B组IL-6治疗前后差值高于A组,以上提示联合应用糖皮质激素后急性期炎症指标hsCRP及IL-6的下降幅度更明显,表明糖皮质激素+IVIG急性期治疗效果肯定,且降低炎症指标方面优于IVIG,对于IVIG不敏感的KD患儿再次应用IVIG时可立即加用糖皮质激素以改善急性期炎症。两组患儿血沉、PLT治疗前后及差值无统计学差异,表明对IVIG无反应型KD患儿急性期应用激素安全、有效,并不会增加冠脉病变发生的风险。至于初始IVIG治疗无反应后直接应用糖皮质激素是否也能缓解症状,因本研究未纳入此方案病例,需临床研究进一步证实。
糖皮质激素抗炎效果肯定,医疗费用低,较之价格昂贵且有输血相关风险的IVIG,其成本效益占有明显优势,可预见糖皮质激素对于IVIG无反应型KD的治疗有广阔的前景。
本研究样本量尚小,结论存在局限性,未进行冠脉病变的远期随访,尚需大样本量及长期随访进一步研究。
[参考文献]
[1] Dimitriades VR,Brown AG,Gedalia A. Kawasaki disease:pathophy-siology,clinical manifestations,and management[J]. Curr Rheumatol Rep,2014,16(6):423.
[2] Jane W. Newburger,Masato Takahashi,Michael A. Gerber,et al. Diagnosis,Treatment,and Long-Term Management of Kawasaki Disease:A Statement for Health Professionals From the Committee on Rheumatic Fever,Endocarditis and Kawasaki Disease,Council on Cardiovascular Disease in the Young[J]. American Heart Associatio,2004,110(17):2747-2771.
[3] Brian W,McCrindle,Anne H. Rowley,Jane W. Newburger,et al. Diagnosis,Treatment,and Long-Term Management of Kawasaki Disease:A Scientific Statement for Health Professionals From the American Heart Association,2017,135(17):e927-e999.
[4] Nobuko Makino,Yosikazu Nakamura,Mayumi Yashiro,et al. Nationwide epidemiologic survey of Kawasaki disease in Japan,2015-2016[J]. Pediatrics International,2019, 61(4):397-403.
[5] Youn Y,Kim J,Hong YM,et al. Infliximab as the first retreatment in patients with Kawasaki disease resistant to initial intravenous immunoglobulin[J]. Pediatr Infect Dis J,2016,35(4):457-459.
[6] Cheung YF. Vascular health late after Kawasaki disease:implications for accelerated atherosclerosis[J]. Korean J Pediatr,2014,57(11):472-478.
[7] Serpytis P,Petntlioniene Z,Gargalskaite U,et a1.Myocardial infar-ction associated with kawasaki disease in adult man:case report and review of literature[J].Am J Med,2015,128(3):1-3.
[8] Khoury M,Kavey RW,St-Pierre J,et al. Incorporating risk stratifica- tion into the practice of pediatric preventive cardiolog[J]. Can J Cardiol,2020:1-12.
[9] Herrington L,Susi A,Gorman G,etal. Factors affecting pediatric dyslipidemia screening and treatment[J]. Clin Pediatr,2019,58(5):502-510.
[10] Tremoulet AH,Jain S,Jaggi P,et al. Infliximab for intensification of primary therapy for Kawasaki disease:a phase 3 randomised,double-blind,placebo-controlled trial[J].Lancet,2014,383(9930):1731-1738.
[11] Hamada H,Suzuki H,Onouchi Y,et al. Efficacy of primary treatment with immunoglobulin plus ciclosporin for prevention of coronary artery abnormalities in patients with Kawasaki disease predicted to be at increased risk of non-response to intravenous immunoglobulin (KAICA):a randomised controlled,open-label,blinded-endpoints,phase 3 trial[J].Lancet,2019,393(10176):1128-1137.
[12] 熊祎,杜忠東.肿瘤坏死因子阻断剂在丙种球蛋白无反应川崎病患儿的应用[J].中华儿科杂志,2020,58(3):248-251.
[13] Eleftheriou D,Levin M,Shingadia D,et a1.Management of Kawasaki disease[J].Arch Dis Child,2013,99(1):74-83.
[14] Rowley AH,Shulman ST.Recent advances in the understanding and management of Kawasaki disease[J].Curr Infect Dis Rep,2010,12(2):96-102.
[15] Son MB,Newburger JW.Management of Kawasaki disease:corticosteroids revisited[J].Lancet,2012,379(9826):1571-1572.
[16] 刘卓勋,谭晓梅,黄清明等.静脉人免疫球蛋白无反应型川崎病患儿的循证治疗[J]. 中国循证儿科杂志,2013,(4):313-315.
[17] 胡秀芬,温宇.丙种球蛋白无反应型川崎病的发病机制及治疗进展[J].中华实用儿科临床杂志,2017,32(21):1612-1616.
[18] Zhu BH,Lv HT,Sun L,et a1.A meta-analysis on the effect of corticosteroid therapy in Kawasaki disease[J].Eur J Pediatr,2012,171(3):571-578.
[19] Yang X,Liu G,Huang Y,et a1.A meta-analysis of retreatnlent for intravenous immunoglobulin-resistant Ka-wasaki disease[J].Cardiol Young,2015,25(6):l182-1190.
[20] 翁海美,项如莲,张园海等.丙种球蛋白无反应型川崎病治疗及随访分析[J].临床儿科杂志,2011,29(3):269-272.
(收稿日期:2021-01-09)
