骨髓间充质干细胞对腹膜间皮细胞凋亡的影响
2024-02-18王扶凝代会博单云俞曼殊盛梅笑
王扶凝 代会博 单云 俞曼殊 盛梅笑

摘要:目的 觀察大鼠骨髓间充质干细胞(BMSCs)对高糖腹透液(PDF)诱导的大鼠腹膜间皮细胞(PMCs)凋亡的影响及可能作用机制。方法 提取并鉴定大鼠原代BMSCs及PMCs,使用高糖腹透液诱导PMCs凋亡,收集BMSCs培养24 h后的细胞上清液作为条件培养基(BMSCs-CM)或通过Transwell小室将PMCs与BMSCs共培养。将PMCs分为空白对照(CON)组、高糖腹透液(PDF)组及间充质干细胞处理(PDF+BMSCs-CM)组,采用CCK-8法测定各组PMCs的增殖活力;JC-1法测定线粒体膜电位的去极化情况;TUNEL染色检测细胞凋亡情况;Western blot检测各组细胞凋亡相关蛋白B细胞淋巴瘤-2(Bcl-2)、Bcl-2相关X蛋白(Bax)、活化的胱天蛋白酶-3(Cleaved Caspase-3)和通路相关蛋白丝氨酸/苏氨酸蛋白激酶(Raf)、丝裂原活化细胞外信号调节激酶(MEK)、细胞外信号调节激酶(ERK)及其磷酸化蛋白的表达水平。结果 与CON组比较,PDF组PMCs增殖活力、线粒体膜电位水平降低,而凋亡率、Bax/Bcl-2、Cleaved Caspase-3/Caspase-3、p-Raf/Raf、p-MEK/MEK、p-ERK/ERK比值升高(P<0.05);与PDF组比较,PDF+BMSCs-CM组PMCs增殖活力、线粒体膜电位升高,而凋亡率、Bax/Bcl-2、Cleaved Caspase-3/Caspase-3、p-Raf/Raf、p-MEK/MEK、p-ERK/ERK比值降低(P<0.05)。结论 BMSCs可减轻高糖腹透液诱导的PMCs凋亡,其机制可能与抑制Raf/MEK/ERK信号通路的活化有关。
关键词:腹膜透析;细胞凋亡;骨髓间充质干细胞;腹膜间皮细胞
中图分类号:R285.5文献标志码:ADOI:10.11958/20231064
Study on the effect and mechanism of bone marrow mesenchymal stem cells on apoptosis of peritoneal mesothelial cells
Abstract: Objective To observe the effect of rat bone marrow mesenchymal stem cells (BMSCs) on the apoptosis of rat peritoneal mesothelium cells (PMCs) induced by high glucose peritoneal dialysis fluid (PDF), and to explore its possible molecular mechanism. Methods The primary BMSCs and PMCs were extracted and identified. Apoptosis of PMCs was induced by high glucose PDF. Cell supernatant from BMSCs after 24 h of culture was collected as the conditioned medium (BMSCs-CM). PMCs were co-cultured with BMSCs by conditioned media or Transwell chambers. PMCs were randomly divided into the control group, the PDF group and the PDF+BMSCs-CM group. The viability of PMCs was measured by CCK-8 in each group. The depolarization of mitochondrial membrane potential was measured by JC-1 method. TUNEL staining was used to detect cell apoptosis. Western blot assay was used to detect the expression levels of apoptosis related proteins B-cell lymphoma-2 (Bcl-2), Bcl-2 associated X protein (Bax), Cleaved cysteine aspartase-3 (Cleaved Caspase-3) and pathway related protein serine/threonine protein kinase (Raf), mitogen-activated extracellular signal-regulated kinase (MEK), extracellular-signal regulated protein kinase (ERK) and their phosphorylated proteins in each group. Results Compared with the control group, the proliferative activity and mitochondrial membrane potential of PMCs were decreased in the PDF group, while the apoptosis rate and the ratio of Bax/Bcl-2, Cleaved Caspase-3/Caspase-3, p-Raf/Raf, p-MEK/MEK and p-ERK/ERK were increased (P<0.05). Compared with the PDF group, the proliferative activity and mitochondrial membrane potential of PMCs were increased in the PDF+BMSCs-CM group, while the apoptosis rate and the ratio of Bax/Bcl-2, Cleaved Caspase-3/Caspase-3, p-Raf/Raf, p-MEK/MEK and p-ERK/ERK were decreased (P<0.05). Conclusion BMSCs can reduce the apoptosis of PMCs induced by high glucose PDF, and its mechanism maybe related to inhibiting the activation of Raf/MEK/ERK signaling pathway.
Key words: peritoneal dialysis; apoptosis; bone marrow mesenchymal stem cells; peritoneal mesothelial cells
腹膜透析(peritoneal dialysis,PD)的长期实施会导致腹膜结构损伤及功能进行性恶化。腹膜损伤以腹膜间皮细胞(peritoneal mesothelial cells,PMCs)的凋亡脱落为主要特征[1-2]。研究显示,腹透液(peritoneal dialysis fluid,PDF)中高浓度葡萄糖及其降解产物是导致PMCs凋亡的关键因素,可引起PMCs细胞膜损伤、线粒体功能障碍,发生氧化应激和炎症反应;PMCs大量凋亡又启动腹膜结构重塑,引发超滤衰竭[3]。因此,抑制PMCs凋亡对维持长程PD具有重要意义。间充质干细胞(mesenchymal stem cell,MSCs)因其强大的免疫调节和再生特性而被广泛应用于细胞治疗[4-5]。研究表明,与骨髓间充质干细胞(bone marrow mesenchymal stromal stem cells,BMSCs)共培养24 h能明显改善高糖培养基诱导的小鼠足细胞凋亡[6]。BMSCs可通过抑制损伤细胞中细胞外信号调节激酶(extracellular-signalregulated protein kinase,ERK)及磷脂酰肌醇3激酶(phosphatidylinositol-3-kinase,PI3K)/蛋白激酶B(protein kinase B,AKT)的磷酸化,促进受损细胞的存活和再生[7]。然而,BMSCs对PDF环境下的PMCs的保护机制尚不明确。本实验以大鼠原代PMCs及BMSCs作为研究对象,探讨BMSCs对PDF诱导的PMCs凋亡的影响及可能作用机制,以期为保护腹膜组织提供新的思路。
1 材料与方法
1.1 实验动物 SPF级雄性Sprague-Dawley(SD)大鼠20只,4周龄,体质量100~150 g,购自济南朋悦实验动物繁育有限公司,动物生产许可证号:SCXK(鲁)2022-0006。
1.2 主要试剂与仪器 4.25%葡萄糖PDF购自广州百特医疗用品有限公司(国药准字H44025216,规格:2 L/袋);DMEM-F12培养液、胎牛血清和胰蛋白酶-EDTA(0.25%)购自美国Gibco公司;大鼠BMSCs成骨诱导分化试剂盒和成脂诱导分化试剂盒购自赛业生物科技有限公司;青霉素-鏈霉素溶液、ECL显影液、CCK-8试剂购自白鲨生物科技有限公司;RIPA裂解缓冲液购自美国Thermo Fisher Scientific公司;4%~20%预制胶购自南京艾思易生物科技有限公司;JC-1线粒体膜电位检测试剂盒、TUNEL细胞凋亡检测试剂盒、抗荧光淬灭剂(含DAPI)、BCA蛋白检测试剂盒、蛋白酶和磷酸酶抑制剂购自上海碧云天生物技术有限公司;兔抗B细胞淋巴瘤-2(Bcl-2)、兔抗Bcl-2相关X蛋白(Bax)抗体购自艾比玛特生物医药(上海)有限公司;鼠抗β-肌动蛋白(β-actin)、兔抗胱天蛋白酶-3(Caspase-3)、鼠抗磷酸化的细胞外信号调节激酶(p-ERK)、鼠抗ERK、鼠抗丝裂原活化的细胞外信号调节激酶(MEK)、鼠抗p-MEK抗体和鼠抗丝氨酸/苏氨酸蛋白激酶(Raf)抗体购自美国Santa Cruz Biotechnology公司;山羊抗兔IgG、辣根过氧化物酶标记山羊抗鼠IgG购自北京中杉金桥生物技术有限公司;兔抗p-Raf、鼠抗角蛋白(Pan-Keratin)抗体购自美国Cell Signaling Technology公司;兔抗波形蛋白(vimentin)抗体、Alex Fluor 488 Goat Anti-Mouse IgG(绿色)标记二抗、Alex Fluor 594 Goat Anti-Rabbit IgG(红色)标记二抗购自武汉三鹰生物技术有限公司。HERAcell 150i二氧化碳细胞培养箱(美国Thermo Fisher Scientific公司);CKX31倒置生物显微镜(日本Olympus公司);SMI3000B荧光倒置显微镜摄像系统(德国Leica公司);LAS400超灵敏化学发光成像仪(美国GE Healthcare Lifescience公司);2300EnSpire酶标仪(美国PerkinElmer公司)。
1.3 研究方法
1.3.1 大鼠原代PMCs的分离培养及鉴定 4%异氟烷过量麻醉处死大鼠,开腹后无菌摘离肠系膜,以含1%青霉素-链霉素溶液的磷酸盐缓冲溶液(PBS)漂洗3遍后转移至新的培养皿。在含EDTA的0.25%胰蛋白酶中均匀剪碎组织,摇床消化30 min,加入等体积含10%胎牛血清的DMEM-F12培养液终止消化,以70 μm细胞滤网过滤,300×g离心5 min,弃去上清液。以含10%胎牛血清+1%青霉素-链霉素溶液的DMEM-F12培养液于37 ℃恒温、5%CO2的细胞培养箱培养24 h后换液。镜下观察PMCs生长情况,采用Pan-Keratin及vimentin免疫荧光双染色对原代PMCs进行鉴定。取第1~2代细胞进行实验。
1.3.2 大鼠原代BMSCs的培养及鉴定 脱颈法处死大鼠,无菌条件下摘取大鼠股骨、胫骨,洗涤并去除所取骨骼上的残留肌肉、血液,以PBS缓慢冲出骨髓腔中的细胞,至骨髓腔发白,将冲出的骨髓细胞悬液离心重悬,接种到培养皿中,48 h后初次换液,后每2~3 d更换一次培养基,接种后5~7 d细胞融合达到70%~80%时传代培养。通过诱导成骨及成脂分化对大鼠原代BMSCs进行鉴定。取第2~4代细胞进行后续实验。收集培养24 h的BMSCs上清液作为条件培养基(BMSCs-conditioned media,BMSCs-CM)。
1.3.3 细胞分组 大鼠原代PMCs分为空白对照(CON)组、高糖PDF组和BMSCs处理(PDF+BMSCs-CM)组。PDF的实验剂量和干预时间参照本课题组前期研究[8]。CON组加入DMEM-F12培养液,PDF组及PDF+BMSCs-CM处理组加入4.25% PDF干预24 h。PDF+BMSCs-CM处理组加入BMSCs-CM处理24 h或通过Transwell小室将PMCs与BMSCs共培养24 h,CON组、PDF组更换DMEM-F12培养液。
1.3.4 CCK-8法检测细胞增殖活力 将PMCs以每孔1×104个接种至96孔板中,每组设5个复孔,分组及给药剂量参照1.3.3。处理24 h后,各孔加入100 μL含10% CCK-8试剂的培养液,37 ℃孵育2 h,酶标仪检测450 nm波长处光密度(OD)值。细胞增殖活力(%)=实验组OD值/空白对照组OD值×100%。
1.3.5 JC-1染色法检测线粒体膜电位 将PMCs以每孔4×104个接种于12孔板过夜培养,分组及给药剂量参照1.3.3。处理24 h后,以PBS洗涤细胞2次,每孔加入JC-1染色工作液500 μL,细胞培养箱中37 ℃孵育20 min。吸弃染色液,用4 ℃预冷的JC-1染色缓冲液洗涤2次,每孔加500 μL细胞培养液,荧光显微镜下随机读取3个视野区,通过红绿荧光比率(JC-1 aggregates/monomers)反映线粒体膜电位变化。
1.3.6 TUNEL染色检测细胞凋亡情况 将PMCs以每孔4×104个接种于12孔板过夜培养,分组及给药剂量参照1.3.3。处理24 h后,PBS洗涤细胞2~3次,每孔加入4%多聚甲醛500 μL,室温固定30 min,吸弃固定液以PBS洗涤3次。加入0.2%的Trition X-100通透30 min,PBS洗涤,每孔滴加50 μL TUNEL检测液,使用圆形塑片使检测液均匀覆盖细胞,37 ℃避光孵育60 min,PBS洗涤,抗荧光淬灭剂封片,荧光显微镜下随机读取3个视野观察。凋亡率(%)=TUNEL阳性细胞数(红色荧光)/总细胞数(DAPI)×100%。
1.3.7 Western blot检测相关蛋白表达 将PMCs以每孔2×105个接种于6孔板培养,CON组加入DMEM-F12培养液,PDF组及PDF+BMSCs-CM组以3% PDF干预24 h,此后PDF组更换DMEM-F12培养液,PDF+BMSCs-CM组加入Transwell小室,上室为2×105个的BMSCs,下室為PDF干预后的PMCs,以DMEM-F12培养液共培养。Transwell共培养24 h后,移去小室,PBS洗涤,将每孔中的PMCs在细胞裂解液(RIPA∶蛋白酶抑制剂∶磷酸酶抑制剂=50∶1∶1)中4 ℃裂解20 min,提取细胞总蛋白并定量。在恒压条件下电泳,采用恒流湿转法将蛋白转移至PVDF膜上。用5%牛血清蛋白室温封闭1 h,分别加入抗Bax、抗Bcl-2、抗Caspase-3、抗ERK1/2、抗p-ERK1/2、抗MEK、抗p-MEK、抗Raf、抗p-Raf和抗β-actin抗体(1∶1 000)在4 ℃下孵育过夜。用TBST溶液洗涤5 min/次×4次,室温孵育二抗(1∶20 000)1 h。TBST溶液洗涤5 min/次×4次,滴加ECL曝光液显影,β-actin作为内参照,使用Image Lab软件对获取的蛋白条带进行灰度分析。
1.4 统计学方法 采用SPSS 26.0软件进行数据分析。符合正态分布的计量资料以[x] ±s表示,多组间比较使用单因素方差分析,组间多重比较采用LSD-t检验,以P<0.05为差异有统计学意义。
2 结果
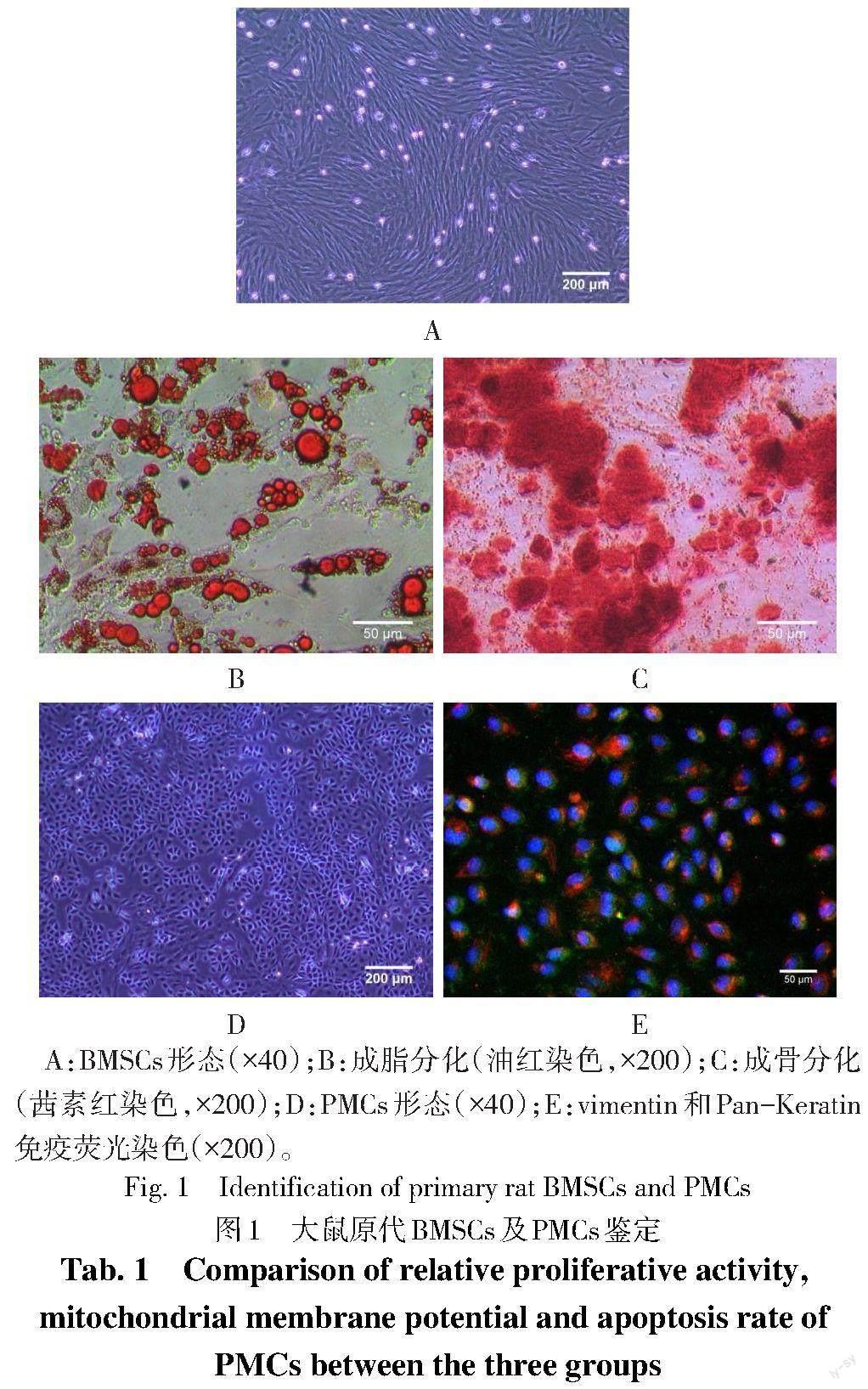
2.1 大鼠原代BMSCs与PMCs的培养与鉴定 光镜下可见BMSCs贴壁生长,融合度大于80%时呈螺旋状排列。成脂诱导2周后镜下可见脂滴,成骨诱导3周后镜下见钙结节,符合BMSCs的分化特点,见图1A—C。光镜下可见PMCs贴壁生长,呈铺路石样,免疫荧光染色显示vimentin和Pan-Keratin阳性,符合PMCs的标记特点,见图1D、E。
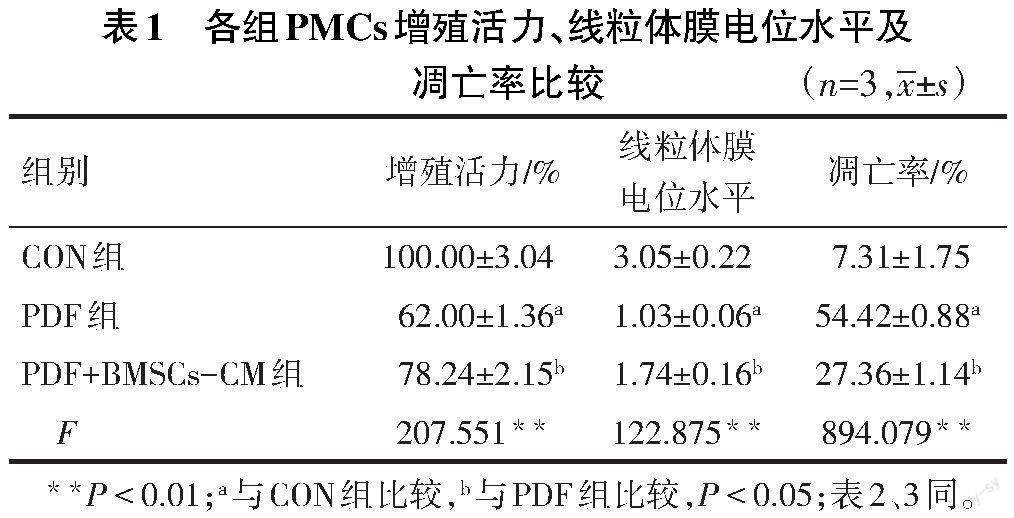
2.2 各组PMCs增殖活力、线粒体膜电位水平及凋亡率比较 与CON组比较,PDF组PMCs增殖活力、线粒体膜电位降低,凋亡率增加(P<0.05);与PDF组比较,PDF+BMSCs-CM组PMCs增殖活力、线粒体膜电位增加,凋亡率降低(P<0.05),见表1,图2、3。
2.3 各组Bcl-2、Bax、Cleaved Caspase-3蛋白表达水平比较 与CON组比较,PDF组Bax/Bcl-2比值、Cleaved Caspase-3蛋白表达水平增加(P<0.05);与PDF组比较,BMSCs干预后Bax/Bcl-2比值、Cleaved Caspase-3蛋白表达水平降低(P<0.05),见图4、表2。
2.4 各组Raf/MEK/ERK信号通路相关蛋白表达水平比较 与CON组比较,PDF组p-Raf、p-MEK和p-ERK蛋白表达水平升高(P<0.05);与PDF组比较,BMSCs干预后p-Raf、p-MEK和p-ERK蛋白表达水平降低(P<0.05),见图5、表3。
3 讨论
腹膜由单层间皮细胞及成纤维细胞、脂肪细胞、胶原纤维、神经、淋巴管和毛细血管构成的间质层组成[9]。PMCs位于腹膜腔表面,是构成腹膜抵御外来损伤的第一道屏障,参与水与溶质的跨膜转运,在维持腹膜稳态中起重要作用[10]。PD治疗过程中,PMCs长期暴露于高糖腹透液,使细胞凋亡异常增加,细胞增殖与凋亡失衡,有效细胞群体数量减少,细胞外基质在间皮下区域过度沉积,导致腹膜结构重塑,功能丧失[11]。因此,抑制PMCs过度凋亡和减少PMCs的脱落对维护正常的腹膜结构和功能、延长腹膜透析治疗时间具有重要意义。
BMSCs具有抗炎、抗凋亡、调节免疫、促进再生等特点,其作用在各领域的基础和临床研究中被证实并受到关注[12-13]。在慢性阻塞性肺病Ⅰ期临床试验中发现,自体输注BMSCs可有效改善患者肺功能,该作用与MSCs的旁分泌功能有关[14-15]。BMSCs-CM中包含多种生长因子、细胞因子、补体调节因子以及微小核糖核酸(microRNAs,miRNAs)等物质,这些因子可以刺激肾小管上皮细胞的再生[16-17]。BMSCs-CM中提取的外泌体(exosome,Exo)通过抑制Akt磷酸化,从而减轻高糖诱导的肾上皮细胞的凋亡[18],上调BMSCs-Exo中miR-126-3p的表达,可进一步降低炎症损伤细胞中ERK1/2、p38/丝裂原活化蛋白激酶(p38/ mitogen-activated protein kinase,p38/MAPK)的磷酸化水平,增强BMSCs对炎症诱导的软骨细胞凋亡的保护作用[19]。此外,BMSCs还可将自身线粒体转移至受损细胞,改善受损细胞线粒体膜电位水平,抑制线粒体介导的凋亡途径的激活,同时改善组织纤维排列和细胞外基质沉积,促进跟腱愈合[20]。上述研究证明BMSCs具有良好的抗凋亡潜力,且可能与线粒体及MAPK信号通路相关。因此,本研究关注BMSCs对PDF诱导的PMCs凋亡的保护作用及机制,采用3%PDF刺激PMCs细胞凋亡并给予BMSCs-CM进行干预,CCK-8实验结果表明,BMSCs-CM能显著改善3% PDF对PMCs增殖的抑制作用。
线粒体是细胞物质代谢、能量转化的重要场所,同时也介导細胞凋亡。生理环境下,线粒体内膜上呼吸链质子转运,形成膜电位,维持膜内外的物质平衡。高糖环境下,线粒体膜通透性增加,膜电位耗散,释放细胞色素C,启动Caspase级联反应,细胞骨架蛋白及DNA裂解,最终细胞凋亡[21]。本研究采用JC-1荧光探针检测PMCs线粒体膜电位水平,发现PDF干预后PMCs线粒体膜电位下降,BMSCs-CM处理后膜电位有所回升,提示BMSCs-CM改善了PDF诱导的PMCs早期凋亡;TUNEL染色分析检测DNA断裂情况显示,BMSCs-CM处理减轻了PDF诱导的PMCs凋亡,进一步证实了BMSCs对PDF诱导的PMCs凋亡的保护作用。
Bcl-2家族参与线粒体膜电位的调节,促凋亡蛋白Bax被激活后在线粒体膜上形成多聚体孔洞,使线粒体外膜通透化,而抗凋亡蛋白Bcl-2可以结合并抑制促凋亡蛋白Bax活性,两者的失衡引发Caspase级联反应,激活Caspase-3,启动细胞分解凋亡[22]。本研究构建Transwell共培养模式以模拟体内环境,发现与BMSCs共培养后,PMCs中Bax/Bcl-2的比值下降,凋亡相关Cleaved Caspase-3的蛋白表达下调,证实了BMSCs通过改善抗凋亡与促凋亡蛋白的失衡,抑制PDF诱导的PMCs细胞凋亡。
Raf/MEK/ERK是MAPK信号通路的主要分支,不仅介导细胞增殖和存活,还介导不同类型细胞的生长停滞和死亡[23]。Raf/MEK/ERK通路介导的生长抑制信号传导的常见标志是ERK1/2的持续激活,当ERK1/2活化高于触发细胞死亡的阈值,将从生长停滞反应切换为Caspase依赖性凋亡,抑制MAPK/ERK的激活可改善线粒体碎片化[24-25]。研究证实,高糖可激活ERK相关信号通路,参与细胞的损伤进程[26]。在人角质形成细胞和大鼠上皮细胞的高糖损伤模型中,抑制ERK1/2的激活均有利于减轻细胞损伤[27-28]。本研究发现,PDF干预诱导PMCs中Raf/MEK/ERK信号通路广泛激活,而与BMSCs共培养后PMCs中Raf、MEK和ERK蛋白磷酸化水平被部分抑制,提示BMSCs对PDF诱导的PMCs凋亡的保护作用可能是通过抑制Raf/MEK/ERK通路的过度活化而实现。
综上所述,大鼠BMSCs对高糖腹透液诱导的PMCs凋亡具有保护作用,其机制可能与抑制Raf/MEK/ERK信号通路活化和线粒体凋亡途径的激活有关。本研究为骨髓间充质干细胞的腹膜保护作用提供了实验依据。
参考文献
[1] MASOLA V,BONOMINI M,BORRELLI S,et al. Fibrosis of peritoneal membrane as target of new therapies in peritoneal dialysis[J]. Int J Mol Sci,2022,23(9):4831. doi:10.3390/ijms23094831.
[2] WANG R,GUO T,LI J. Mechanisms of peritoneal mesothelial cells in peritoneal adhesion[J]. Biomolecules,2022,12(10):1498. doi:10.3390/biom12101498.
[3] ROUMELIOTIS S,DOUNOUSI E,SALMAS M,et al. Unfavorable effects of peritoneal dialysis solutions on the peritoneal membrane: the role of oxidative stress [J]. Biomolecules,2020,10(5):768. doi:10.3390/biom10050768.
[4] LOTFY A,ABOQUELLA N M,WANG H. Mesenchymal stromal/stem cell(MSC)-derived exosomes in clinical trials[J]. Stem Cell Res Ther,2023,14(1):66. doi:10.1186/s13287-023-03287-7.
[5] HOANG D M,PHAM P T,BACH T Q,et al. Stem cell-based therapy for human diseases[J]. Signal Transduct Target Ther,2022,7(1):272. doi:10.1038/s41392-022-01134-4.
[6] SUN J,ZHAO F,ZHANG W,et al. BMSCs and miR-124a ameliorated diabetic nephropathy via inhibiting notch signalling pathway[J]. J Cell Mol Med,2018,22(10):4840-4855. doi:10.1111/jcmm.13747.
[7] LIN M,LIU X,ZHENG H,et al. IGF-1 enhances BMSC viability,migration,and anti-apoptosis in myocardial infarction via secreted frizzled-related protein 2 pathway[J]. Stem Cell Res Ther,2020,11(1):22. doi:10.1186/s13287-019-1544-y.
[8] 趙君谊,单云,朱晓琳,等. 黄芪多糖对高糖腹透液诱导HMrSV5细胞凋亡的影响[J]. 中华中医药学刊,2020,38(10):113-117,280-281. ZHAO J Y,SHAN Y,ZHU X L,et al. Effect of astragalus polysaccharide on apoptosis of hmrsv5 induced by peritoneal dialysis solution[J]. Chin Arch Tradit Chin Med,2020,38(10):113-117,280-281. doi:10.13193/j.issn.1673-7717.2020.10.026.
[9] TEITELBAUM I. Peritoneal dialysis[J]. N Engl J Med,2021,385(19):1786-1795. doi:10.1056/NEJMra2100152.
[10] HUANG Q,SUN Y,PENG L,et al. Extracellular vesicle-packaged ILK from mesothelial cells promotes fibroblast activation in peritoneal fibrosis[J]. J Extracell Vesicles,2023,12(7):e12334. doi:10.1002/jev2.12334.
[11] HU Q,XIA X,KANG X,et al. A review of physiological and cellular mechanisms underlying fibrotic postoperative adhesion[J]. Int J Biol Sci,2021,17(1):298-306. doi:10.7150/ijbs.54403.
[12] ZHOU T,YUAN Z,WENG J,et al. Challenges and advances in clinical applications of mesenchymal stromal cells[J]. J Hematol Oncol,2021,14(1):24. doi:10.1186/s13045-021-01037-x.
[13] ZHOU L,ZHU H,BAI X,et al. Potential mechanisms and therapeutic targets of mesenchymal stem cell transplantation for ischemic stroke[J]. Stem Cell Res Ther,2022,13(1):195. doi:10.1186/s13287-022-02876-2.
[14] SQUASSONI S D,SEKIYA E J,FISS E,et al. Autologous infusion of bone marrow and mesenchymal stromal cells in patients with chronic obstructive pulmonary disease: phase I randomized clinical trial[J]. Int J Chron Obstruct Pulmon Dis,2021,16:3561-3574. doi:10.2147/copd.S332613.
[15] CHEN T Y,LIU C H,CHEN T H,et al. Conditioned media of adipose-derived stem cells suppresses sidestream cigarette smoke extract induced cell death and epithelial-mesenchymal transition in lung epithelial cells[J]. Int J Mol Sci,2021,22(21):12069. doi:10.3390/ijms222112069.
[16] BEHZADIFARD M,ABOUTALEB N,DOLATSHAHI M,et al. Neuroprotective effects of conditioned medium of mesenchymal stem cells (MSC-CM) as a therapy for ischemic stroke recovery: a systematic review[J]. Neurochem Res,2023,48(5):1280-1292. doi:10.1007/s11064-022-03848-x.
[17] CALCAT I C S,SANZ-NOGU?S C,O'BRIEN T. When origin matters: properties of mesenchymal stromal cells from different sources for clinical translation in kidney disease[J]. Front Med (Lausanne),2021,8:728496. doi:10.3389/fmed.2021.728496.
[18] WANG H,WANG J,LIU T,et al. Stem cell-derived exosomal MicroRNAs: potential therapies in diabetic kidney disease[J]. Biomed Pharmacother,2023,164:114961. doi:10.1016/j.biopha.2023.114961.
[19] LI S,ST?CKL S,LUKAS C,et al. Curcumin-primed human BMSC-derived extracellular vesicles reverse IL-1β-induced catabolic responses of OA chondrocytes by upregulating miR-126-3p[J]. Stem Cell Res Ther,2021,12(1):252. doi:10.1186/s13287-021-02317-6.
[20] WEI B,JI M,LIN Y,et al. Mitochondrial transfer from bone mesenchymal stem cells protects against tendinopathy both in vitro and in vivo[J]. Stem Cell Res Ther,2023,14(1):104. doi:10.1186/s13287-023-03329-0.
[21] RAMIL-G?MEZ O,L?PEZ-PARDO M,FERN?NDEZ-RODR?GUEZ J A,et al. Involvement of mitochondrial dysfunction in the inflammatory response in human mesothelial cells from peritoneal dialysis effluent[J]. Antioxidants (Basel),2022,11(11):2184. doi:10.3390/antiox11112184.
[22] GREEN D R. The mitochondrial pathway of apoptosis: part I: MOMP and beyond[J]. Cold Spring Harb Perspect Biol,2022,14(5):a041038. doi:10.1101/cshperspect.a041038.
[23] WU P K,BECKER A,PARK J I. Growth inhibitory signaling of the Raf/MEK/ERK pathway[J]. Int J Mol Sci,2020,21(15):5436. doi:10.3390/ijms21155436.
[24] ZHONG Y,LI M Y,HAN L,et al. Galangin inhibits programmed cell death-ligand 1 expression by suppressing STAT3 and MYC and enhances T cell tumor-killing activity[J]. Phytomedicine,2023,116:154877. doi:10.1016/j.phymed.2023.154877.
[25] KUMARI S,DHAPOLA R,REDDY D H. Apoptosis in Alzheimer's disease: insight into the signaling pathways and therapeutic avenues[J]. Apoptosis,2023,28(7/8):943-957. doi:10.1007/s10495-023-01848-y.
[26] LIU Y,CHEN J,LIANG H,et al. Human umbilical cord-derived mesenchymal stem cells not only ameliorate blood glucose but also protect vascular endothelium from diabetic damage through a paracrine mechanism mediated by MAPK/ERK signaling[J]. Stem Cell Res Ther,2022,13(1):258. doi:10.1186/s13287-022-02927-8.
[27] LANG J,YANG C,LIU L,et al. High glucose activates ERK1/2 to stabilize AP1 and increase MMP9 expression in diabetic foot ulcers[J]. Exp Cell Res,2021,403(1):112550. doi:10.1016/j.yexcr.2021.112550.
[28] PAN L,ZHANG X,GAO Q. Histatin-1 alleviates high-glucose injury to skin keratinocytes through MAPK signaling pathway[J]. J Cosmet Dermatol,2022,21(11):6281-6291. doi:10.1111/jocd.15235.
