阿帕替尼对胰腺癌细胞株AsPC-1增殖、迁移和凋亡的影响
2017-02-20顾晓霞李洁吴梅红彭小波湛先保
顾晓霞 李洁 吴梅红 彭小波 湛先保
·论著·
阿帕替尼对胰腺癌细胞株AsPC-1增殖、迁移和凋亡的影响
顾晓霞 李洁 吴梅红 彭小波 湛先保
目的 探讨阿帕替尼(Apatinib)对体外培养的胰腺癌AsPC-1细胞增殖、凋亡、迁移的影响。方法 以不同浓度Apatinib干预胰腺癌AsPC-1细胞,采用CCK-8法和流式细胞技术检测细胞的增殖和凋亡,通过划痕实验观察Apatinib对胰腺癌细胞迁移能力的影响。结果 对照组及10、20、30、40、50μmol/L Apatinib处理组AsPC-1细胞的增殖抑制率分别为0、(1.45±0.68)%、(16.92±0.70)%、(23.84±0.84)%、(34.35±1.55)%、(37.33±0.81)%,细胞增殖随Apatinib浓度的增加而显著被抑制,差异有统计学意义(P<0.05)。对照组及20、40 μmol/L Apatinib处理组AsPC-1细胞的凋亡率分别为(9.44±0.18)%、(16.62±0.19)%、(25.42±0.41)%,细胞凋亡数量随Apatinib浓度增加而显著增多,差异具有统计学意义(P<0.05)。对照组及20、40 μmol/L Apatinib处理组AsPC-1细胞的迁移率分别为(29.5±0.7)%、(17.4±0.9)%、(6.6±0.5)%,细胞迁移能力随Apatinib浓度增加而显著下降,差异有统计学意义(P<0.05)。结论 Apatinib能有效抑制胰腺癌AsPC-1细胞的增殖、迁移,并诱导其凋亡。
阿帕替尼; 胰腺肿瘤; 细胞增殖; 细胞运动; 细胞凋亡
胰腺癌是高度恶性的肿瘤,5年生存率低于5%[1],这主要与它早期局部或者远处转移、临床症状出现较晚、对传统的化疗和放射治疗高度抵抗等因素有关[2-3]。吉西他滨是目前用于局部进展和转移性胰腺癌患者的标准化疗药物,但也只能延长患者几周的生存期[4]。Apatinib是一种新的、通过口服给予的血管上皮生长因子受体-2(VEGFR-2)的选择性抑制剂,可以有效抑制细胞内VEGFR-2的磷酸化,也能抑制c-Kit和c-Src的激酶活性以及c-Kit和血小板源生长因子受体(platelet derived growth factor receptor,PDGFR)的磷酸化[5]。研究显示[6-9],Apatinib在体外能抑制多种肿瘤细胞,如非小细胞肺癌、乳腺癌、胆管细胞癌等的增殖,也能明显减缓裸鼠移植瘤的生长,且毒性较小。但目前尚未见其对胰腺癌的研究报道。本研究应用Apatinib干预胰腺癌AsPC-1细胞,观察其对癌细胞增殖、凋亡、迁移的影响。
材料和方法
一、CCK-8法测定细胞增殖抑制率
人胰腺癌AsPC-1细胞由上海长海医院消化内科实验室提供,常规复苏、培养、传代。取对数生长期细胞,调整细胞密度为5×104/ml,在96孔板每孔中加入100 μl细胞悬液,培养至细胞贴壁后更换含1% FBS的DMEM饥饿培养过夜,然后加入10、20、30、40、50 μmol/L的Apatinib,以不加Apatinib作为对照组,每组设6个复孔。培养24 h后每孔加入10 μl CCK-8液继续培养2 h,上酶标仪测定450 nm处的吸光度值(A450值),绘制细胞生长曲线。Apatinib由恒瑞医药有限公司提供,CCK-8试剂盒购自上海威奥生物科技有限公司。
二、流式细胞技术检测细胞凋亡
取对数生长期细胞,以每孔5×105个AsPC-1细胞接种于6孔板,培养至细胞80%融合后饥饿培养过夜,然后加入20、40 μmol/L的Apatinib,以不加Apatinib作为对照组。培养24 h后用胰酶消化收集细胞,PBS清洗1次后重悬于300 μl PBS,加入300 μl的binding buffer、3μl AV-FITC和3 μl PI,混均后避光10 min,上流式细胞仪检测细胞的凋亡比例。
三、细胞划痕实验检测细胞迁移能力
取对数生长期细胞,以每孔5×105个AsPC-1细胞接种于6孔板,培养至细胞90%融合后饥饿培养过夜,用200 μl的移液枪头在每孔中间划一条横线, PBS冲洗3次,然后加入20、40 μmol/L Apatinib,以不加Apatinib作为对照组。在培养即刻(0 h)及培养24 h时置显微镜下照相,每个样本测量3个划痕距离。细胞迁移率= [(0 h划痕距离-24 h划痕距离)/0 h划痕距离]×100%。实验重复3次,取均值。
四、统计学处理
结 果
一、Apatinib对AsPC-1细胞增殖的影响
对照组及10、20、30、40、50 μmol/L Apatinib处理组AsPC-1细胞的增殖抑制率分别为0、(1.45±0.68)%、(16.92±0.70)%、(23.84±0.84)%、(34.35±1.55)%、(37.33±0.81)%,细胞增殖随Apatinib浓度的增加而显著被抑制,差异有统计学意义(P<0.05)。
二、Apatinib对AsPC-1细胞凋亡的影响
对照组及20、40 μmol/L Apatinib处理组AsPC-1细胞培养24 h后的凋亡率分别为(9.44±0.18)%、(16.62±0.19)%、(25.42±0.41)%,细胞凋亡数量随Apatinib浓度增加而显著增多,差异具有统计学意义(P<0.05,图1)。
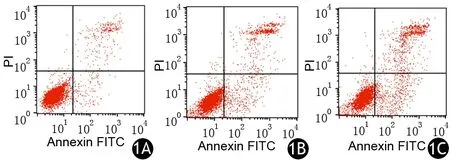
图1 对照组(1A)及20、40 μmol/L Apatinib处理组(1B、1C)AsPC-1细胞的凋亡
三、Apatinib 对AsPC-1细胞迁移能力的影响
对照组及20、40 μmol/L Apatinib处理组AsPC-1细胞培养24 h后的迁移率分别为(29.5±0.7)%、(17.4±0.9)%、(6.6±0.5)%,细胞迁移能力随Apatinib浓度增加而显著下降,差异有统计学意义(P<0.05,图2)。
讨 论
血管形成是肿瘤的标志之一,能够促进恶性肿瘤的生长和转移[10],而肿瘤转移是引起肿瘤相关死亡的主要原因。胰腺癌预后不良也和肿瘤转移密切相关,大约50%的胰腺癌患者被确诊时已有远处转移。促进肿瘤血管形成的通路主要有3条:VEGF/VEGFR、血管生成素(ANGPT)家族以及Notch-Notch配体通路,其中VEGF是血管形成的重要调节因子。VEGF通过和VEGFR-2结合发挥促血管生成的作用以及诱导VEGFR-2的下游分子的激活。VEGFR-2主要是在血管内皮细胞上表达的,在很多肿瘤表达都是上调的,包括胰腺癌[11]。VEGF和VEGFR的过表达与肿瘤生长速率加快、微血管密度增加、肿瘤增殖和转移能力增强以及不良预后相关[12]。
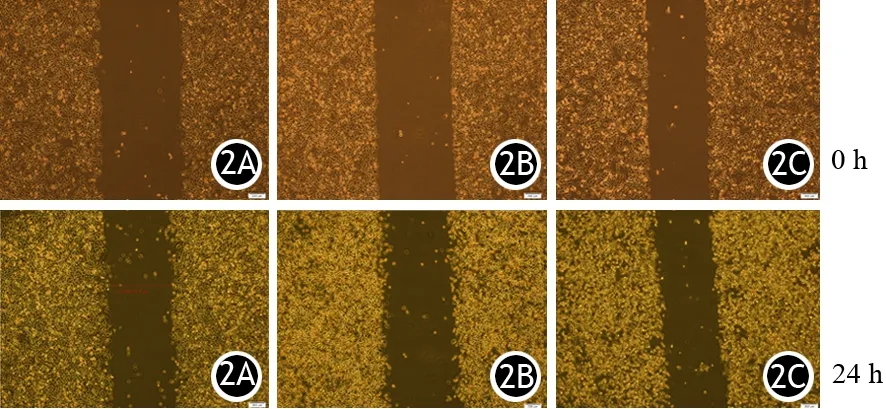
图2 对照组(2A)及20、40 μmol/L Apatinib处理组(2B、2C)AsPC-1细胞的迁移
近年来拮抗血管形成成为肿瘤治疗的研究热点之一,抗血管药物在很多实体肿瘤,如肺癌、乳腺癌、结肠癌、胃癌等的治疗中效果显著,目前很多抗血管药物也已用于胰腺癌患者,如贝伐单抗(抗VEGF的单克隆抗体)。贝伐单抗联合吉西他滨用于晚期胰腺癌患者的Ⅱ期临床研究显示,联合治疗的总生存期、无进展生存期、客观反应率均较单用吉西他滨有较明显改善,但随后的Ⅲ期研究结果显示在总生存期方面并没有明显改善[13]。多种VEGF信号通路抑制剂,如sunitinib、axitinib、sorafenib、vatalanib等也已在晚期胰腺癌患者中开展Ⅱ或Ⅲ期临床研究。Apatinib在胃癌的Ⅲ期以及非小细胞肺癌的Ⅱ期试验中显示有生存效益,且不良反应较少,在肺癌、乳腺癌患者中能明显改善患者预后。Peng等[14]研究结果显示,Apatinib通过VEGF/VEGFR-2/PI3K/AKT信号通路抑制肝内胆管癌细胞的增殖并诱导其凋亡。Doi等[15]也报道VEGF-A/VEGFR-2信号在多个胰腺癌细胞系的迁移和侵袭中起到了重要的作用。本研究结果显示,Apatinib在体外可以抑制AsPC-1细胞的增殖、迁移并诱导其凋亡,且浓度越高,抑制作用越明显。
[1] Hidalgo M. Pancreatic cancer[J]. New Engl J Med, 2010, 362(17): 1605-1617.DOI: 10.1056/NEJMra0901557.
[2] 赵玉沛.外科医师要重视胰腺癌的临床研究[J].中华消化外科杂志,2016,15(6):534-536.DOI:10.3760/cma.j.issn.1673-9752.2016.06.002.
[3] 张太平,曹喆,赵玉沛.胰腺癌的化疗与放疗[J].中华消化外科杂志,2015,14(8):619-622.DOI:10.3760/cma.j.issn.1673-9752.2015.08.006.
[4] Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial[J]. J Clin Oncol, 1997, 15(6): 2403-2413.
[5] Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo[J]. Cancer Sci, 2011, 102(7): 1374-1380.DOI: 10.1111/j.1349-7006.2011.01939.x.
[6] Roviello G, Ravelli A, Polom K, et al. Apatinib: A novel receptor tyrosine kinase inhibitor for the treatment of gastric cancer[J]. Cancer Lett,2016, 372(2): 187-191. DOI: 10.1016/j.canlet.2016.01.014.
[7] Langer CJ, Mok T, Postmus PE. Target agents in the third-/fourth-line treatment of patients with advanced(stage Ⅲ/Ⅳ) non-small cell lung cancer(NSCLC)[J]. Cancer TreatRev t, 2013,39(3): 252-260. DOI: 10.1016/j.ctrv.2012.05.003.
[8] Zhang H. Apatinib for molecular targeted therapy in tumor [J]. Drug Des Devel Ther, 2015,9(3): 6075-6081. DOI: 10.2147/DDDT.S97235.
[9] Hu X,Zhang J, Xu B, et al. Multicenter phase Ⅱ study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer [J]. Int J Cancer,2014, 135(8): 1961-1969.DOI: 10.1002/ijc.28829.
[10] Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease[J]. Nat Med, 1995, 1(1): 27-31.
[11] Doi Y, Yashiro M, Yamada N, et al. Significance of phospho-vascular endothelial growth factor receptor-2 expression in pancreatic cancer[J]. Cancer Sci, 2010, 101(6): 1529-1535.DOI: 10.1111/j.1349-7006.2010.01547.x.
[12] Longo R, Gasparini G. Challenges for patient selection with VEGF inhibitors[J]. Cancer Chemother Pharmacol, 2007, 60(2): 151-170.
[13] Ko AH, Dito E, Schillinger B, et al. A phase II study evaluating bevacizumab in combination with fixed-dose rate gemcitabine and low-dose cisplatin for metastatic pancreatic cancer: is an anti-VEGF strategy still applicable? [J]. Invest New Drugs, 2008, 26(5): 463-471.DOI: 10.1007/s10637-008-9127-2.
[14] Peng H, Li J, Hua Y, et al. Apatinib inhibits VEGFsignaling and promotes apoptosis in intrahepatic cholangiocarcinoma[J]. Oncotarget, 2016,7(13): 220-229.
[15] Doi Y, Yashiro M, Yamada N, et al. VEGF-A/VEGFR-2 signaling plays an important role for the motility of pancreas cancer cells[J]. Ann Surg Oncol, 2012, 19(8): 2733-2743.DOI: 10.1245/s10434-011-2181-6.
(本文编辑:屠振兴)
Effect of apatinib on cell proliferation, migration and apoptosis in pancreatic cancer cell line AsPC-1
GuXiaoxia,LiJie,WuMeihong,PengXiaobo,ZhanXianbao.
DepartmentofOncology,ChanghaiHospital,SecondMilitaryMedicalUniversity,Shanghai200433,China
ZhanXianbao,Email:zhanxianbao@126.com
Objective To investigate the effect of apatinib on the proliferation, apoptosis and migration of pancreatic cancer cell line AsPC-1 in vitro. Methods Pancreatic cancer AsPC-1 cells were treated by apatinib in different concentrations. Cell proliferation and apoptosis were measured by CCK-8 and flow cytometry, and the effect of apatinib on cell migration ability was observed by wound healing assay. Results In control and 10, 20, 30, 40 and 50umol/L apatinib treatment group, the inhibitory rates of AsPC-1 cells were 0,(1.45±0.68)%,(16.92±0.70)%,(23.84±0.84)%,(34.35±1.55)% and (37.33±0.81)%,respectively. Cell proliferation was obviously inhibited by apatinib as the concentration increased, and the differences were statistically significant (P<0.05). In control and 20, 40 umol/L apatinib treatment group, the apoptotic rates were (9.44±0.18)%,(16.62±0.19)% and (25.42±0.41)%, respectively. Number of apoptotic cells was obviously increased by apatinib as the concentration increased, and the differences were statistically significant (P<0.05). In control and 20, 40 umol/L apatinib treatment group, the migration ability was (29.5±0.7)% ,(17.4±0.9)% and (6.6±0.5)%, which was greatly decreased as the concentration increased, and the differences were statistically significant (P<0.05). Conclusions Apatinib can effectively inhibit the proliferation and migration of pancreatic cancer AsPC-1 cells and induce apoptosis.
Apatinib; Pancreatic neoplasms; Cell proliferation; Cell movement; Apoptosis
10.3760/cma.j.issn.1674-1935.2017.01.004
200433 上海,第二军医大学长海医院肿瘤科
湛先保,Email: zhanxianbao@126.com
2016-11-13)
