胰腺脂肪沉积的超声评估及其与糖尿病高危因素的关系
2017-02-20冯银平谷丽萍冯亮李维梅马方魏丽祝超瑜
冯银平 谷丽萍 冯亮 李维梅 马方 魏丽 祝超瑜
·论著·
胰腺脂肪沉积的超声评估及其与糖尿病高危因素的关系
冯银平 谷丽萍 冯亮 李维梅 马方 魏丽 祝超瑜
目的 探讨超声评估胰腺脂肪沉积的可行性,分析胰腺脂肪沉积与糖尿病高危因素的关系。方法 2015年10月至2016年1月间上海交通大学附属第六人民医院东院进行糖尿病流行病学调查,共调查294人,其中糖尿病(DM)111人,糖代谢异常(IGR)54人,健康者129人。记录受检者一般资料,采集空腹血行ALT、AST等生物化学指标及胰岛素检测,计算胰岛素抵抗指数(HOMA2-IR)和胰岛素分泌指数(HOMA-IS)。采用超声评估胰腺脂肪沉积,以腹直肌为参照物将胰腺回声分为1、2、3级。各组间胰腺脂肪沉积检出率应用χ2检验分析,影响胰腺脂肪沉积的糖尿病高危因素采用多因素Logistic回归分析。结果 健康组检出胰腺脂肪沉积42例(33.3%),其中1级19例,2级16例,3级8例;IGR组检出30例(55.6%),其中1、2、3级分别为14、11、5例;DM组检出67例(60.4%),其中1、2、3级分别为37、18、12例。3组间胰腺脂肪沉积检出率的差异有统计学意义(P<0.01) 。多因素Logistic回归分析显示,体重指数(BMI)、HOMA2-IR、三酰甘油(TG)、总胆固醇(TC)可能是IGR、DM患者胰腺脂肪沉积的高危因素。结论 超声评估胰腺脂肪沉积具有可行性,胰腺脂肪沉积与糖尿病的多个高危因素相关。
胰腺; 超声检查; 脂肪沉积; 糖尿病; 危险因素
Fund program:The Technology Development Innovation Grant of Pudong New District(PKJ2013-Y70);Shanghai Municipal Commission of Health and Family Planning(20134023);The Key Discipline Group Program in Health of Pudong New Distribution, Shanghai(PWZxq2014-07)
近年来胰腺脂肪沉积与糖代谢异常的关系日趋受到重视。有关前瞻性糖尿病研究的数据表明,在血糖升高的前几年胰腺功能已明显降低[1-2],胰腺脂肪沉积并不是由于糖尿病的存在[3],其含量与体重指数的增加、胰岛素抵抗、代谢综合征[4-7]及肝脂肪含量密切相关。然而,有关胰腺脂肪含量与胰岛B细胞功能关系的报道结果并不一致。有研究表明,空腹血糖受损(impaired fasting glucose,IFG)及葡萄糖耐量(impaired glucose tolerance, IGT)低下人群的胰脂肪含量与胰岛素分泌呈负相关。也有研究报道,B细胞功能与胰腺脂肪在IFG或IGT异常者[8]或糖尿病受试者之间没有关联。本研究采用超声评估糖代谢异常人群的胰腺脂肪沉积,分析其与糖尿病高危因素的相关性。
资料与方法
一、研究对象
2015年10月至2016年1月间上海交通大学附属第六人民医院东院进行糖尿病流行病学调查,共调查294人,其中糖尿病(diabetes mellitus,DM)111人,平均年龄(62±3)岁;糖代谢异常(impaired glucose regulation, IGR)54人,平均年龄(58±2)岁;健康者129人,平均年龄(58±0)岁。DM及IGR诊断标准均符合中国2型糖尿病防治指南(2013年版) 的标准[9]。
二、观察指标
记录受检者性别、年龄、身高、体重、腰围(腰部肋下缘与髂前上棘连线中点处周径)、臀围(臀部最大周径),计算体重指数(BMI)、腰臀比(WHR)。
抽取受检者空腹血,应用全自动生化分析仪检测血谷丙转氨酶(ALT)、谷草转氨酶(AST)、总胆固醇(TC)、三酰甘油(TG)、高密度脂蛋白胆固醇(HDL-C)、低密度脂蛋白胆固醇(LDL-C)、空腹血糖(FPG)、C反应蛋白(CRP)。应用放射免疫法测定空腹血胰岛素(FINS),稳态模型评估法计算胰岛素抵抗指数(insulin resistance index,HOMA2-IR)和胰岛素分泌指数(insulin secretion index,HOMA-IS)。HOMA2-IR=空腹胰岛素×FPG÷22.5),HOMA-IS=20×FPG÷(FPG-3.5)。
三、胰腺超声检查
使用Philips iU Elite实时超声诊断仪进行检查,凸阵探头频率3.5MHz。受检者空腹12 h,取平卧位,常规扫查胰腺区域,观察胰腺形态、大小、边界及内部回声强度。以腹直肌为参照物对胰腺回声分级:胰腺回声与腹直肌相等或较肝脏回声稍强为1级,胰腺回声高于腹直肌低于腹后壁脂肪为2级,胰腺回声与腹后壁脂肪相等为3级(图1)。
四、统计学处理
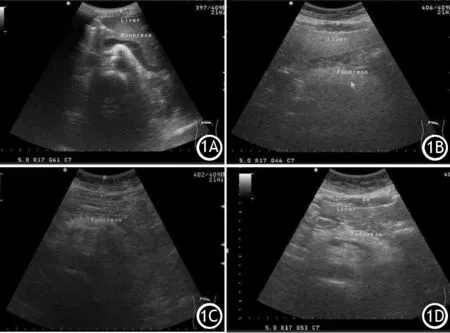
图1 正常胰腺回声(1A)及胰腺回声强度1级(1B)、2级(1C)、3级(1D)的超声图像
结 果
一、胰腺脂肪沉积检出情况
健康组检出胰腺脂肪沉积42例,其中1级19例,2级16例,3级8例;IGR组检出30例,其中1、2、3级分别为14、11、5例;DM组检出67例,其中1、2、3级分别为37、18、12例。健康组、IGR组、DM组胰腺脂肪沉积检出率分别为33.3%、55.6%、60.4%,胰腺脂肪沉积检出率随糖尿病病程的进展逐渐增加(χ2=19.14,P<0.01) 。
二、各组观察指标的变化
健康组、IGR组、DM组间TC、TG、LDL-C、FPG、HOMA2-IR、HOMA-IS的差异均有统计学意义,而AST、ALT、HDL-C、CRP、FINS的差异均无统计学意义(表1)。
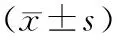
表1 健康组、IGR组、DM组的各观察指标的变化
三、胰腺脂肪沉积的多因素Logistic回归分析
健康组的血清TC、TG水平与胰腺脂肪沉积检出率有关,且TC、TG每增加一个单位,受检者患胰腺脂肪沉积的概率会增加44%、34%(表2);IGR组的BMI、HOMA2-IR、HOMA-IS、ALT、AST、TG、TC与胰腺脂肪沉积检出率有关 (表3) ;DM组的BMI、HOMA2-IR TG、TC、LDL-C与胰腺脂肪沉积检出率有关(表4) 。BMI、HOMA2-IR、TG、TC可能是IGR、DM患者胰腺形成脂肪沉积的高危因素。
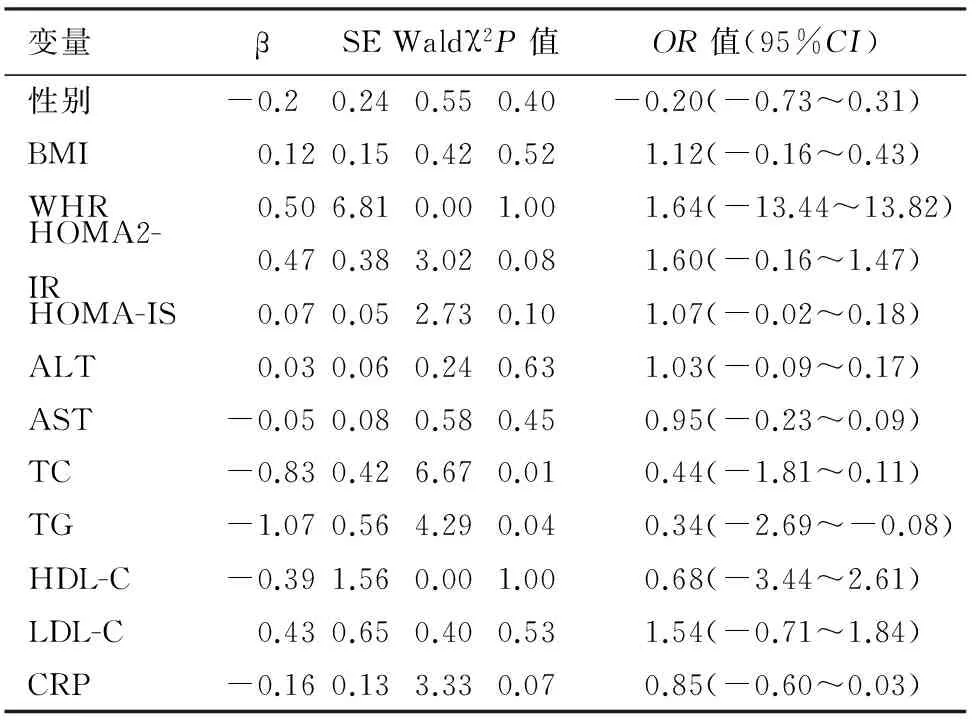
表2 健康组胰腺脂肪沉积的多因素Logistic回归分析
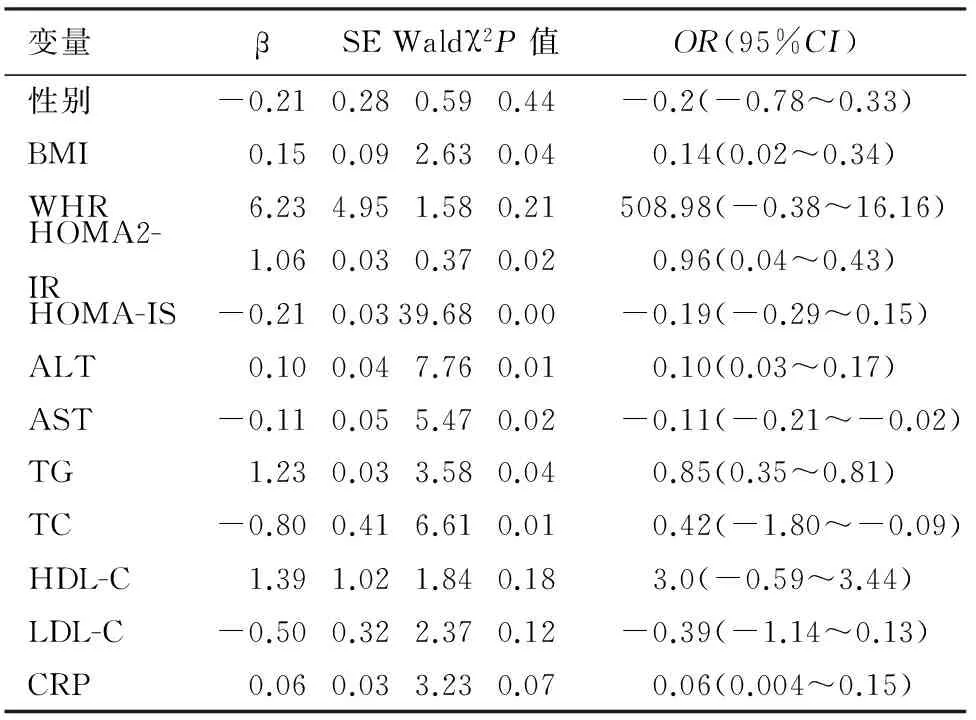
表3 IGR组胰腺脂肪沉积的多因素Logistic回归分析
讨 论
糖尿病是常见的代谢性疾病,与内脏脂肪的沉积关系密切。研究发现[10-11],有胰腺脂肪浸润者发生糖尿病的风险增加,认为脂肪浸润的胰腺可能引起胰岛细胞功能异常,B细胞质量和功能丧失,从而导致糖尿病的发生和发展。Heni等[12]的研究表明,与糖尿病患者内脏脂肪相比,胰腺脂肪是更强的胰岛素分泌受损的决定因素。Lee等[13]的研究表明,
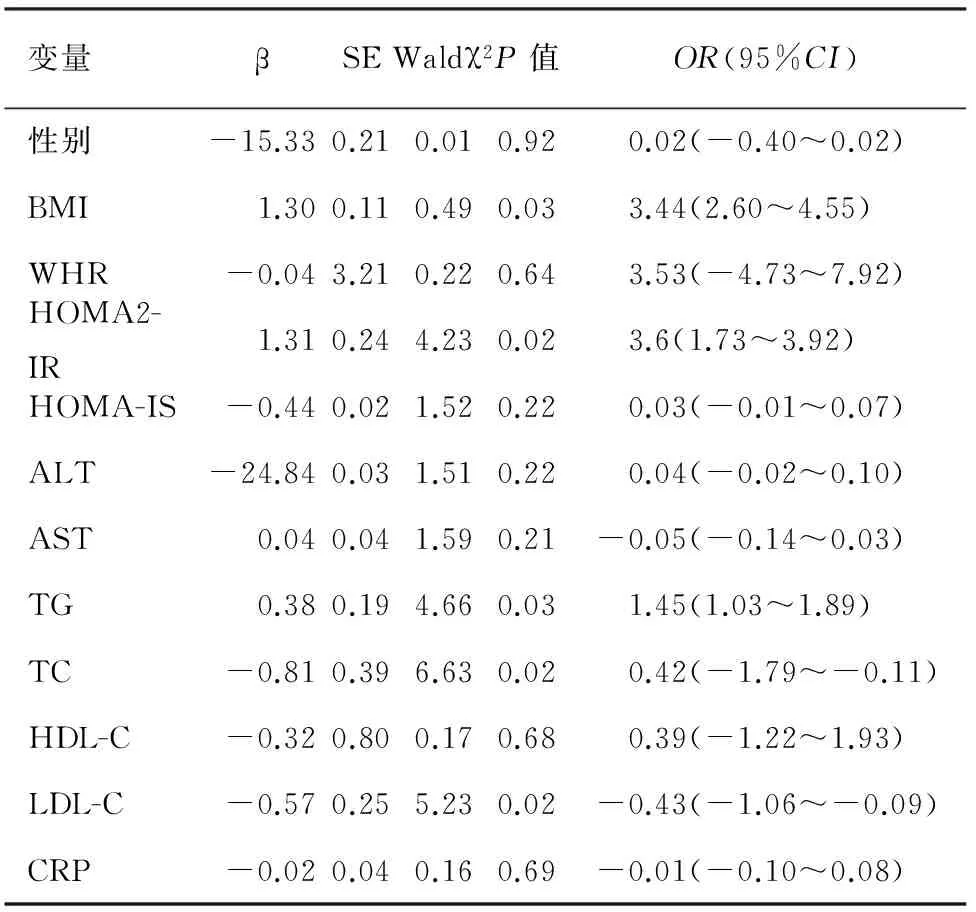
表4 DM组胰腺胰腺脂肪沉积的多因素Logistic回归分析
糖代谢异常大鼠胰岛三酰甘油含量显著增加先于糖尿病发生。本研究结果显示,HOMA2-IR是胰腺脂肪沉积的相关因素(OR=3.6,95%CI=1.73~3.92),而胰岛素抵抗与代谢综合征和2型糖尿病的发生、发展有直接关系。因此,早期诊断胰腺脂肪沉积,对其危险因素进行早期干预,可能对延缓糖尿病的进程有积极的意义。
关于评估胰腺脂肪沉积方法的探讨国内外报道较少,且有争议[14]。质子核磁共振光谱学(MRS)长期以来一直被视为非侵入性胰腺脂肪量化的金标准[14],但因其价格昂贵,临床上难以大规模开展。Marks等[15]曾用超声检查评估28例胰腺回声,并与CT图像的胰腺外观形态进行比较,结果表明脂肪沉积是胰腺回声增强的主要决定因素。Worthen和Beabeau[16]的研究结果表明,年龄增长和体脂含量也是胰腺回声增强的因素。本研究采用超声检查正常人群、IGR者、DM者的胰腺,以胰腺超声回声增强强度评估脂肪沉积的程度,发现IGR及DM患者胰腺脂肪沉积的检出率显著增加,并与IGR、DM患者的 BMI、HOMA2-IR、TG、TC因素均相关。其中IGR患者的HOMA-IS与胰腺脂肪沉积呈负相关,这与患者在糖尿病早期胰岛素分泌增加、糖尿病期胰岛素分泌减少的临床特征相符。
因超声具有无创、简便、快捷、可重复等独特优点, 应用该技术估测胰腺脂肪沉积不失为一种实用、有效影像学手段。
[1] Holman RR. Assessing the potential for alpha-glucosidase inhibitors in prediabetic states[J].Diabetes Res Clin Prac,1998,40:S21-S25.
[2] Matthews DR, Cull CA, Stratton IM, et al. UKPDS 26: Sulphonylurea failure in non-insulin-dependent diabetic patients over six years. UK Prospective Diabetes Study(UKPDS)Group[J].Diabet Med,1998,15(4):297-303.DOI:10.1002/(SICI)1096-9136(199804)15:4<297::AID-DIA572>3.0.CO;2-W.
[3] Hori M,Kitahashi T,Imai T,et al.Enhancement of carcinogenesis and fatty infiltration in the pancreas in N-nitrosobis(2-oxopropyl)amine-treated hamsters by high-fat diet[J].Pancreas,2011,40(8):1234-1240.DOI:10.1097/MPA.0b013e318220e742.
[4] Pezzilli R, Calculli L.Pancreatic steatosis: Is it related to either obesity or diabetes mellitus[J]?World J Diabetes,2014,5(4):415-419.DOI:10.4239/wjd.v5.i4.415.
[5] Della Corte C,Mosca A,Majo F,et al.Nonalcoholic fatty pancreas disease and Nonalcoholic fatty liver disease: more than ectopic fat[J].Clin Endocrinol (Oxf),2015,83(5):656-662.DOI:10.1111/cen.12862.
[6] Hannukainen JC, Borra R, Linderborg K, et al. Liver and pancreatic fat content and metabolism in healthy monozygotic twins with discordant physical activity[J].J Hepatol,2011,54(3):545-552.DOI:10.1016/j.jhep.2010.07.029.
[7] Sepe PS, Ohri A, Sanaka S, et al.A prospective evaluation of fatty pancreas by using EUS[J].Gastrointest Endosc,2011,73(5):987-993.DOI:10.1016/j.gie.2011.01.015.
[8] van der Zijl NJ, Goossens GH, Moors CC, et al.Ectopic fat storage in the pancreas, liver, and abdominal fat depots: impact on beta-cell function in individuals with impaired glucose metabolism[J]. Clin Endocrinol Metab,2011,96(2):459-467.DOI:10.1210/jc.2010-1722.DOI: 10.3969/j.issn.1006-6187.2014.08.027.42.
[9] 中华医学会糖尿病学分会.中国2型糖尿病防治指南(2013年版)[J].中国糖尿病杂志,2014,22(8):2-42.DOI: 10.3760/cma.j.issn.1674-5809.2014.07.004.
[10] Anuradha R, Saraswati M, Kumar KG, et al.Apoptosis of beta cells in diabetes mellitus[J].DNA Cell Biol, 2014, 33(11):743-748.DOI:10.1089/dna.2014.2352.
[11] Tang C, Naassan AE, Chamson-Reig A,et al.Susceptibility to fatty acid-induced β-cell dysfunction is enhanced in prediabetic diabetes-prone biobreeding rats: a potential link between β-cell lipotoxicity and islet inflammation[J].Endocrinology,2013,154(1):89-101.DOI:10.1210/en.2012-1720.
[12] Heni M,Machann J,Staiger H,et al.Pancreatic fat is negatively associated with insulin secretion in individuals with impaired fasting glucose and/or impaired glucose tolerance: a nuclear magnetic resonance study[J].Diabetes Metab Res Rev,2010,26(3):200-205.DOI:10.1002/dmrr.1073.
[13] Lee Y, Hirose H, Ohneda M, et al. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships[J]. Proc Natl Acad Sci U S A,1994,91(23):10878-10882.
[14] Hu HH, Kim HW, Nayak KS, et al. Comparison of fat-water MRI and single-voxel MRS in the assessment of hepatic and pancreatic fat fractions in humans[J]. Obesity (Silver Spring),2010,18(4): 841-847.DOI:10.1038/oby.2009.352.
[15] Marks WM,Filly RA,Callen PW.Ultrasonic evaluation of normal pancreatic echogenicity and its relationship to fat deposition[J].Radiology,1980,137(2):475-479.DOI:10.1148/radiology.137.2.7433680.
[16] Worthen NJ,Beabeau D.Normal pancreatic echogenicity: relation to age and body fat[J].AJR Am J Roentgenol,1982,139(6):1095-1098.DOI:10.2214/ajr.139.6.1095.
(本文编辑:吕芳萍)
Ultrasonic estimation on pancreatic fat deposition and its relationship with risk factor for diabetes
FengYinping,GuLiping,FengLiang,LiWeimei,MaFang,WeiLi,ZhuChaoyu.
DepartmentofUltrosound,ShanghaiSixthPeoples′Hospital,ShanghaiJiaotongUniversity,Shanghai201306,China
MaFang,Email:mafang59@126.com
Objective To investigate the feasibility of evaluating the pancreatic fat deposition with ultrasound, and analyze the relationship of pancreatic fat deposition with the risk factors for diabetes. Methods Two hundred and ninety-four subjects were recruited in the diabetes epidemic survey of Shanghai Sixth Peoples′ Hospital,Shanghai Jiaotong University from Oct 2015 to Jan 2016, including 111 diabetes mellitus (DM) cases, 54 sugar metabolic abnormalities (IGR) cases, and 129 healthy cases as control group. The general data were collected. Biochemical indicators including ALT, AST and insulin in fasting blood samples were detected, and insulin resistance index (HOMA2-IR) and insulin secretion index (HOMA-IS) were calculated. The pancreatic fat deposition was evaluated by the ultrasound, and pancreas echoes were divided into three levels (1, 2, 3), by comparing with the echo of the rectus. The Chi-square test was used to analyze the pancreatic fat deposition rate, while the Logistic regression was used to figure out the diabetes high-risk factors influencing the pancreatic fat deposition. Results Forty-two subjects were found to have the pancreatic fat deposition in the healthy group (33.3%), of whom 19 cases, 16 cases and 8 cases were categorized into level 1, 2 and 3, respectively. Thirty subjects were found to have the pancreatic fat deposition in the IGR group (55.6%), of whom 14 cases, 11 cases and 5 cases were categorized into level 1, 2 and 3, respectively. Sixty-seven subjects were found to have the pancreatic fat deposition in the DM group (60.4%), of whom 37 cases, 18 cases and 12 cases, were categorized into level 1, 2, 3, respectively. The pancreatic fat deposition rates of the 3 groups were statistically significantly different (P<0.01).Multivariate Logistic regression analysis showed that body mass index (BMI), HOMA2-IR, high triglycerides (TG), and total cholesterol (TC) may be the-risk factors for the pancreatic fat deposition in IGR and T2DM patients. Conclusions The ultrasound evaluation on pancreatic fat deposition was feasible, and the pancreatic fat deposition was related to multiple high-risk factors for diabetes.
Pancreas; Ultrasonography; Fat deposition; Diabetes mellitus; Risk factors
10.3760/cma.j.issn.1674-1935.2017.01.006
201306 上海,上海交通大学附属第六人民医院(冯银平、谷丽萍、冯亮、李维梅、马方、魏丽、祝超瑜);河南新乡医学院(冯银平)
马方,Email: mafang59@126.com
浦东新区科技发展基金创新资金项目(PKJ2013-Y70);上海市卫计委重点项目(20134023);上海市浦东新区卫生系统重点学科群建设项目(PWZxq 2014-07)
2016-07-29)
