Intervention of riboflavin metabolism improves sensitivity of ovarian cancer HO8910 cells to cisplatin in vitro
2016-02-14YANGRuhuiSONGYuWUSongquanSHENXiangchun
YANG Ru-hui,SONG Yu,WU Song-quan,SHEN Xiang-chun
(1.College of Medicine and Health,Lishui University,Lishui 323000,China;2.The Third Affiliated Hospital of Southern Medical University,Guangzhou 510630,China;3.Key Laboratory of Optimal Utilization of Natural Medicine Resources,Guizhou Medical University,Guiyang 550004,China)
Intervention of riboflavin metabolism improves sensitivity of ovarian cancer HO8910 cells to cisplatin in vitro
YANG Ru-hui1,3,SONG Yu2,WU Song-quan1,SHEN Xiang-chun3
(1.College of Medicine and Health,Lishui University,Lishui 323000,China;2.The Third Affiliated Hospital of Southern Medical University,Guangzhou 510630,China;3.Key Laboratory of Optimal Utilization of Natural Medicine Resources,Guizhou Medical University,Guiyang 550004,China)
OBJECTIVE To investigate the effect of intervention with riboflavin(RIB)metabolic path⁃ways on the sensitivity of human ovarian cancer cells HO8910 to cisplatin(DDP).METHODS HO8910 cells were infected with a lentivirus vector harboring the short hairpin(sh)RNA of riboflavin transporter 2(RFT2)gene or chlorpromazine(CHL,a competitive inhibitor of RIB).According to divid⁃ed groups,HO8910 cells were treated alone or in combination withRFT2shRNA,CHL 50 μmol·L-1,DDP 20 μmol·L-1or RIB 20 μmol·L-1.48 h after treatment,cell viabilities were detected by CCK-8 assay,the colony formation number was calculated through crystal violet staining,and apoptosis rates,mito⁃chondrial membrane potential(MMP),content of reactive oxygen species(ROS)and percentage of CD44+CD133+cells were analyzed by flow cytometry.RESULTS Compared with the sole DDP treat⁃ment group,viabilities,colony formation abilities,MMP and percentage of CD44+CD133+cells inRFT2shRNA or CHL+DDP group were significantly decreased(P<0.01),while the contents of ROS and percentage of apoptotic cells were increased(P<0.01).RIB treatment could reduce the effect of DDP on HO8910 cells(P<0.01).CONCLUSION Intervention with RIB metabolism can enhance the sensitivity of HO8910 cells to DDP by promoting DDP-induced oxidative damage and reducing the percentage of stem cell-like cancer cell(CD44+CD133+)in tumor cells.
riboflavin;chlorpromazine;riboflavin transporter 2;cisplatin;ovarian cancer
In the process of cancer treatment,cell apoptosis induced by chemotherapeutic agents involves excessive production of reactive oxygen species(ROS)and DNA damage[1].Recent studieshave showed thatantioxidantsmay accelerate the growth of early tumors in mice[2].Therefore,we assume that reducing anti-oxidant capability of cancer cells may increase the effect of chemotherapy.Riboflavin(RIB)participates in cell biological oxidation and increases cell antioxidation.RIB insufficiency reduces the antioxi⁃dant capability of cells against ROS[3].Tumor cells, especially stem cell-like cancer cells(CSCs),canenrichwithRIB[4].However,the relation between this phenomenon and resis⁃tance of tumor cells or CSCs to chemotherapeu⁃tic agents remains unclear.
Cisplatin(DDP),which is commonly used for ovarian cancer,interferes with replication of nuclear DNA and mitochondrial DNA.DDP dis⁃rupts the cell metabolic balance of oxygen-free radicals by damaging the mitochondria,which leads to oxidative stress[5-6].The concentration of DDP in the mitochondrial DNA is higher than that in the nuclear DNA,and its clearance in the mito⁃chondrial DNA is significantly later than that in the nuclear DNA[7].Therefore,DDP not only can lead to energy metabolic disorders but also induce the generation of free radicals in cells.RIB,A coenzyme of flavin mononucleotide and flavin adenine dinucleotide,can reduce free radicals and improve mitochondrial energy metabolism[8]. Thus,RIB attenuates mitochondrial ultrastruc⁃ture damage caused by DDP.And there were reports that the ameliorative effect of RIB on the DDP induced nephrotoxicity,hepatotoxicityor nephrotoxicity[9-10].In the present study,we inves⁃tigated the effects of the interference with the RIB metabolic pathway by using RIB transporter 2(RFT2)or chlorpromazine(CHL),which is a competitive inhibitor of RIB,on the sensitivity of ovarian carcinoma cells to DDP.This research will shed light on the underlying mechanisms of RIB in ovarian cancer drug resistance and pro⁃vide a new strategy for ovarian cancer chemo⁃therapy.
1 METHODS AND MATERIALS
1.1 Cells,drugs,reagents and equipments
Human ovarian cancer cell lines HO8910(Shanghai Cell Bank of Chinese Academy of Sciences);RIB and CHL(National Institutes for Food and Drug Control of China);CCK-8 detection kit(Dojindo Laboratories,Japan);mitochondrial membrane potential(MMP)assay kit with JC-1 and ROS assay kit(DCFH-DA)(Beyotime Institute of Biotechnology,Jiangsu,China);apoptosis detection kit(Multi Sciences Biotech Co.,Ltd.,Zhejiang,China);mouse anti-human CD44-FITC IgG antibody and isotype control antibody(BD Biosciences,USA);mouse anti-human CD133-PE antibody and isotype control antibody(MiltenyiBiotec,Gemany).3111 Carbon dioxide incubator(Thermo,USA);Accuri C6 flow cytometry(BD Biosciences,USA);qPCR System,and Western blot system(Bio-Rad,USA).
1.2 Cell culture and treatment
HO8910 cells and 293T cells were cultured in DMEM containing 10%fetal bovine serum supplemented with penicillin(1×105U·L-1)and streptomycin(0.1 g·L-1)at 37℃in 5%CO2atmo⁃sphere.The medium was changed every 2-3 d depending on cell growth.Exponentially growing cells were seeded in 6-well plates(1×106cells perwell).The cellswererandomlydivided into eight groups:normal cell control group(control),nonspecific target sequence short hairpin(sh)RNA control group(shRNAcon),RFT2shRNA group,CHL 50 μmol·L-1group,DDP 20 μmol·L-1group,RFT2shRNA+DDP group,CHL+ DDP group,and DDP+RIB 20 μmol·L-1group. Cells were collected 48 h after treatment.The experiments were totally repeated for 3 times for each group.
1.3 Lentiviral vector harboring shRNA ofRFT2
Three interference sequences and one non⁃specific target sequence were screened and syn⁃thesized using a design software in accordance with the nucleotide sequence oftheRFT2gene(NC_000020.11)and design principles of shrank.The sequences forRFT2-shRNA1783, shRNA1901,and shRNA2078 were 5′-GCCT⁃GAGTAATTGAATCATGA-3′,5′-GCTCAGATCTCCTTTGCTTTG-3′and 5′-GGATTCCAGTAAGTACCAACT-3′.Interference DNA sequences were designed and synthesized by Shanghai GenePh⁃arma Co.,Ltd..Lentivirus skeleton plasmid pll3.7 was digested using restriction enzymesXhoⅠandHpaⅠand then connected with double-stranded DNA to produce shRNA expression vectors. After transfection,supernatants of 293T cells containing lentivirus were obtained.The lentiviral vector containing shRNA was used for the following experiments.HO8910 cells transfected by lentiviral vectors containingRFT2shRNA were named shRNA1783,shRNA1901,andshRNA2708,respectively.Lentiviral vector containing invalid sequence shRNA served as control(shRNAcon). After 48 h treatment,interference efficiency ofeach shRNA was detected by Western blotting and qPCR methods.Image J software was used to calculate the integrated absorbance(IA)of identified bands,and the expression of RFT2 was calculated using the following formula:the relative expression of RFT2 protein=IARFT2/IAβ-actin. Relative mRNA expression ofRFT2was calculated and expressed as 2-ΔΔCtmethod by CFX Man⁃ager software(Bio-Rad).
1.4 Cell vitalities detected by CCK-8
Cells were seeded at a density of 1×105cells per well with 100 μL DMEM medium.Cell viability was determined by CCK-8 assay kit according to the instructions after 48 h treat⁃ment.The absorbance(A)value of each well was read at 450 nm(A450nm).Each group had six well plates.Cell viability was calculated using the following formula:cell viability(%)=(A450nmof experiment group-A450nmof blank)/(A450nmof control group-A450nmof blank)×100%.
1.5 Apoptosis assay by flow cytometry
1×105cells were collected through centrifu⁃gation after 48 h treatment.The cells were digested by 0.25%trypsin(excluding EDTA),washed twice(centrifugation at 100×gfor 5 min)with4℃ precooledPBS,addedwith5 μL AnnexinⅤ-FITC and 10 μL PI,and then incubated at 4℃for 5 min.Rate of apoptosis was assayed via flow cytometry.
1.6 Colony formation ability detected by crystal violet staining
HO8910 cells in good condition were digested and centrifuged 1500×gfor 5 min.Then,they were repeatedly suspended and seeded in 6-well plates. Shaken,and then incubated at 37℃ in 5%CO2under saturated humidity.The colonies were stained by crystal violet at 48 h after treatment. Up to 200 cells per plate were counted as a cell counter using a microscope.
1.7 Cell mitochondrial membrane potential detected by flow cytometry
1×105cells from each group were digested,collected through centrifugation, suspended,and mixed with a JC-1 working liquid probe in accordance with the manufacturer′s protocol. The cells were incubated at 37℃ for 20 min,and centrifugal cells were washed twice with 4℃buffer.The MMP(Δψm) was determined by the JC-1 probe according to the manufacturer′s specifications and the data were analyzed using Accuri C6 flow cytometry.And the stained cells were analyzed by flow cytometry equipped with CellQuest software.
1.8 ROS level detected by flow cytometry
2×105cells from each group were collected 48 h after drug treatment through 1500×gfor 5 min. Cells were suspended with serum-free medium,and then incubated at 37℃ for 20 min.The supernatants were removed after centrifugation 1500×gfor 5 min,and then the cells were washed three times with serum-free medium.Finally,ROS levels were detected using DCFH-DA kit according to the manufacturer′s instructions.Levels of ROS(104DCF-H)were detected using an Accuri C6 flow cytometry with CellQuest software.
1.9 Percentage of CD44+CD133+cells ana⁃lyzed by flow cytometry
2×105cells from each group were collected 48 h after treatment through centrifugation 1500×gfor 5 min,and suspended with PBS twice. CD44-FITC and CD133-PE antibodies or isotype control antibodies were mixed and incubated at 4℃ for 20 min.CD44+CD133+cells were ana⁃lyzed using a flow cytometry after adding PBS to 500 μL volume.The percentage of CD44+CD133+cells was analyzed by flow cytometry equipped with CellQuest software.
1.10 Statistic analysis
Data were analyzed using SPSS 11.5 soft⁃ware,and the results were shown asx±s.TheFtest for homogeneity of variance was conducted,and difference between groups was analyzed usingttest.P<0.05 was considered to indicate significant difference.
2 RESULTS
2.1 Interference efficiency ofRFT2shRNA lentiviral vector
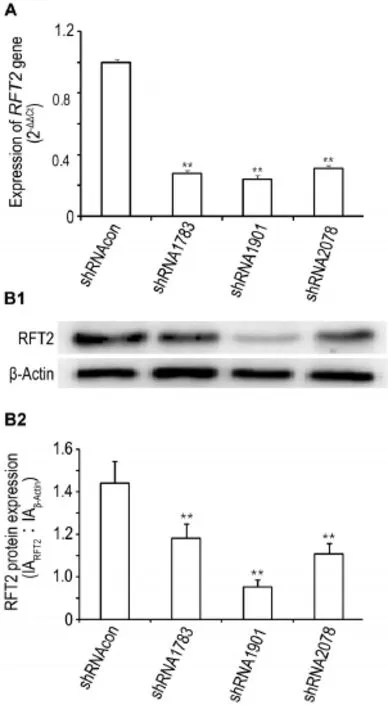
Fig.1 Gene and protein expression of riboflavin transporter 2(RFT2)in HO8910 cells after treatment with shRNA lentiviral vector detected by Western blotting(A)and qPCR(B).HO8910 cells were transfected with lentiviral vectors containingRFT2shRNAs〔shRNA1783, shRNA1901,shRNA2708,and the lentiviral vector containing nonspecific target sequence shRNA as shRNA control(shRNAcon)〕respectivly.48 h later,expression of RFT2 were detected.B2 was semi-quantitative of B1.x±s,n=3.**P<0.01,compared with shRNAcon group.
As shown in Fig.1,compared with shRNAcon group,expressions of gene and protein of RFT2 in shRNA1783,shRNA1901 and shRNA2078 group were decreased(P<0.01),suggesting thatRFT2shRNA could effectively inhibitRFT2gene and protein expression,and that shRNA1901 might have the best interference effectiveness in genes or proteins and is used in follow-up experiments.
2.2 Effects ofRFT2shRNA or CHL combined with DDP on the viability of HO8910 cells
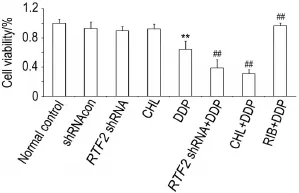
Fig.2 Synergistic effect ofRFT2shRNA or chlor⁃promazine(CHL)combined with cisplatin(DDP)on via⁃bility of HO8910 cells detected by CCK-8.HO8910 cells were collected from each group 48 h after treatment;shRNAcon group:treated with lentiviral vector containing nonspecific target sequence shRNA;RFT2shRNA group:treated with lentiviral vector containingRFT2shRNA;CHL group:treated with CHL 50 μmol·L-1;DDP group:treated with DDP 20 μmol·L-1;RFT2shRNA+DDP group:treated with lentiviral vector containingRFT2shRNA and DDP 20 μmol·L-1;CHL+DDP group:treated with CHL 50 μmol·L-1and DDP 20 μmol·L-1;RIB+DDP group:treated with RIB 20 μmol·L-1and DDP 20 μmol·L-1.x±s,n=3. **P<0.01,compared with normal control group;##P<0.01,compared with DDP group.
As shown in Fig.2,compared with normal control group,viability of the cells in DDP group was decreased(P<0.01),as inRFT2shRNA group or CHL group.Compared with DDP group, viability of the cells was further decreased in the presence of DDP(RFT2shRNA+DDP group or CHL+DDP group)(P<0.01).By contrast,viability of the cells in RIB+DDP group was increased(P<0.01),indicateing that interference of the RIB metabolism could aggravate HO8910 cells′damage induced by DDP,and the effect of DDP on cell viability was abrogated by RIB treatment(P<0.01).
2.3 Effects ofRFT2shRNA or CHL combined withDDP oncolonyformationabilityof HO8910 cells
As shown in Fig.3,compared with normal control group,the number of cell colonies in CHL and DDP groups was decreased(P<0.05 orP<0.01),but there was no statistically signifi⁃cant decrease in shRNAcon orRFT2shRNA group.Compared with DDP group,the number of cell colonies was decreased in the presence of DDP(RFT2shRNA+DDP and CHL+DDP group)(P<0.01).However,the number of cell colonies in RIB+DDP group was increased(P<0.01),suggesting that the effect of DDP could be abolished by RIB treatment.
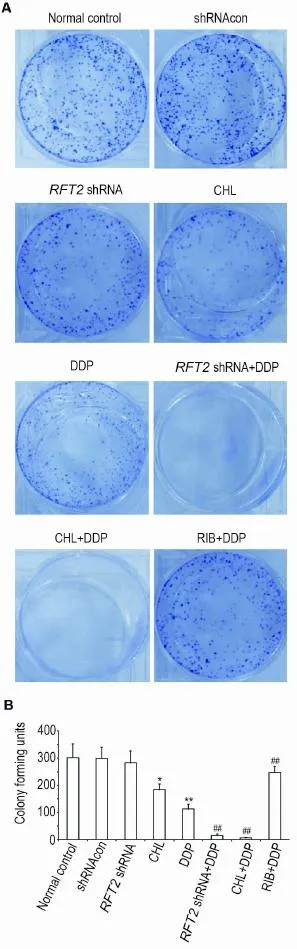
Fig.3 Synergistic effects ofRFT2shRNA or CHL combined with DDP oncolony formation ability of HO8910 cells detected by crystal violet staining.See Fig.2 for the treatment.CFU:colony formation unit.B was quanti⁃tative result of A.x±s,n=3.*P<0.05,**P<0.01,compared with normal control group;##P<0.01,compared with DDP group.
2.4 Effects ofRFT2shRNA or CHL combined with DDP on mitochondrial membrane potential of HO8910 cells
As shown in Fig.4,48 h after treatment,MMP of mitochondria was decreased in DDP and CHL groups(P<0.05,P<0.01)compared with normal control group,but there was no sta⁃tistically significant decrease in shRNAcon group andRFT2shRNA group.Compared with DDPgroup,MMP of mitochondria was further decreased inRFT2shRNA+DDP group and CHL+DDP group(P<0.01),while MMP of mitochondria was increased in RIB+DDP group(P<0.01).

Fig.4 Effects ofRFT2shRNA or CHL combined with DDP onmitochondrialmembranepotential(MMP)(Δψm)of H08910 cells detected by flow cytometry.See Fig.2 for the treatment.B was semi-quantitative result of A.x±s, n=3. *P<0.05, **P<0.01, comparedwith a normalcontrol group;##P<0.01,compared with DDP group.
2.5 Effects ofRFT2shRNA or CHL combined with DDP on levels of ROS in HO8910 cells
As shown in Fig.5,48 h after treatment,the level of ROS was increased in DDP group(P<0.01)compared with normal control group,but there was no statistically significant decrease in shRNAcon,RFT2shRNA or CHL groups. Compared with DDP group,the level of ROS was increased inRFT2shRNA+DDP and CHL+DDP groups(P<0.01),while the level of ROS was decreased in RIB+DDP group(P<0.01).
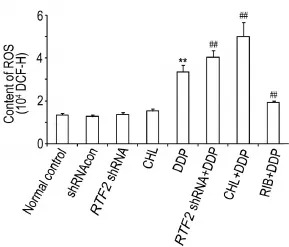
Fig.5 Synergistic effects ofRFT2shRNA or CHL combined with DDP on level of reactive oxygen species(ROS)in HO8910 cells by flow cytometry.See Fig.2 for the treatment.B was semi-quantative result of A.x±s,n=3. **P<0.01,compared with normal control group;##P<0.01,compared with DDP group.
2.6 Effects ofRFT2shRNA or CHL combined with DDP on apoptosis of HO8910 cells
As shown in Fig.6,compared with normal control group,the apoptotic level was increased in all shRNAcon,RFT2shRNA,CHL and DDP groups(P<0.01).Compared with DDP group,the apoptotic level was further increased inRFT2shRNA+DDP group and CHL+DDP group(P<0.01),but was decreased in RIB+DDP group.
2.7 Effects ofRFT2shRNA or CHL combined with DDP on percentage of CD44+CD133+HO8910 cells
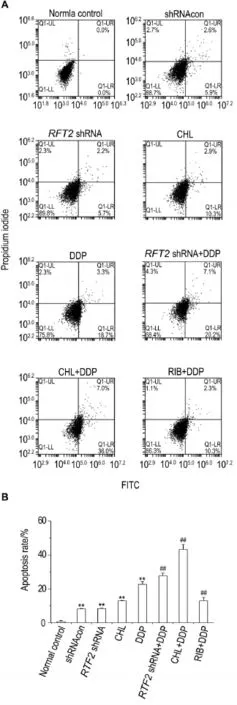
Fig.6 Effects ofRFT2shRNA or CHL combined with DDP on apoptosis rate of HO8910 cells by flow cytometry.See Fig.2 for the treatment.B was the semi-quan⁃titative result of A.x±s,n=3.**P<0.01,compared with normal control group;##P<0.01,compared with DDP group.
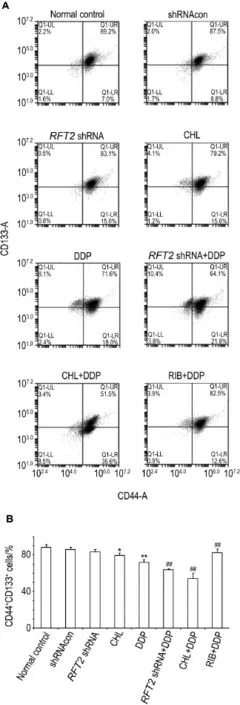
Fig.7 Synergistic effects ofRFT2shRNA or CHL com⁃bined with DDP on percentage of CD44+CD133+HO8910 cell detected by flow cytometer.See Fig.2 for the treatment.B was semi-quantitative result of A.x±s,n=3.*P< 0.05,**P<0.01,compared with normal control group;##P<0.01,compared with DDP group.
As shown in Fig.7,compared with normal control group,the percentage of CD44+CD133+HO8910 cells was decreased in CHL or DDP group(P<0.05,P<0.01).Compared with DDP group,the percentage of CD44+CD133+HO8910 cells was further decreased inRFT2shRNA+DDP and CHL+DDP groups(P<0.01), butwas increased in RIB+DDP group.
3 DISCUSSION
The concentration of DDP in the mtDNA is higher than that in the nuclear DNA,and its clearance in the mtDNA is significantly later than that in the nuclear DNA[8].Therefore,DDP not only can lead to energy metabolic disorders but also induce the generation of free radicals in cells.RIB,a coenzyme of flavin mononucleotide and flavin adenine dinucleotide,can reduce free radicals and improve mitochondrialenergy metabolism[8].Thus,RIB can attenuate mito⁃chondrial ultrastructure damage caused by DDP. Also,there are report that ameliorative effect of RIB on the DDP induced nephrotoxicity,hepato⁃toxicity or nephrotoxicity[9-10].So,in this study,we assumed that RIB could interfere with the antitumor effect of DDP and blocking RIB would enhance the antitumor effect of chemotherapeutic agents.To test this hypothesis,we explored whether interference with protein of RFT2 and CHL can block metabolic pathway of RIB.The transport of RIB in cells can be reduced by inter⁃ference withRFT2expression because RFT2 is the main RIB transporter,and it is at the central core during the transfer process[11].RIB is con⁃verted into flavin mononucleotide by flavoki⁃nase,and flavin mononucleotide is transformed into flavin adenine dinucleotide by flavin adenine dinucleotide synthetase.Given its similar struc⁃ture to RIB,CHL can inhibit RIB physiological function by suppressing the transformation of RIB to flavin adenine dinucleotide[12].In the present study,RFT2interference and CHL treatment exerted no effects on the viability of HO8910 cells at 48 h,but CHL reduced the clone formation ability of the cells.However,RFT2interferenceor CHL treatment combined with DDP exerted obvious synergistic effects on the viability and clone formation ability of the cells.These data suggest that the effects of RIB are very impor⁃tant for viability and proliferation of cells.More⁃over,the results of RIB combination with DDP showed that RIB can weaken the effects of DDP on the viability and clone formation ability of the cells. Thus,intervention with the RIB metabolic path⁃way could improve the sensibility of ovarian can⁃cer cells to the influence of DDP on viability and clone formation ability.
Mitochondrial damage is the key event in cell apoptosis.It promotes ROS accumulation,which strengthens cell mitochondrial damage. Changes of in mitochondrial permeability and its transmembrane potential decline after mitochon⁃drial damage are the initial events in the apopto⁃sis cascade,and mitochondrial dissipation trig⁃gers the irreversible process of apoptosis[13].In the present study,RFT2interference or CHL assisted DDP in reduceing mitochondrial trans⁃membrane potential,increased the ROS content and the apoptotic rate of ovarian cancer cells sig⁃nificantly.However,RFT2interference or CHL alone exerted no obvious influence on the mito⁃chondrial transmembrane potential or ROS level in the cells.These results indicate that the inter⁃vention with the metabolic pathways of RIB alone exerted minimal effects on the mitochondri⁃al function of the cells.However,the combina⁃tion ofRFT2or CHL with DDP considerably in⁃creased the sensitivity of ovarian cancer cells to DDP.On the other hand,the results from the RIB+DDP group demonstrated the important effect of RIB on the mitochondrial function and apoptosis of cells.Surprisingly,CHL increased the apoptosis rate of HO8910 cells,and no significant differ⁃ence was observed between theRFT2shRNA group and the shRNA control group.Thus,CHL may have other mechanisms in HO8910 ovarian cancer cells.
CSCs are tumor cells that possess some of the characteristics of normal stem cells.CSCs maintain an undifferentiated state with infinite proliferation ability and differentiation potential. CSCs are the main cause of recurrence,metas⁃tasis,and poor prognosis of tumor[14-15].CD133 is an extremely valuable screening marker of CSCs,and CD133+ovarian cancer cells display remarkable proliferation,self-renewal,and differ⁃entiation abilities.Thus,CD133 is an important target in the chemotherapy of ovarian cancer[16]. CD44 is a transmembrane receptorprotein belonging to the family of adhesion molecules.It not only serves as a surface marker of CSCs but also plays a role in maintaining CSCs properties and multi-drug resistance.CD44 is also a key factor contributing to the invasion,metastasis,and antioxidant ability of tumor cells[17-18].Recent studies have confirmed that CSCs of ovarian cancer are sensitive to mitochondrial damage caused by chemotherapy because CSCs have a higher proportion of split ratio,energy metabo⁃lism,and sensitivity to chemotherapy than com⁃mon tumor[19].In the present study,RFT2inter⁃ference or CHL combined with DDP significantly reduced the rate of CD44+CD133+cells,and RIB reversed the effect of DDP on HO8910 cells. Thus,interference with the RIB metabolic path⁃way could enhance the sensitivity of the cells to DDP by reducing the proportion of CSCs that reportedly can enrich RIB[4].However,whether or not the effect of the interference with the RIB metabolic pathway is related to the enrichment of RIB in HO8910 cells warrants further research.
The preliminary results of this study showed that intervention with the RIB metabolic pathway can enhance the sensitivity of ovarian tumor cells to DDP by promoting of DDP mitochondrial damage and reducting of CSCs rate.This research providesa new strategyforovarian cancer chemotherapy.
REFERENCES:
[1] Ashoori M,Saedisomeolia A.Riboflavin(vitamin B2)and oxidative stress:a review[J].Br J Nutr,2014,111(11):1985-1991.
[2]Sayin VI,Ibrahim MX,Larsson E,Nilsson JA,Lindahl P,Bergo MO.Antioxidants accelerate lungcancer progression in mice[J].Sci Transl Med,2014,6(221):221ra15.
[3]Molina-López J,Florea D, Quintero-Osso B,de la Cruz AP,Rodríguez-Elvira M,Del Pozo EP. Pyridoxal-5′-phosphate deficiency is associated with hyperhomocysteinemia regardless of antioxi⁃dant,thiamine,riboflavin,cobalamine,and folate status in critically ill patients[J].Clin Nutr,2015,35(3):706-712.
[4]Miranda-Lorenzo I,Dorado J,Lonardo E,Alcala S,Serrano AG,Clausell-Tormos J,et al.Intracellular autofluorescence:a biomarker for epithelial cancer stem cells[J].Nat Methods,2014,11(11):1161-1169.
[5]Zhang L,Fan H,Improvement effect and antioxidative stress mechanism of rosiglitazone against cisplatin-induced nephropathy in rats[J].Chin J Pharmacol Toxicol(中国药理学与毒理学杂志),2014,28(4):525-530.
[6]Kohno K,Wang KY,Takahashi M,Kurita T,Yoshida Y,Hirakawa M,et al.Mitochondrial tran⁃scription factor a and mitochondrial genome as molecular targets for cisplatin-based cancer chemotherapy[J].Int J Mol Sci,2015,16(8):19836-19850.
[7]Yang Z,Schumaker LM,Egorin MJ,Zuhowski EG,Guo Z,Cullen KJ.Cisplatin preferentially binds mitochondrial DNA and voltage-dependent anion channel protein in the mitochondrial membrane of head and neck squamous cell carcinoma:possible role in apoptosis[J].Clin Cancer Res,2006,12(19):5817-5825.
[8]Salman M,Naseem I.Riboflavin as adjuvant with cisplatin:study in mouse skin cancer model[J].Front Biosci(Elite Ed),2015,7(7):242-254.
[9]Hassan I,Chibber S,Naseem I.Ameliorative effect of riboflavin on the cisplatin induced nephro⁃toxicity and hepatotoxicity under photoillumination[J].Food Chem Toxicol,2010,48(8-9):2052-2058.
[10]Naseem I,Hassan I,Alhazza IM,Chibber S. Protective effect of riboflavin on cisplatin induced toxicities:a gender-dependent study[J].J Trace Elem Med Biol,2015,29:303-314.
[11]Moriyama Y.Riboflavin transporter is finally identified[J].J Biochem,2011,150(4):341-343.
[12]Pinto J,Huang YP,Rivlin RS.Inhibition of riboflavin metabolism in rattissuesby chlorpromazine,imipramine,and amitriptyline[J].J Clin Invest,1981,67(5):1500-1506.
[13]Chang CH,Wang Y,Gupta P,Goldenberg DM. Extensive crosslinking of CD22 by epratuzumab triggers BCR signaling and caspase-dependent apoptosis in human lymphoma cells[J].MAbs,2015,7(1):199-211.
[14]Ahmed N,Abubaker K,Findlay JK.Ovarian cancer stem cells:Molecular concepts and relevance as therapeutic targets[J].Mol Aspects Med,2014,39:110-125.
[15]Song DY,Wang Y,Sun L,Zhang YG.Progress in tumor-stem cell theory and tumor stem cells isolation and identification[J].Chin J Pharmacol Toxicol(中国药理学与毒理学杂志),2012,26(5):674-679.
[16]Battle S L,Larjo A,Liao J,Lähdesmäki H,Lieber A,Hawkins RD.Abstract AS04:Epigenomic charac⁃terization of gene regulatory networks in human ovarian cancer stem cells[J].Clin Cancer Res,2015,21(16 Suppl):AS04-AS04.
[17]Chanmee T,Ontong P,Kimata K,Itano N.Key roles of hyaluronan and its CD44 receptor in the stemness and survival of cancer stem cells[J].Front Oncol,2015,5:180.
[18]Gao Y,Foster R,Yang X,Feng Y,Shen JK,Mankin HJ,et al.Up-regulation of CD44 in the development of metastasis,recurrence and drug resistance ofovarian cancer[J].Oncotarget,2015,6(11):9313-9326.
[19]Abubaker K,Latifi A,Luwor R,Nazaretian S,Zhu H,Quinn MA,et al.Short-term single treatment of chemotherapy results in the enrichment of ovarian cancer stem cell-like cells leading to an increased tumor burden[J].MolCancer, 2013,12(1):24.
干扰核黄素代谢对细胞卵巢癌HO8910细胞顺铂敏感性的影响
杨如会1,3,宋 羽2,吴松泉1,沈祥春3
(1.丽水学院医学与健康学院,浙江丽水323000;2.南方医科大学第三附属医院,广东广州 510630;3.贵州医科大学天然药物资源优效利用重点实验室,贵州贵阳 550004)
目的 研究干预核黄素(RIB)代谢途径对卵巢癌HO8910细胞顺铂(DDP)敏感性的影响。方法 采用慢病毒包装shRNA载体干扰RIB转运体2(RFT2)和RIB竞争性抑制剂氯丙嗪(CHL)干预RIB代谢途径。HO8910卵巢癌细胞分为正常细胞对照组、shRNA对照组、RFT2shRNA组、CHL 50 μmol·L-1组、DDP 20 μmol·L-1组、RFT2shRNA+DDP组、CHL+DDP组和DDP+RIB 20 μmol·L-1组。各组细胞处理48 h后CCK-8方法检测细胞存活;结晶紫染色方法检测克隆形成能力;流式细胞仪检测凋亡、线粒体膜电位、活性氧(ROS)含量和CD44+CD133+细胞比例。结果 与单独DDP处理组对比,RFT2shRNA或CHL联合DDP能明显降低HO8910细胞活性(P<0.01),减少细胞集落形成能力(P<0.01),降低细胞线粒体膜电位(P<0.01),增加细胞ROS含量和细胞凋亡比例(P<0.01),同时减少CD44+CD133+细胞比例(P<0.01),RIB可减弱DDP对HO8910细胞的作用(P<0.01)。结论 干扰RIB代谢途径能增强DDP对卵巢癌HO8910细胞的杀伤作用,该作用与增强DDP氧化损伤和降低干细胞样肿瘤细胞比例有关;RIB可减弱DDP对HO8910细胞的作用。
核黄素;氯丙嗪;核黄素转运体2;顺铂;卵巢癌
浙江省医药卫生科学研究基金项目(2013KYA236);国家自然科学基金(81570013)
沈祥春,E-mail:shenxiangchun@126.com
2016-01-25接受日期:2016-07-04)
R979.1 < class="emphasis_bold">Document code:A Article ID:
A Article ID:1000-3002-(2016)08-0823-10
10.3867/j.issn.1000-3002.2016.08.005
(本文编辑:贺云霞)
Foundation item:The project supported by Medical Sci⁃ence Research Fund of Zhejiang Province(2013KYA236);and National Natural Science Foundation of China(81570013)
Biography:YANG Ru-hui,lecturer,Doctor of pharmacology,main research field is the molecular and biochemical phar⁃macology,E-mail:yangruhui2008@163.com
SHEN Xiang-chun, E-mail:shenxiangchun@126.com
