高频超声对强直性脊柱炎亚临床附着点炎的筛查评估价值
2024-10-30李玲申英灏杨洁马雯娟张学兰


摘要:目的" 探讨高频超声对强直性脊柱炎(AS)不同部位亚临床附着点炎早期诊断的价值。方法 选取2017年1月~2023年11月甘肃省人民医院就诊的AS患者100例 ,依据2022年全球首个附着点炎筛查与评估临床实践指南进行分组:临床附着点炎患者50例(分为有主观症状和无主观症状但有压痛2组);亚临床附着点炎患者50例(无主观症状也无压痛,需借助MRI或超声识别确诊)及50例健康志愿者,分别对其近端足底筋膜、远端跟腱、远端髌韧带、近端髌韧带、远端股四头肌腱和肱三头肌腱附着点行高频超声检查,根据不同部位附着点的厚度、结构、钙化、骨侵蚀、滑囊的厚度及能量多普勒信号,分析超声检查的特异性、敏感度和准确性。结果 受检AS患者超声检查其中有主观症状异常附着点(阳性率为54%),以股四头肌腱附着点为著;无主观症状但有压痛异常附着点(阳性率为46%),以足底筋膜附着点为著;亚临床附着点炎AS患者异常附着点(阳性率为61%),以跟腱附着点为著。高频超声不同亚临床附着点超声检查病变情况显示,与临床检查相较,AS患者亚临床附着点炎的检出率更高(Plt;0.05);与正常对照组比较,高频超声对亚临床附着点检出率更高,阳性表现以肌腱增厚和滑囊增厚最为常见(Plt;0.05)。与临床检查比较,高频超声在AS不同部位亚临床附着点早期诊断中,特异性、敏感度、准确性均较高。结论 相较于单一的临床检查,高频超声在AS患者不同部位亚临床附着点早期诊断中,可有效提高检出率,提升诊断价值,弥补了临床检查一直以来对亚临床附着点炎的低估性。
关键词:高频超声;强直性脊柱炎;附着点炎;亚临床
Value of high‑frequency ultrasound in screening for subclinical attachment point inflammation of ankylosing spondylitis
LI Ling1, SHEN Yinghao1, YANG Jie2, MA Wenjuan2, ZHANG Xuelan2
1The First Clinical Medical College of Gansu University of Chinese Medicine, Lanzhou 730000, China; 2Department of Ultrasound, Gansu Provincial People's Hospital, Lanzhou 730000, China
Abstract: Objective To investigate the value of high-frequency ultrasound for the early diagnosis of subclinical adhesion pointitis in different parts of ankylosing spondylitis (AS). Methods" A total of 100 patients with AS attending Gansu Provincial People's Hospital from January 2017 to November 2023 were selected. Fifty patients with clinical adhesion pachydermatitis (divided into 2 groups of patients with subjective symptoms and those with no subjective symptoms but with tenderness), 50 patients with subclinical adhesion pachydermatitis (with no subjective symptoms and tenderness, and need to be diagnosed with the help of MRI or ultrasound) and 50 healthy volunteers were grouped according to the world's first clinical practice guideline on screening and assessment of adhesion pachydermatitis in 2022. High-frequency ultrasonography was performed on the proximal plantar fascia, distal Achilles tendon, distal patellar ligament, proximal patellar ligament, distal quadriceps tendon, and triceps tendon attachment points, and the specificity, sensitivity, and accuracy of ultrasonography were analyzed according to the thickness, structure, calcification, bone erosion, bursal thickness, and energy Doppler signals of the attachment points at different sites. Results Ultrasonography of the examined AS patients included abnormal attachment points with subjective symptoms (positive rate of 54%), mainly quadriceps tendon attachment points; abnormal attachment points without subjective symptoms but with pressure pain (positive rate of 46%), mainly plantar fascia attachment points; abnormal attachment points of the AS patients with subclinical attachment point inflammation (positive rate of 61%), mainly Achilles tendon attachment points. High-frequency ultrasound ultrasonography of different subclinical attachment point lesions had a higher detection rate of subclinical attachment point inflammation in AS patients compared with clinical examination (Plt;0.05). Compared with normal controls, high-frequency ultrasound had a high detection rate of subclinical attachment points, with tendon thickening and bursa thickening being the most common positive manifestations (Plt;0.05). Compared with clinical examination, high-frequency ultrasound had higher specificity, sensitivity, and accuracy in the early diagnosis of subclinical attachment points in different parts of AS. Conclusion Compared with unitary clinical examination, high-frequency ultrasound can effectively improve the detection rate and diagnostic value in the early diagnosis of subclinical attachment points in different parts of AS patients, making up for the underestimation of subclinical attachment point inflammation that clinical examination has always been.
Keywords: high-frequency ultrasound; ankylosing spondylitis; attachment point inflammation; subclinical
收稿日期:2023-10-27
基金项目:甘肃省人民医院院内科研基金项目(23GSSYD-2)
作者简介:李" 玲,在读硕士研究生,E-mail: 1306084943@qq.com
通信作者:杨" 洁,教授,硕士生导师,E-mail: yangjie997@163.com
强直性脊柱炎(AS)是一种以中轴关节、外周关节的慢性炎症为主要表现的系统性、慢性、自身免疫性疾病[1]。患者多为中青年男性,病变主要累及中轴关节及下肢关节,其基本病理改变为附着点炎,及时客观地对AS附着点炎进行早期诊断,对临床治疗方案的选择具有重要指导意义。临床检查是评价AS患者附着点炎最常用的方法,常用评分方法有 Mander附着点炎指数、马德里超声附着点炎指数(MASEI)、Maastricht 强直性脊柱炎评分、Leeds附着点炎指数、加拿大脊柱关节炎研究联盟附着点炎指数、Berlin指数和Gladman指数。在这些评分方法中,MASEI被认为是最方便的,因此在临床实践和临床试验中应用最广泛[2-5],但与其他影像学检查相比,临床检查的敏感度相对较低,约为20%[6]。AS患者可通过超声或MRI等影像学方法发现附着点炎,但在临床检查中未发现压痛[7-8]。另一方面,附着点压痛并不能证实其存在肌腱附着点炎,因为疼痛可归因于滑膜炎、关节炎或邻近结构的其他病理情况。临床检查只能作为发现肌腱附着点炎的线索,而不是确定肌腱附着点炎的证据[9]。临床检查完成后,仍建议采用US或MRI作为后续检查方式。
由于US方便价廉、无辐射等优点,近年来已成为评估附着点炎的一线方法[10-11]。与临床检查和X线、MR检查相比,US检查对附着点炎的检测具有更高的敏感度,能够同时发现炎性病变和结构性病变。现有多种评分或分级系统用来评估附着点炎,其中MASEI对足底筋膜近端、跟腱远端、髌韧带远端和近端、股四头肌远端和肱三头肌肌腱进行灰阶超声和能量多普勒超声评分[12]。因其具有能量多普勒超声评估的优势以及包括上肢附着点,被认为可能更胜一筹。目前,利用MASEI评分系统对亚临床附着点炎[13]的研究较少,数量上相对有限,且研究方向相对局限,目前开发的许多评分或分级系统,旨在评估具有临床症状的附着点炎,附着点炎不同评分或分级系统的研究表明,不同系统之间的敏感度和特异性不同[14-15],MASEI评分系统可用于更广泛的AS患者群体,MASEI评分在临床试验和日常医疗实践中均是可靠的工具,超声检查作为其补充诊断的另一种可靠工具,来提高对亚临床附着点炎的诊断敏感性和特异性,结合MASEI评分在亚临床附着点炎中的早期诊断,以及对于下肢附着点的纳入,MASEI可能更优。本研究不是简单地重复已有研究,而是旨在填补现有研究中对亚临床附着点评估的空白,具有新颖性及补充性,旨在利用高频超声对AS患者临床容易低估和忽视的亚临床附着点炎进行MASEI评分评估,为亚临床附着点炎的早期诊断评估方法提供参考价值,为临床早期采取干预提供客观依据。
1" 资料与方法
1.1" 一般资料
选取2017年1月~2023年11 月于甘肃省人民医院就诊的AS患者100例,其中男性72例,女性28例,年龄20~55(33.22±5.23)岁;另选取50例健康志愿者。纳入标准:采用1984 年国际强直性脊柱炎评价工作组纽约标准及2010年SPA外周分类标准[16];研究对象均接受超声检查,且超声检查资料完整完备;3月内未进行糖皮质激素或其他生物制剂治疗。排除标准:有关节外伤史;有四肢外伤史;有其他严重风湿疾病导致关节病变。本研究经甘肃省人民医院伦理委员会审批(审批号:2023-273)。
1.2" 方法
1.2.1" 超声检查" 选用GEs8超声检查仪(ML6-15探头频率为6~15 MHz)及Siemens healthineers 超声检查仪(18L6 探头频率为6~18 MHz)。对所有受检者行高频超声检查,检查时根据不同检查部位选取不同的探查体位,所有附着点在肌腱处于松弛状态下,减轻探头压力的条件下探测多普勒血流信号。超声检查者均具有10年以上肌骨超声经验。
1.2.2" 评分标准" 根据MASEI附着点炎超声评估方法[17]:低回声:校正各异性后,附着点处(距骨皮质lt;2 mm)均匀的纤维信号缺失,且周围紧密包绕的回声线消失。附着点处增厚:与肌腱体相比,嵌入骨骼处的肌腱增厚(距骨皮质lt;2 mm),伴或不伴肌腱边缘模糊。骨侵蚀:在附着点嵌入处可在两个垂直平面上观察到骨皮质中断并出现逐渐模糊的边缘轮廓缺损。钙化:在附着点处(距骨皮质lt;2 mm)检测到高回声灶伴或不伴声影。骨赘:在两个垂直平面上观察到附着点骨端边缘逐渐增厚的骨性隆起。附着点嵌入处多普勒信号:嵌入骨骼处观察到多普勒信号(距骨皮质lt;2 mm),与反射界面伪影或营养血管信号不同,伴或不伴骨皮质不规则、侵蚀或骨赘。
1.3" 统计学分析
采用SPSS27.0软件进行统计分析。对所有计量资料进行正态性和方差齐性检验,符合正态分布的计量资料以均数±标准差表示,组间比较采用独立样本t检验;计数资料以n(%)表示,采用卡方检验进行组间分析,多组间比较采用单因素方差分析,以Plt;0.05为差异有统计学意义。
2" 结果
2.1" AS患者不同部位亚临床附着点炎超声评估
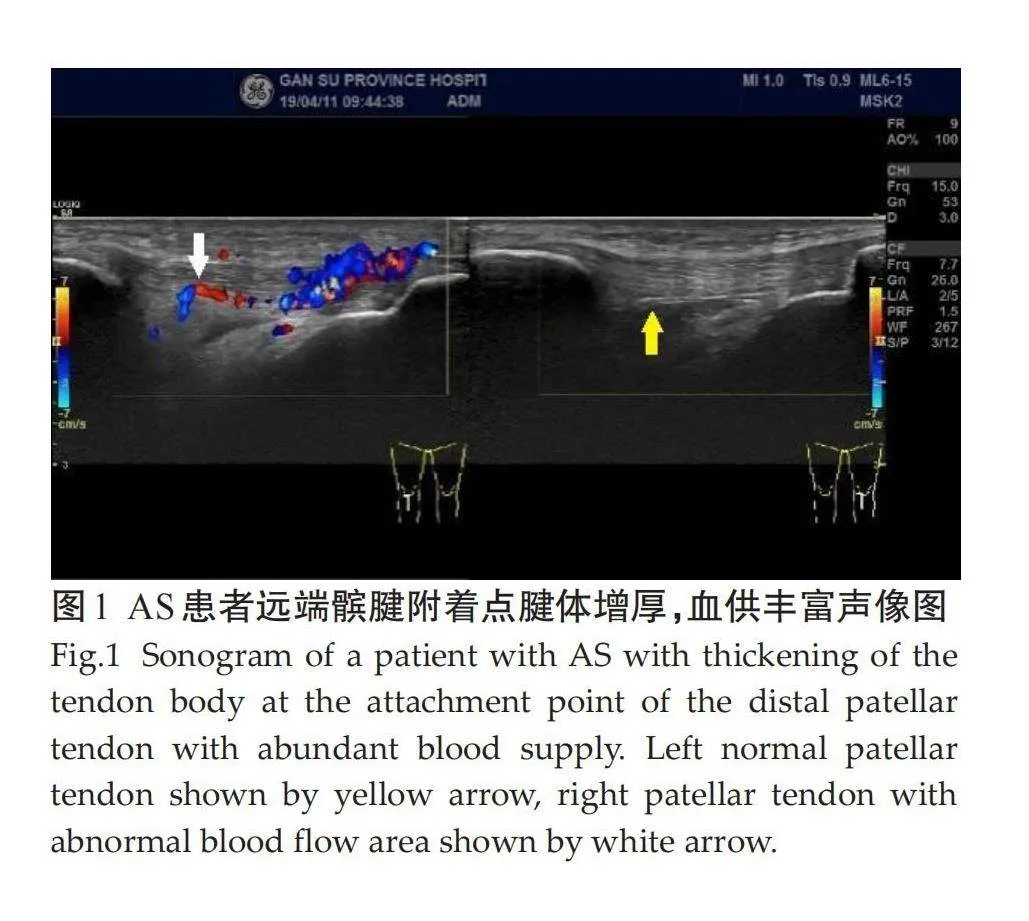
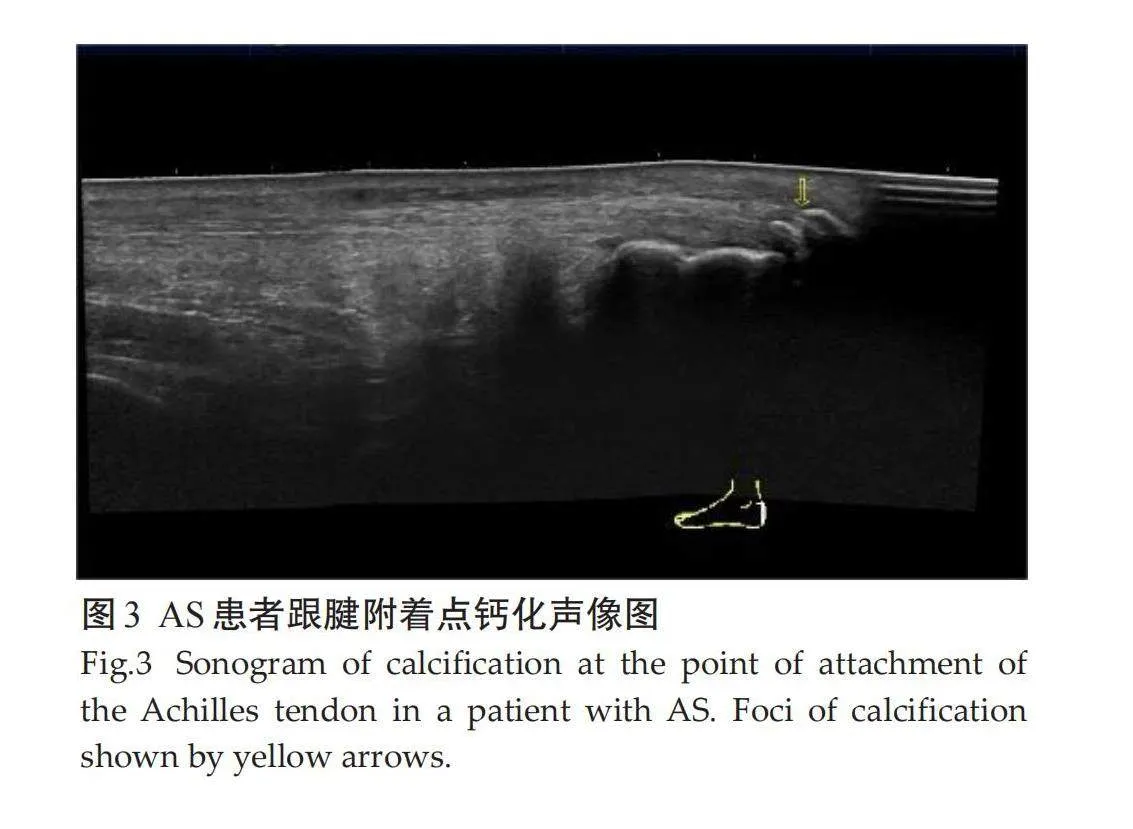
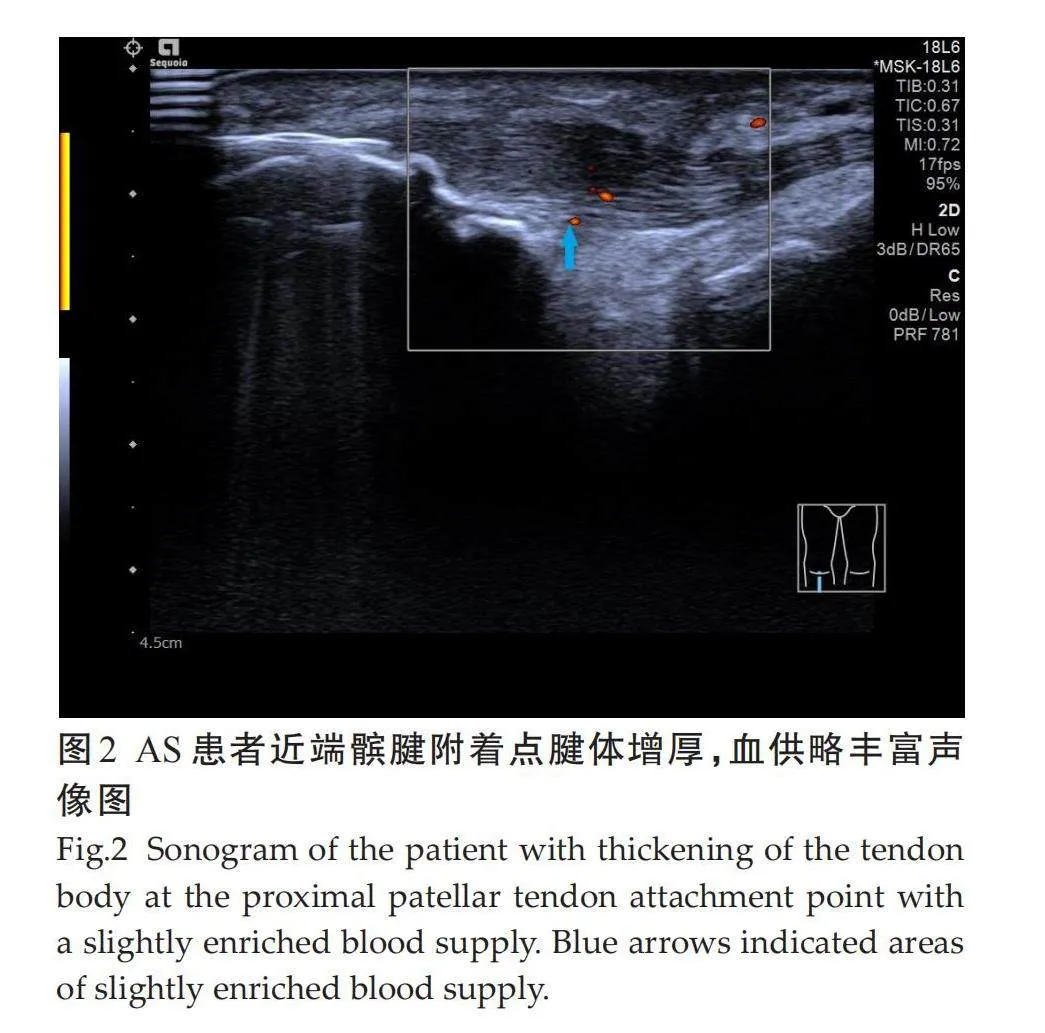
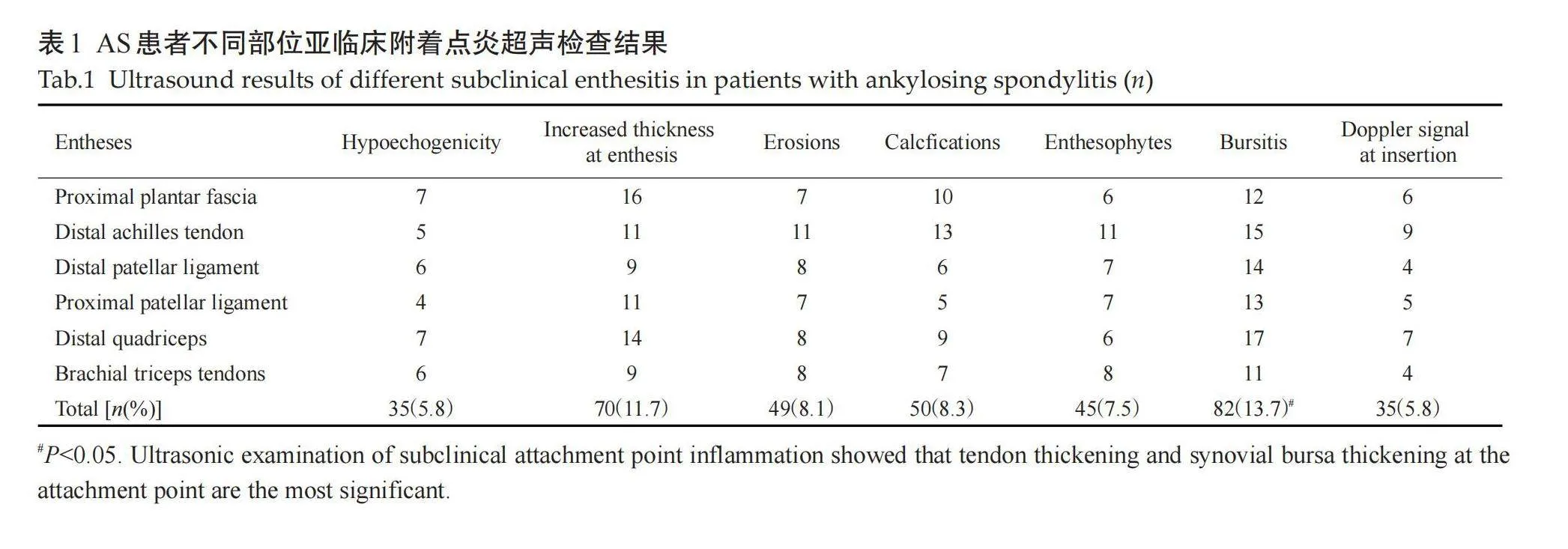
受检的100例AS患者共1200个附着点,超声检查50例有临床症状附着点炎患者共600个附着点,有主观症状附着点324个(54%),无主观症状但有压痛组附着点276个(46%);50例亚临床症状患者共600个附着点,异常附着点个366(61%)。不同亚临床附着点超声检查病变情况差异有统计学意义。检查不同亚临床附着点,各异常声像图阳性表现:肌腱附着端异常低回声(5.8%),附着点处肌腱增厚(11.7%),骨侵蚀(8.1%),钙化(8.3%),多发骨赘(7.5%),滑囊增厚(13.7%),附着点嵌入处多普勒信号(5.8%)。病变声像图中尤以附着点处肌腱增厚和滑囊增厚为著(表1、图1~3)。
2.2" "临床附着点炎组与亚临床附着点炎组超声检查结果比较
有主观症状组内尤以股四头肌腱附着点炎检出率最高;有主观症状组与无主观症状但有压痛组相比较,无主观症状但有压痛组以足底筋膜附着点炎检出率最高,超声更能全面的检出已产生炎性或结构性病变的附着点炎;亚临床附着点炎组与无主观症状但有压痛组相比较,以跟腱附着点炎为著(表2)。
2.3" AS患者临床附着点炎组与正常对照组超声评估比较
亚临床附着点炎组各附着点的异常附着点数均明显高于正常对照组(表3)。
3" 讨论
附着点是肌腱和韧带嵌入骨表面的部位,是人体运动的关节结构。附着点炎是附着点处发生炎症,是脊柱关节炎的特征性损伤,是AS和相关病症的特征性表现。在原发性 AS中,外周附着点炎的发生率为25%~58%[18] ,有附着点炎的AS患者,疾病活动度更高,患者结局的报告更差[19-20]。附着点炎的管理对改善AS的整体疾病活动度至关重要。但是,实际临床中附着点炎的存在常被低估[21],主要是因为检查方法的缺失,主要依赖于患者主诉和医生经验。既往国内外缺乏专门筛查和评估附着点炎的指南,仅提到可以针对附着点炎进行超声、MRI等检查,但是并未对具体筛查和评估方法做详细解释,也未对各方法之间的优劣势做明确介绍。2022年全球首个附着点炎指南发布[3],指南针对患者的临床检查、超声检查、MRI和X线等具体检查方法和评估手段进行了详细描述,并介绍了各方法和手段之间的优劣势。高分辨率MRI可帮助临床医生鉴别附着点炎与其他造成局部疼痛的疾病[22],但MRI存在较多局限性,如花费高、可及性较差,传统MRI序列只可显示特定部位的附着点[23],而高频超声因其敏感度高、可重复强等优点,指南强烈推荐灰阶超声和能量多普勒超声用于评估附着点炎。
本研究在综合考虑成本、可行性和证据强度的情况下,使用指南推荐的MASEI指数进行量化评分,对AS患者常见附着点炎部位(跖腱膜近端、跟腱远端、髌韧带远端和近端、股四头肌远端、肱三头肌肌腱等)进行超声评估,更准确的反应炎症程度。既往有文献报道高频超声早期筛查亚临床银屑病关节炎的临床价值[24-25],但鲜有文献报道高频超声对AS患者不同部位附着点的厚度、结构、钙化、骨侵蚀、滑囊的厚度及能量多普勒信号等特征的分析及检查效能评估。本研究发现附着点炎不同超声表现的发生率低于部分Meta 分析结果[3],这可能与本研究病例的样本量相对较少或与患者的发病年限有关,后续仍需大样本量研究及发病年限的差异性对比。有研究表明,高度血管化的附着点更易产生疼痛,临床体格检查易查出,而声像图显示AS患者附着点插入处存在异常血管形成解释了这一表现[12]。并且高度血管化[26]仅存在于AS患者中,健康对照组中未有此发现,这些特征表现与临床特征分析具有一致性[27-28]。
本研究采用的MASEI评分法重点纳入下肢附着点,而对伸肌总腱、屈肌总腱和股骨大转子等附着点尚未纳入,可能存在上肢附着点炎评估的缺失,后续仍需对多种评分系统进行对比分析,以期寻找更全面准确的评分方式。
综上所述,利用高频超声对AS患者亚临床附着点炎进行MASEI评分评估,是附着点炎量化评估的可靠可行的诊断方式,弥补了临床检查一直以来对亚临床附着点炎的低估性,虽然附着点炎半定量评分目前还不足以成为诊断AS的依据, 且本研究尚未对多个超声评分系统进行对比分析,但预期通过长期随访验证后可以对AS的早期诊断提供重要价值。
参考文献:
[1]" "Zhu W, He XX, Cheng KY, et al. Ankylosing spondylitis: etiology, pathogenesis, and treatments[J]. Bone Res, 2019, 7: 22.
[2]" "Hartung W, Nigg A, Strunk J, et al. Clinical assessment and ultrasonography in the follow‑up of enthesitis in patients with spondyloarthritis: a multicenter ultrasound study in daily clinical practice[J]. Open Access Rheumatol, 2018, 10: 161-9.
[3]" "Gladman DD, Kavanaugh A, Gómez-Reino JJ, et al. Therapeutic benefit of apremilast on enthesitis and dactylitis in patients with psoriatic arthritis: a pooled analysis of the PALACE 1-3 studies[J]. RMD Open, 2018, 4(1): e000669.
[4]" " Lee SH, Park W, Won Lee S, et al. Frequency of peripheral diseases in Korean patients with ankylosing spondylitis and the effectiveness of adalimumab[J]. Int J Rheum Dis, 2020, 23(9): 1175-83.
[5]" "Rudwaleit M, Claudepierre P, Kron M, et al. Effectiveness of adalimumab in treating patients with ankylosing spondylitis associated with enthesitis and peripheral arthritis[J]. Arthritis Res Ther, 2010, 12(2): R43.
[6]" "Wu XY, Liu D, Li YF, et al. A clinical practice guideline for the screening and assessment of enthesitis in patients with spondyloarthritis[J]. Front Immunol, 2022, 13: 978504.
[7]" Spadaro A, Iagnocco A, Perrotta FM, et al. Clinical and ultrasonography assessment of peripheral enthesitis in ankylosing spondylitis[J]. Rheumatology, 2011, 50(11): 2080-6.
[8]" Wiell C, Szkudlarek M, Hasselquist M, et al. Power Doppler ultrasonography of painful Achilles tendons and entheses in patients with and without spondyloarthropathy: a comparison with clinical examination and contrast‑enhanced MRI[J]. Clin Rheumatol, 2013, 32(3): 301-8.
[9]" "Nadon V, Moltó A, Etcheto A, et al. Clinical peripheral enthesitis in the DESIR prospective longitudinal axial spondyloarthritis cohort[J]. Clin Exp Rheumatol, 2019, 37(4): 561-5.
[10] Gandjbakhch F, Terslev L, Joshua F, et al. Ultrasound in the evaluation of enthesitis: status and perspectives[J]. Arthritis Res Ther, 2011, 13(6): R188.
[11] Terslev L, Naredo E, Iagnocco A, et al. Defining enthesitis in spondyloarthritis by ultrasound: results of a Delphi process and of a reliability reading exercise[J]. Arthritis Care Res, 2014, 66(5): 741-8.
[12] de Miguel E, Cobo T, Muñoz-Fernández S, et al. Validity of enthesis ultrasound assessment in spondyloarthropathy[J]. Ann Rheum Dis, 2009, 68(2): 169-74.
[13] Krabbe S, Eshed I, Sørensen IJ, et al. Whole-body magnetic resonance imaging inflammation in peripheral joints and entheses in axial spondyloarthritis: distribution and changes during adalimumab treatment[J]. J Rheumatol, 2020, 47(1): 50-8.
[14]" Vahidfar S, Sunar İ, Ataman Ş, et al. Ultrasonographic evaluation of Achilles tendon: is there any difference between ankylosing spondylitis, non‑radiographic axial spondyloarthropathy and controls[J].? Int J Rheum Dis, 2020, 23(4): 511-9.
[15]" Yurdakul OV, Rezvani A. Can ultrasound be an assessment tool for sagittal spine mobility and chest expansion in patients with ankylosing spondylitis[J]? Medicine, 2018, 97(39): e12609.
[16] Dejaco C, Duftner C, Schirmer M. Bildgebende verfahren in der Frühphase des morbus bechterew[J]. Wien Med Wochenschr, 2008, 158(7): 191-4.
[17]" Huang YH, Hu YC, Liao CH, et al. Utilizing ultrasound findings of a single indicator joint to assess non-systemic juvenile idiopathic arthritis[J]. Pediatr Rheumatol Online J, 2021, 19(1): 60.
[18] Mease PJ, Heijde DV, Karki C, et al. Characterization of patients with ankylosing spondylitis and nonradiographic axial spondyloarthritis in the US-based corrona registry[J]. Arthritis Care Res, 2018, 70(11): 1661-70.
[19] Mease PJ, Liu M, Rebello S, et al. Characterization of patients with axial spondyloarthritis by enthesitis presence: data from the corrona psoriatic arthritis/spondyloarthritis registry[J]. ACR Open Rheumatol, 2020, 2(7): 449-56.
[20] Strand V, Deodhar A, Conaghan PG, et al. Assessing the humanistic and economic burden of enthesitis among patients with peripheral and axial spondyloarthritis: Results from a multi-national real world survey database [J]. Arthritis Rheumatol, 2019, 71: 1082-5.
[21] McGonagle D, Aydin SZ, Marzo-Ortega H, et al. Hidden in plain sight: is there a crucial role for enthesitis assessment in the treatment and monitoring of axial spondyloarthritis?[J]. Semin Arthritis Rheum, 2021, 51(6): 1147-61.
[22]Aydin SZ, Tan AL, Hodsgon R, et al. Comparison of ultrasonography and magnetic resonance imaging for the assessment of clinically defined knee enthesitis in spondyloarthritis[J]. Clin Exp Rheumatol, 2013, 31(6): 933-6.
[23] Du J, Chiang AJT, Chung CB, et al. Orientational analysis of the Achilles tendon and enthesis using an ultrashort echo time spectroscopic imaging sequence[J]. Magn Reson Imaging, 2010, 28(2): 178-84.
[24]" 龚艺燃, 陈树强, 何聚馨, 等. 高频超声早期筛查亚临床银屑病关节炎的临床价值[J]. 中华超声影像学杂志, 2022, 31(6): 532-6.
[25]" 宋志博, 耿" 研, 邓雪蓉, 等. 肌肉骨骼超声在指导银屑病关节炎临床分型中的价值[J]. 北京大学学报: 医学版, 2021, 53(6): 1061-6.
[26] Krabbe S, Eshed I, Sørensen IJ, et al. Novel whole-body magnetic resonance imaging response and remission criteria document diminished inflammation during golimumab treatment in axial spondyloarthritis[J]. Rheumatology, 2020, 59(11): 3358-68.
[27]" 张奕楠, 冀肖健, 胡嘉文, 等. 强直性脊柱炎患者合并附着点炎的临床特征分析[J]. 解放军医学院学报, 2022, 43(3): 264-9.
[28]D'Agostino MA, Said‑Nahal R, Hacquard‑Bouder C, et al. Assessment of peripheral enthesitis in the spondylarthropathies by ultrasonography combined with power Doppler: a cross-sectional study[J]. Arthritis Rheum, 2003, 48(2): 523-33.
(编辑:林" 萍)
