哺乳动物卵母细胞的DNA损伤与修复研究进展
2023-05-23张楠张珏林戈
张楠,张珏,林戈,
综 述
哺乳动物卵母细胞的DNA损伤与修复研究进展
张楠1,张珏2,林戈1,2
1. 中南大学基础医学院生殖与干细胞工程研究所,长沙 410000 2. 中信湘雅生殖与遗传专科医院,长沙 410000
DNA损伤是影响配子发生和胚胎发育的关键因素之一。卵母细胞容易被各种内外源因素(如活性氧、辐射、化疗药物等)诱发DNA损伤。目前研究发现,对于各类DNA损伤,各发育阶段的卵母细胞能够做出相应的DNA损伤反应,通过复杂的机制对DNA进行修复或者启动细胞凋亡。相比于进入生长阶段的卵母细胞,原始卵泡卵母细胞更容易被DNA损伤诱导凋亡。DNA损伤不易诱导卵母细胞减数分裂成熟进程停滞,然而携带DNA损伤的卵母细胞的发育能力明显下降。在临床上,衰老、放疗和化疗是导致女性卵母细胞DNA损伤、卵巢储备降低和不孕的常见原因。为此,人们尝试了能够减轻卵母细胞DNA损伤和增强DNA修复能力的多种方法,试图保护卵母细胞。本文对哺乳动物的各发育阶段卵母细胞的DNA损伤与修复的相关研究进行了梳理和总结,并讨论了其潜在的临床价值,以期为生育力保护提供新的策略。
卵母细胞;DNA损伤;DNA修复;DNA损伤反应
DNA作为遗传信息的核心,是细胞存活和发挥功能的基石。对于生殖细胞而言,DNA的完好性是其正常发育和形成健康胚胎的基础[1]。然而,DNA是脆弱的,许多有害物质都会造成细胞DNA损伤。细胞能够通过复杂的DNA修复机制对损伤的DNA进行修复。如果损伤的DNA没有被成功修复,则有可能导致基因突变、细胞功能改变和疾病的发生[2]。哺乳动物卵母细胞的质量是早期胚胎发育能力的关键决定因素[3]。卵母细胞的DNA损伤可能导致机体的生殖功能下降甚至丧失[1,4]。近年来,关于卵母细胞的DNA损伤与修复机制的研究逐渐成为一个热点。在各种DNA损伤类型中,对DNA双链断裂(double-strand breaks,DSBs)的研究是最多的。目前研究认为,对于各种内外源因素诱导的DNA损伤,哺乳动物卵母细胞是敏感的,能够及时启动DNA损伤反应(DNA damage response,DDR),发生DNA修复或凋亡等一系列活动[5~7]。这对于保障卵母细胞质量及早期胚胎的正常发育是至关重要的。本文对哺乳动物的各发育阶段卵母细胞的DNA损伤与修复机制的研究现状进行了阐述,强调了卵母细胞DNA损伤在衰老和放化疗导致的生育力下降中的重要作用,并简要总结了目前关于减轻卵母细胞DNA损伤和改善卵母细胞质量的可能方法。
1 体细胞的经典DDR与DNA修复机制
细胞的DNA损伤可由各种外源性或内源性因素引起,前者即环境因素,如紫外线、电离辐射、毒性化学物质,后者即细胞内代谢活动的产物,如氧化呼吸、脂质过氧化等活动产生的活性氧(reactive oxygen species,ROS)。此外,DNA损伤也可以自发产生[2]。DNA损伤的形式包括碱基改变、单链断裂、DSBs、加合物病变、链间交联(interstrand crosslinks,ICLs)等[8]。这些损伤可能干扰DNA复制或转录过程进而损害细胞功能。细胞通常能够积极地做出DDR以应对DNA损伤。DDR途径由多种蛋白质组成。根据功能的不同,这些蛋白质可分为传感器、调解器、转导器和效应器。DDR涉及许多细胞反应,包括细胞周期停滞、染色质重塑、损伤修复和细胞凋亡,是应对刺激较全面的细胞反应之一[2,8]。细胞主要在G1/S期和G2/M期激活DDR机制。共济失调毛细血管扩张突变蛋白(ataxia telangiectasia mutated,ATM)和ATM与Rad3相关蛋白(ATM and Rad3 related,ATR)是重要的DNA损伤检查点激酶。在G1期,ATM和ATR激酶被招募至DNA损伤位点并发生磷酸化,随即激活下游的细胞周期检查点激酶1(checkpoint kinase 1,CHK1)和细胞周期检查点激酶2(checkpoint kinase 2,CHK2)[9~11]。CHK1和CHK2激酶继续激活下游效应器p53。在p53的介导下,p21结合并抑制了细胞周期依赖性激酶(cyclin- dependent kinase,CDK)的活性,从而使细胞周期暂停[12,13]。在G2期,DNA损伤位点同样会招募并激活ATM/ATR激酶和CHK1/CHK2激酶。不同的是,CHK1/CHK2激酶通过抑制细胞分裂周期因子25(cell division cyclin 25,CDC25)磷酸酶进而抑制CDK1的激活,从而使细胞周期停滞[13~15]。在细胞周期停滞期间,细胞将通过复杂的机制对损伤的DNA进行修复。在DNA修复完成后,DNA损伤检查点激酶发生去磷酸化,细胞周期恢复[13~15]。当DNA损伤不能被完全修复时,p53将激活促凋亡基因如p53上调凋亡调节基因(p53-upregulated modulator of apoptosis,)和佛波醇-12-豆蔻酸-13-乙酸诱导蛋白1基因(phorbol-12-myristate-13-acetate- induced protein 1,/)的转录,从而诱导细胞凋亡[2,16,17]。
对于不同类型的DNA损伤,细胞能够通过相应的DNA修复机制进行修复。DNA复制过程中发生的碱基错配能够通过错配修复(mismatch repair,MMR)机制得到纠正;发生微小化学变化的碱基能够通过碱基切除修复(base excise repair,BER)机制被去除;较大的DNA病变则可通过核苷酸切除修复(nucleotide excision repair,NER)机制被去除;DNA单链断裂的修复过程涉及一组酶级联反应;同源重组(homologous recombination,HR)与非同源末端连接(non-homologous end joining,NHEJ)是修复DSBs的两种机制;ICLs病变的去除机制涉及一组与范可尼贫血蛋白相关的复杂反应[2,18]。普遍认为,DSBs是DNA损伤类型中最严重的,可导致基因组重排和结构变化,如缺失、易位、融合等[2,8,19]。DSBs的修复机制包括HR和NHEJ。在HR修复过程中,首先MRN复合物被招募至DSBs末端并对DNA末端进行处理和切割以产生单链DNA[20,21]。之后,复制蛋白A(replication protein A,RPA)将单链DNA包裹,使其免受核酸酶作用并去除其二级结构。在乳腺癌蛋白2(breast cancer 2,BRCA2)的介导下,RPA被DNA修复蛋白RAD51(DNA repair protein RAD51)替换。随后,RAD51介导单链DNA侵入未受损的姐妹染色体[22,23]。最后在聚合酶、核酸酶、螺旋酶和其他分子的作用下,DNA进行延伸并完成修复[24,25]。不同于HR,NHEJ是通过DNA连接酶将DSBs末端直接进行连接。首先Ku70/Ku80蛋白识别并结合至DSBs末端,随后招募并激活DNA依赖性蛋白激酶催化亚基蛋白(DNA-dependent protein kinase catalytic subunit,DNA-PKcs)[26,27]。之后,DNA-PKcs招募重组酶Artemis对DNA末端进行处理,同时招募由X射线修复交叉互补蛋白4(X-ray repair cross-complementing protein 4,XRCC4)和DNA连接酶4(DNA ligase 4,LIG4)组成的蛋白复合物对DNA末端进行连接[27,28]。HR发生在细胞周期的S期和G2期,利用未受损的姐妹染色体进行修复,因而更加精准。NHEJ是将DSBs的两端直接连接在一起,虽不精准,但可以在整个细胞周期运作[25,29]。普遍认为,DNA损伤的累积和错误的DNA修复容易引起基因突变和染色体畸变,导致细胞功能的减退和丧失,进而可能促进衰老和疾病的发生[2,8,30]。因此,细胞保持其基因组的稳定性和完整性是至关重要的。
2 各发育阶段卵母细胞的DNA损伤与修复机制
2.1 原始卵泡卵母细胞
胎儿期的卵母细胞开始了第一次减数分裂,在前期的细线期至粗线期阶段,同源染色体发生了联会和重组事件。在同源染色体重组完成后,卵母细胞即停滞在第一次减数分裂前期的双线期。在同源染色体联会过程中,染色体内主动发生DNA DSBs,从而允许同源非姐妹染色体之间进行基因重组和形成染色体交叉。染色体交叉对同源染色体的中期对齐和后期正确分离至关重要[31,32]。卵母细胞的减数分裂重组过程受到了时间和空间的严格调控,重组过程中的DSBs修复失败会导致卵母细胞凋亡或卵母细胞染色体错误[31~33]。哺乳动物出生后的卵母细胞与周围的单层扁平颗粒细胞共同形成原始卵泡结构。原始卵泡卵母细胞处于第一次减数分裂前期的双线期,此时卵母细胞中的同源染色体通过染色体交叉连接在一起。在被招募进入生长发育轨道前,原始卵泡卵母细胞长期维持在第一次减数分裂前期的双线期,并保持代谢不活跃的状态[34]。这一维持时间在小鼠()中可达数月,在人类()中则长达50年[35]。由于长期停滞在第一次减数分裂前期的双线期,原始卵泡卵母细胞发生并累积DNA损伤的风险较高。原始卵泡卵母细胞的DNA完好是卵母细胞后续发育的基本保障,此外原始卵泡库代表着哺乳动物生命中全部的生殖储备。因此原始卵泡卵母细胞对DNA损伤有着敏感和严格的监控反应机制,以能够修复DNA损伤或清除存在损伤的卵母细胞,从而确保个体排出最优质的卵母细胞用于受精和胚胎发育。
大量动物实验和临床数据表明,辐射和化疗药物等外源性因素很容易诱导原始卵泡卵母细胞出现广泛DNA损伤和发生凋亡[36~40]。在临床上,化疗和卵巢局部放疗导致的原始卵泡库的损耗容易诱发早发性卵巢功能不全(premature ovarian insufficiency,POI)、不孕或过早绝经等疾病[36,37,41]。在体细胞DNA损伤时,p53是介导细胞凋亡的关键因子。与体细胞不同的是,由基因(人类为基因)编码的反式激活p63蛋白(trans-activating p63,TAp63)是介导DNA损伤的卵母细胞发生凋亡的关键因子,TAp63特异性表达于原始卵泡和初级卵泡的卵母细胞中。是迄今为止发现的唯一参与卵母细胞DDR的家族成员[42~47]。人类基因功能获得性突变导致卵母细胞凋亡,最终导致POI的发生[48]。当原始卵泡卵母细胞发生DNA损伤时,在ATM激酶和CHK2激酶的调控下,TAp63被磷酸化激活。TAp63由封闭的、无活性的二聚体构象转变为开放的、有活性的四聚体构象,其与DNA的结合亲和力显著提高[42,49]。磷酸化的TAp63继而激活和等基因的转录和翻译。之后PUMA和NOXA与促凋亡的BCL2相关的X蛋白(BCL2-associated X protein,BAX)和BCL2拮抗剂/杀伤蛋白(BCL2- antagonist/killer,BAK)相互作用,最终诱导细胞凋亡[36~40]。通过进一步构建、和等基因敲除的小鼠,研究发现经γ辐射或化疗药物处理的基因敲除小鼠能够维持原始卵泡数量,并生育正常的子代[38,40,45,50~52]。综上所述,自然情况下,大部分原始卵泡卵母细胞在发生广泛的DNA损伤时,选择启动凋亡程序以消除自身;然而当人为阻断其凋亡时,这些基因受损的卵母细胞能够存活,甚至可以完成生育的使命,表明原始卵泡卵母细胞有能力对DNA损伤进行有效修复。
在对于原始卵泡卵母细胞的DNA修复的相关研究中,关于DSBs修复的研究是最多的。由于原始卵泡卵母细胞长期停滞在第一次减数分裂前期,细胞中存在的姐妹染色体可以提供准确修复的模板,因此原始卵泡卵母细胞主要通过复杂而精确的HR机制对DSBs进行修复,以保障卵母细胞的基因组完整性。RAD51是重要的HR修复因子,Kujjo等[53]首次在小鼠的原始卵泡卵母细胞内发现了RAD51。后续研究进一步证明,小鼠原始卵泡卵母细胞通过激活ATM,磷酸化组蛋白H2AX(组蛋白H2A变体)的139位丝氨酸,即形成γH2AX位点,将RAD51定位于DNA断裂位点,从而通过HR机制对γ辐射和化疗药物诱导的DSBs进行快速修复[50,52]。乳腺癌蛋白1(breast cancer 1,BRCA1)也是HR修复因子家族的重要成员[25]。BRCA1在小鼠和人类的原始卵泡卵母细胞的DSBs修复中发挥关键作用,基因突变人群的原始卵泡储备减少[54,55]。NHEJ作为另一种DSBs修复机制,在原始卵泡卵母细胞中可能不发挥主要作用[52,56]。除DSBs损伤外,范可尼贫血互补群E型基因(Fanconi anemia complementation group E,)在小鼠原始卵泡卵母细胞的ICLs损伤修复中发挥关键作用,基因敲除小鼠的原始卵泡几乎耗竭[57]。关于原始卵泡卵母细胞的其他类型DNA损伤与修复机制有待进一步探索。
2.2 生长阶段的卵母细胞
在卵泡刺激素的作用下,原始卵泡被激活进入生长发育轨道,成为生长卵泡。生长卵泡进一步可分为初级卵泡、次级卵泡和窦卵泡。生长阶段的卵母细胞依然维持在第一次减数分裂前期的双线期。此阶段的卵母细胞生长发育十分活跃,表现为细胞体积增大,基因转录和蛋白质合成显著增加。这些母源RNA和蛋白质被储存在生发泡(germinal vesicle,GV)和胞质中,以支持卵母细胞减数分裂成熟和早期胚胎发育[58]。类似于原始卵泡卵母细胞,在应对DNA损伤时,初级卵泡卵母细胞也会发生由TAp63介导的细胞凋亡反应。基因敲除小鼠的初级卵泡卵母细胞能抵抗一定剂量的辐射而存活[42]。因此,在原始卵泡和初级卵泡中,DNA损伤的卵母细胞均倾向于通过TAp63介导的凋亡程序清除自身。然而,在初级卵泡之后的生长阶段,卵母细胞中TAp63表达水平下降,因此生长阶段的卵母细胞在发生DNA损伤后不易凋亡。Puy等[59]报道,0.5 Gy的射线诱发了小鼠原始卵泡的大量损失,而大量生长卵泡在经8 Gy的射线照射后仍然存活。Luan等[40]也表明,与休眠的原始卵泡卵母细胞相比,激活后的卵母细胞对化疗药物(环磷酰胺)的敏感性更差。研究认为,生长阶段的卵母细胞更倾向于对DNA损伤进行修复,此时卵母细胞中与DNA修复相关的基因表达增加[60~64]。对于DSBs损伤,与原始卵泡卵母细胞一样,生长阶段的卵母细胞也主要通过HR机制进行修复[50]。然而,如果DNA损伤超过了一定的限度,卵母细胞也将发生凋亡。多柔比星(doxorubicin,DOX)和依托泊苷(etoposide,ETP)是广泛使用的DNA损伤诱导药物。低剂量DOX和ETP对小鼠卵泡的存活和生长影响较小,而高剂量DOX和ETP则会导致生长卵泡存活率大幅下降,卵母细胞凋亡率增加[65,66]。
2.3 完全生长的卵母细胞
卵泡生长发育的最后阶段为成熟卵泡。成熟卵泡的卵母细胞是完全生长的卵母细胞。在完全生长的卵母细胞中,染色质结构已从非包围核仁状态转变为包围核仁状态,基因转录沉默[67]。在黄体生成素(luteinizing hormone,LH)峰的作用下,处于第一次减数分裂前期的完全生长的GV卵母细胞恢复减数分裂,发生生发泡破裂(germinal vesicle breakdown,GVBD),进入第一次减数分裂中期(metaphase I,MI),此时通过交叉连接的同源染色体排列在赤道板上。随后同源染色体在纺锤丝的牵引下分离并向两极移动。移至两极后,染色体解旋,核膜重建,同时细胞质分裂,卵母细胞排出第一极体,卵母细胞中剩余每对同源染色体中的一条,至此第一次减数分裂完成。卵母细胞随后进入第二次减数分裂,并停滞在第二次减数分裂中期(metaphase II,MII),此时通过着丝粒连接的高度螺旋化的姐妹染色体排列在赤道板上。以上的过程被称为卵母细胞减数分裂成熟[58]。
转录沉默的卵母细胞的DNA修复依赖于细胞中储存的相关mRNA和蛋白质。在完全生长的GV和MII卵母细胞中,参与各种DNA修复途径的mRNA和蛋白质都已被确定[7,60~63,68,69]。研究已证明,完全生长的卵母细胞能够通过HR和NHEJ机制对DSBs进行修复[70]。在小鼠完全生长的GV卵母细胞中敲降、、等HR关键基因造成了卵母细胞DSBs增加,卵母细胞存活率降低[54]。蛋白磷酸酶4催化亚基基因(protein phosphatase 4 catalytic subunit,)是促进HR修复的重要基因,条件性敲除小鼠的GV和MII卵母细胞的HR修复功能障碍,细胞出现大量DNA损伤[71]。Lee等[70]的研究进一步表明,在减数分裂成熟期间,DSBs修复途径从HR过渡到NHEJ,HR在GV卵母细胞中占优势,NHEJ在MII卵母细胞中占优势。此外,两项研究也证明小鼠MII卵母细胞能够通过NHEJ机制,而不是HR机制,对DSBs进行修复[72,73]。原因可能是相比于长期停滞在第一次减数分裂前期双线期的GV卵母细胞,一方面MII卵母细胞的染色体处于高度螺旋化的状态,HR机制在细胞分裂中期发挥的作用受限,另一方面减数分裂成熟期间的卵母细胞可能不具备进行HR修复所需的较长时间,因此能够在整个细胞周期进行快速修复的NHEJ机制是MII卵母细胞修复DSBs的主要选择[74,75]。此外,BER途径能够修复氧化诱导的DNA损伤,即8-羟基脱氧鸟苷(8-hydroxy-2'-deoxyguanosine,8-OHdG)[76]。Lord等[77]发现,小鼠MII卵母细胞具有参与BER途径的几乎所有蛋白质,并且卵母细胞受精后的一系列翻译后修饰活动能够激活BER途径的关键蛋白,增强细胞的DNA修复能力。ICLs修复途径的FANCE蛋白也能够保护小鼠GV和MII卵母细胞的DNA[57]。以上研究共同表明,哺乳动物完全生长的卵母细胞中存在活跃的DNA修复活动。
3 DNA损伤对卵母细胞减数分裂成熟的影响及机制
前文已阐述,DNA损伤在一定程度上影响卵母细胞的存活和发育。考虑到卵母细胞的功能,随之而来的问题是,DNA损伤是否对卵母细胞的减数分裂成熟有所影响?众所周知,卵母细胞长期处于第一次减数分裂的G2/前期。直到排卵时,卵母细胞恢复减数分裂,发育至MII期[58]。一些研究共同表明,外源诱导的DNA损伤不易导致GV卵母细胞停滞在G2/前期,大多数卵母细胞能够恢复甚至完成第一次减数分裂。DNA损伤对卵母细胞的减数分裂成熟进程影响较小,除非DNA损伤严重[6,70,78~82]。这与体细胞应对DNA损伤时所发生的G2/M期停滞反应有所不同。当体细胞在G2/M期发生DNA损伤时,细胞启动DDR,激活DNA损伤检查点激酶,通过抑制CDC25磷酸酶最终抑制CDK1的激活,从而使细胞停滞在G2/M期,为修复病变提供时间[83]。卵母细胞不能建立强有力的G2/前期停滞反应的原因可能是DNA损伤检查点激酶被激活的强度不够[78]。近期的两项研究作出了更多的解释。野生型P53诱导的磷酸酶1(wild-type p53-induced phosphatase 1,WIP1)能够对DDR途径的许多蛋白进行去磷酸化[84]。Leem等[85]发现,在卵母细胞减数分裂成熟过程中持续表达的WIP1阻碍了ATM的磷酸化,从而抑制了DNA损伤检查点的激活,细胞周期无法暂停。另一种解释是小鼠卵母细胞的DNA损伤检查点的激活是延迟的,在DNA损伤发生数小时(20 小时以上)后才启动[86]。
在经典的G2/M DNA损伤检查点不能被有效激活的情况下,卵母细胞依然有能力诱导细胞周期停滞以应对DNA损伤,这与纺锤体组装检查点(spindle assembly checkpoint,SAC)有关。SAC在纺锤体组装过程中负责监测着丝粒与微管的附着状态,从而保障染色体的正确分离[87,88]。毒性药物(如DOX、ETP)、紫外线B和电离辐射诱导的基因组损伤可激活SAC,诱导卵母细胞停滞在MI期。而抑制SAC则可以阻止DNA损伤诱导的MI期停滞反应。然而,卵母细胞的基因组轻中度损伤可能不足以激活SAC,卵母细胞能够发育至MII期。需注意的是,逃逸的卵母细胞存在DNA损伤、纺锤体变形、染色体紊乱等异常[70,78,80,87,88]。这些异常可能对卵母细胞受精及后续胚胎发育过程造成影响。研究发现,有DNA损伤的小鼠MII卵母细胞受精后形成的胚胎发育异常,囊胚率显著下降[71,72]。近期,Leem等[89]发现,在小鼠卵母细胞中存在一个依赖于DNA损伤检查点1的介质蛋白(mediator of DNA damage checkpoint 1,MDC1)的非经典G2/M DNA损伤检查点。在卵母细胞DNA损伤时,MDC1与后期促进复合物/环状体(anaphase promoting complex/cyclosome,APC/C)- APC/C激活蛋白CDH1(APC/C activator protein CDH1,CDH1)发生解离,游离的APC/C-CDH1介导了细胞周期蛋白B1(cyclin B1)降解从而推迟M期的进入。
4 卵母细胞DNA损伤与修复相关研究的临床价值
4.1 生殖衰老与抗氧化
当今社会人们推迟生育的趋势越来越明显。随着生育年龄的增长,女性的卵巢储备和卵母细胞质量大幅降低[90]。生殖衰老引起的卵母细胞质量下降与卵母细胞的DNA损伤积累和DNA修复能力下降密切相关[1,91~95]。在人类、猪()、小鼠等多个物种中,随着机体年龄增长,卵母细胞的DSBs损伤逐渐积累,关键的DNA修复蛋白如BRCA1、RAD51、ATM、卵母细胞表达蛋白(oocyte expressed protein,OOEP)等的表达水平也逐渐下降[54,96~98]。Horta等[99]发现与年轻小鼠相比,老年小鼠的卵母细胞修复精子DNA损伤的能力下降。在人类中也发现,高龄女性的卵母细胞在注射高精子DNA碎片指数的精子后会发育成质量较差的胚胎,从而导致较低的植入率、怀孕率以及较高的流产率[100]。此外,全基因组关联研究表明,基因组不稳定性可能是绝经发生的一个因素,进一步提供了卵巢衰老与卵母细胞DNA修复能力密切相关的证据[93,101]。以衰老为例,机体内源性因素诱导的DNA损伤在很大程度上影响了卵母细胞的质量和发育能力。研究表明,诱导卵母细胞DNA损伤的内源性因素主要是以ROS为代表的细胞内代谢活动的产物[38,54,77,102,103]。因此,研究如何通过抗氧化方法减轻卵母细胞内源性DNA损伤具有重要意义,有助于保护临床中有需求人群的生育能力[91,104]。
目前研究已发现多种能够减轻卵母细胞DNA损伤的抗氧化剂。褪黑素是一种能够清除超氧阴离子和过氧化氢的强效抗氧化剂,能够维持抗氧化代谢物的水平,减少ROS的积累。关于小鼠、大鼠()、猪等的研究发现,褪黑素能够抑制卵母细胞ROS的产生,减轻化疗药物或衰老引起的DNA损伤,改善卵母细胞质量[105~108]。褪黑素还能够增强细胞的NHEJ修复活动,减轻DSBs损伤,促进小鼠卵母细胞成熟[73]。辅酶Q10是一种天然抗氧化剂,能够通过降低超氧化物和DNA损伤的水平来抑制氧化应激和细胞凋亡,从而有效提高小鼠和大鼠的卵母细胞质量[109,110]。N-乙酰-L-半胱氨酸是一种合成制剂,能够刺激细胞合成谷胱甘肽。谷胱甘肽是由谷氨酸、半胱氨酸和甘氨酸组合而成的含巯基三肽,能够通过巯基与氧自由基及其他亲电体相互作用,也可作为各种酶的辅助因子来发挥抗氧化作用[111]。服用N-乙酰-L-半胱氨酸可以减轻小鼠卵母细胞的氧化DNA损伤,提高卵母细胞发育能力[112]。此外,烟酰胺单核苷酸(nicotinamide mononucleotide,NMN)和烟酰胺核糖苷(nicotinamide riboside,NR)也具有抗氧化功能。NMN和NR是生物合成烟酰胺腺嘌呤二核苷酸(nicotinamide adenine dinucleotide,NAD+)的必要前体。NAD+是一种重要的氧化还原辅助因子和酶底物,参与能量代谢和DNA修复等过程[113]。衰老和代谢异常引起机体NAD+水平下降。补充NMN和NR可降低ROS水平、减轻DNA损伤和抑制细胞凋亡,从而改善卵母细胞的发育潜力[114,115]。除使用药物干预外,选择健康的生活方式也是减轻机体氧化应激的有效方法[116]。已有研究证明体育活动能显著降低肥胖大鼠卵母细胞的NADPH氧化酶的活性,减少ROS的产生,减轻卵母细胞损伤,改善卵母细胞质量[117,118]。
4.2 放化疗患者的生育力保存
在临床上,化疗和放疗是针对癌症的常用治疗手段。然而,在治疗癌症的同时,放疗和化疗对卵巢造成了严重的损害,直接损伤了卵母细胞DNA并诱导卵母细胞凋亡,进而导致患者发生POI、不孕和提前绝经等疾病[36,37]。对于接受放化疗的有生育需求的女性来说,如何保存其生育力是值得研究且具有重要临床意义的。目前人们提出了一些药物治疗的建议,包括性激素、鞘氨醇-1-磷酸、地塞米松等药物。然而,没有足够的证据支持这些药物的有效性和安全性[36,119]。更好的办法是预先手术切除部分卵巢并冷冻保存,在癌症治疗结束后再将其重新植入患者体内,然而这种方法有重新引入肿瘤细胞的风险且受到手术时间的限制[120]。鉴于放化疗诱导原始卵泡卵母细胞DNA损伤进而导致卵母细胞凋亡的分子机制,靶向卵母细胞的DDR机制进行药物研发或许是保护患者生育力的有效策略。
抑制卵母细胞凋亡可能是保护放化疗患者卵母细胞的潜在靶点之一。研究表示,通过敲除介导卵母细胞凋亡的基因,如、、等基因,小鼠的大量卵母细胞能够在辐射后存活,并且小鼠能够生育健康的子代[38,40,45,50,51]。通过对、、等基因敲除小鼠的卵母细胞进行γH2AX(DSBs标志物)免疫荧光染色,研究发现辐射诱导的卵母细胞的DSBs损伤在5天后基本消失。且在辐射发生后的24小时,基因敲除小鼠卵母细胞的RAD51蛋白水平明显增加[38,50,51]。这说明,在人为阻止凋亡发生后,小鼠的卵母细胞激活了DNA修复途径,对损伤的DNA进行了修复。此外,Luan等[40]报道,在基因敲除小鼠的原始卵泡卵母细胞中,标志线粒体活性的OPA1线粒体动力蛋白样GTP酶(OPA1 mitochondrial dynamin like GTPase,OPA1)蛋白水平增加,标志着卵母细胞生存能力增强。因此,抑制卵母细胞凋亡似乎可以间接促进DNA修复和增强细胞生存能力。相较于基因编辑,使用抑制凋亡的小分子药物是更可行的办法。研究报道,靶向CHK2或ATM的小分子抑制剂可以阻止TAp63磷酸化,抑制辐射和化疗药物诱导的卵母细胞凋亡,对卵母细胞具有保护作用[40,45,121~124]。综上所述,这些研究为放化疗患者的生育力保护提供了理论依据和干预靶点,然而这些小分子制剂作为生育保护剂的潜力和安全性仍需进一步研究。
此外,激活DNA修复途径、补充关键的DNA修复因子等方法也显现出了增强卵母细胞DNA修复能力的潜能。Sirtuins(SIRT)家族的许多成员是DNA修复途径的关键因子[125],SIRT1、SIRT6和SIRT7蛋白分别在HR、BER和NHEJ修复中发挥了重要作用[126~130]。因此,针对SIRT的靶向激活药物可能具有改善卵母细胞质量的潜能。白藜芦醇是一种SIRT1激活剂。研究报道,在小鼠、猪、牛的卵母细胞体外培养基添加白藜芦醇能够减轻卵母细胞DNA损伤、改善卵母细胞质量和提高早期胚胎发育能力[131~133]。此外,哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin, mTOR)信号通路的抑制剂雷帕霉素似乎也能够增强细胞DNA修复能力。研究报道,在人类和小鼠的卵母细胞中,雷帕霉素能够增强DDR相关基因的表达,减轻DNA损伤,提高卵母细胞的质量和发育潜力[134~136]。此外,人们尝试了对卵母细胞直接补充关键的DNA修复因子。Kujjo等[53]报道,向小鼠的老化卵母细胞中注射重组RAD51蛋白能够减轻DSBs损伤,抑制卵母细胞凋亡,提高胚胎的发育能力。
5 结语与展望
与体细胞类似,卵母细胞也容易被各种因素诱发DNA损伤。在基础研究和临床治疗中,辐射和化疗药物经常造成卵母细胞DNA损伤和卵巢储备损失。除了外源因素,卵母细胞也容易被ROS等内源因素诱发DNA损伤。对于DNA损伤,卵母细胞能够启动DDR,发生包括DNA修复在内的一系列活动。图1总结了各发育阶段卵母细胞的DDR与DNA修复机制。在卵母细胞中,有关DSBs损伤与修复的研究是最多的。研究已经证明,各发育阶段的卵母细胞均能通过HR或NHEJ机制修复DSBs损伤。然而其他DNA损伤类型的卵母细胞相关研究仍比较少。有趣的是,在应对DNA损伤时,不同发育阶段卵母细胞的DDR有所不同。原始卵泡卵母细胞和初级卵泡卵母细胞倾向于通过TAp63途径启动凋亡。然而,进入生长阶段后的卵母细胞更倾向于选择修复,而不是凋亡,除非DNA损伤严重。卵母细胞这种选择策略背后的原因不明。从进化的角度看,在卵母细胞发育的最初阶段,清除基因受损的卵母细胞能够大大降低子代基因突变和染色体变异的风险。此外,轻中度DNA损伤对卵母细胞的减数分裂成熟影响较小,而严重的DNA损伤则可以通过激活DNA损伤检查点诱导卵母细胞G2/前期停滞,或者通过激活SAC诱导卵母细胞MI期停滞。需注意的是,即使逃避了细胞周期检查点的卵母细胞能够完成第一次减数分裂,然而DNA损伤、染色体变异等异常可能持续存在。这提示人们,在科研及临床工作中,获得的形态正常的MII卵母细胞可能存在DNA和染色体的异常,这些异常可能对卵母细胞受精及早期胚胎发育造成影响。
生育高龄女性的卵巢储备减少和卵母细胞质量下降是生殖临床中的常见现象。衰老引起卵母细胞DNA损伤积累和DNA修复能力下降,而ROS增多是衰老损伤细胞DNA的主要机制之一。研究表明,褪黑素等抗氧化剂能够有效减轻ROS诱导的DNA损伤和提高卵母细胞发育能力。此外,在癌症的临床治疗中,放化疗容易造成卵母细胞DNA损伤和诱导卵母细胞凋亡,由此引起的POI对患者的生育能力造成了极大威胁。关于卵母细胞的DNA损伤与修复机制的研究似乎为此类患者保存生育力提供了新的策略。研究发现,通过基因编辑或小分子靶向制剂阻止卵母细胞凋亡可以间接促进DNA修复,从而保护暴露于辐射和化疗药物的动物的生育能力。其次,向卵母细胞直接补充重要DNA修复因子以及使用小分子制剂激活DNA修复途径等方法也初步在动物实验中显现出有效效果。总体而言,这些研究呈现出了较好的结果,然而涉及的药物制剂的效果及作用机制仍需更多的评价和研究。
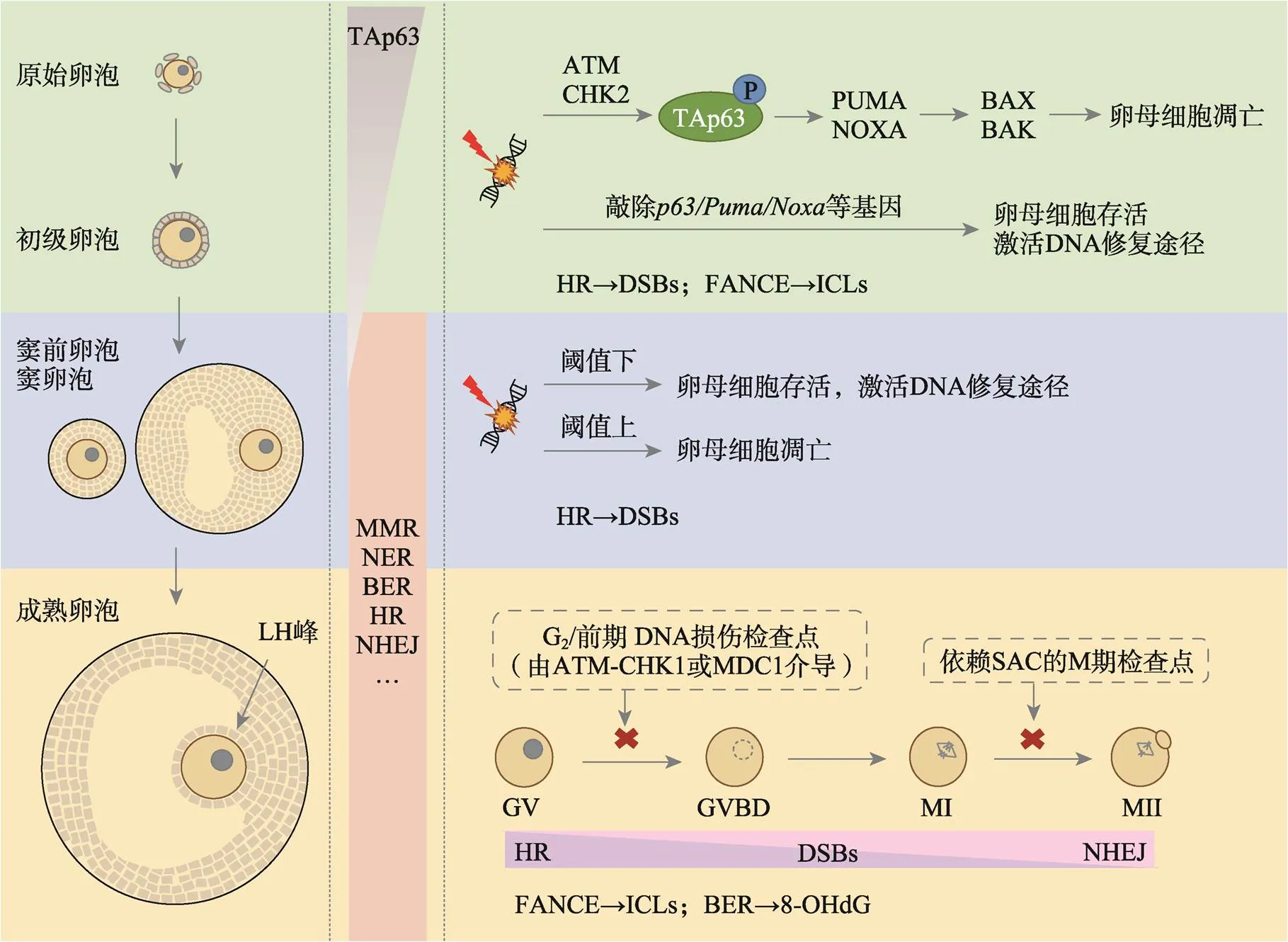
图1 各发育阶段卵母细胞的DDR与DNA修复机制
DNA损伤发生时,原始卵泡和初级卵泡的卵母细胞均通过TAp63介导的反应发生凋亡。卵母细胞进入生长阶段后,TAp63水平下降,参与MMR、BER、NER、HR和NHEJ等DNA修复途径的基因表达水平增加。HR和NHEJ机制在卵母细胞的DSBs修复中发挥重要作用。在卵母细胞减数分裂成熟期间,DSBs修复途径从HR过渡到NHEJ。对于ICLs和8-OHdG损伤,卵母细胞可分别通过FANCE和BER途径进行修复。卵母细胞的其他类型DNA损伤的修复机制有待验证。卵母细胞缺乏经典的由ATM-CHK1介导的G2/前期DNA损伤检查点,但似乎具有一个由MDC1介导的非经典G2/前期DNA损伤检查点。此外,SAC介导了卵母细胞的MI期停滞反应。
综上所述,卵母细胞的DDR与DNA修复机制十分复杂,许多问题迄今只在动物研究中得到了初步回答,关于人类的相关研究仍很缺乏。伴随着高通量技术、基因编辑技术、实验技术等的快速发展,人们将不断拓宽和加深对于哺乳动物卵母细胞的DNA损伤与修复机制的认识。此领域的研究将为开发生育力保护策略提供理论基础和新的方向,具有重要意义。
[1] Musson R, Gąsior Ł, Bisogno S, Ptak GE. DNA damage in preimplantation embryos and gametes: specification, clinical relevance and repair strategies., 2022, 28(3): 376–399.
[2] Jackson SP, Bartek J. The DNA-damage response in human biology and disease., 2009, 461(7267): 1071–1078.
[3] Keefe D, Kumar M, Kalmbach K. Oocyte competency is the key to embryo potential., 2015, 103(2): 317–322.
[4] Winship AL, Stringer JM, Liew SH, Hutt KJ. The importance of DNA repair for maintaining oocyte quality in response to anti-cancer treatments, environmental toxins and maternal ageing., 2018, 24(2): 119–134.
[5] Collins JK, Jones KT. DNA damage responses in mammalian oocytes., 2016, 152(1): R15– R22.
[6] Pailas A, Niaka K, Zorzompokou C, Marangos P. The DNA damage response in fully grown mammalian oocytes., 2022, 11(5): 798.
[7] Martin JH, Aitken RJ, Bromfield EG, Nixon B. DNA damage and repair in the female germline: contributions to ART., 2019, 25(2): 180–201.
[8] Carusillo A, Mussolino C. DNA damage: from threat to treatment., 2020, 9(7): 1665.
[9] Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER 3rd, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, Shiloh Y, Gygi SP, Elledge SJ. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage., 2007, 316(5828): 1160–1166.
[10] Blackford AN, Jackson SP. ATM, ATR, and DNA-PK: the trinity at the heart of the DNA damage response., 2017, 66(6): 801–817.
[11] Collins PL, Purman C, Porter SI, Nganga V, Saini A, Hayer KE, Gurewitz GL, Sleckman BP, Bednarski JJ, Bassing CH, Oltz EM. DNA double-strand breaks induce H2Ax phosphorylation domains in a contact-dependent manner., 2020, 11(1): 3158.
[12] Jeggo PA, Pearl LH, Carr AM. DNA repair, genome stability and cancer: a historical perspective., 2016, 16(1): 35–42.
[13] Kciuk M, Gielecińska A, Mujwar S, Mojzych M, Kontek R. Cyclin-dependent kinases in DNA damage response., 2022, 1877(3): 188716.
[14] Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H, Elledge SJ. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25., 1997, 277(5331): 1497–1501.
[15] Mailand N, Falck J, Lukas C, Syljuâsen RG, Welcker M, Bartek J, Lukas J. Rapid destruction of human Cdc25A in response to DNA damage., 2000, 288(5470): 1425–1429.
[16] Blackford AN, Stucki M. How cells respond to DNA breaks in mitosis., 2020, 45(4): 321–331.
[17] Groelly FJ, Fawkes M, Dagg RA, Blackford AN, Tarsounas M. Targeting DNA damage response pathways in cancer., 2023, 23(2): 78–94.
[18] He Y, Xie MN, Yu L, Ren Z, Zhu F, Fu C. The roles of Fanconi anemia genes in the regulation of follicle development., 2017, 39(6): 469–481. 贺燕, 谢梦女, 余立, 任真, 朱芳, 符淳. 范可尼贫血基因在卵泡发育中的调节作用. 遗传, 2017, 39(6): 469–481.
[19] Takata M, Sasaki MS, Sonoda E, Morrison C, Hashimoto M, Utsumi H, Yamaguchi-Iwai Y, Shinohara A, Takeda S. Homologous recombination and non-homologous end-joining pathways of DNA double-strand break repair have overlapping roles in the maintenance of chromosomal integrity in vertebrate cells., 1998, 17(18): 5497–5508.
[20] Lee JH, Paull TT. ATM activation by DNA double- strand breaks through the Mre11-Rad50-Nbs1 complex., 2005, 308(5721): 551–554.
[21] Williams RS, Moncalian G, Williams JS, Yamada Y, Limbo O, Shin DS, Groocock LM, Cahill D, Hitomi C, Guenther G, Moiani D, Carney JP, Russell P, Tainer JA. Mre11 dimers coordinate DNA end bridging and nuclease processing in double-strand-break repair., 2008, 135(1): 97–109.
[22] Yang HJ, Li QB, Fan J, Holloman WK, Pavletich NP. The BRCA2 homologue Brh2 nucleates RAD51 filament formation at a dsDNA-ssDNA junction., 2005, 433(7026): 653–657.
[23] Thorslund T, McIlwraith MJ, Compton SA, Lekomtsev S, Petronczki M, Griffith JD, West SC. The breast cancer tumor suppressor BRCA2 promotes the specific targeting of RAD51 to single-stranded DNA., 2010, 17(10): 1263–1265.
[24] McVey M, Khodaverdian VY, Meyer D, Cerqueira PG, Heyer WD. Eukaryotic DNA polymerases in homologous recombination., 2016, 50: 393–421.
[25] Scully R, Panday A, Elango R, Willis NA. DNA double-strand break repair-pathway choice in somatic mammalian cells., 2019, 20(11): 698–714.
[26] Gottlieb TM, Jackson SP. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen., 1993, 72(1): 131–142.
[27] Ochi T, Blackford AN, Coates J, Jhujh S, Mehmood S, Tamura N, Travers J, Wu Q, Draviam VM, Robinson CV, Blundell TL, Jackson SP. DNA repair. PAXX, a paralog of XRCC4 and XLF, interacts with Ku to promote DNA double-strand break repair., 2015, 347(6218): 185–188.
[28] Pannunzio NR, Watanabe G, Lieber MR. Nonhomologous DNA end-joining for repair of DNA double- strand breaks., 2018, 293(27): 10512– 10523.
[29] Arnoult N, Correia A, Ma J, Merlo A, Garcia-Gomez S, Maric M, Tognetti M, Benner CW, Boulton SJ, Saghatelian A, Karlseder J. Regulation of DNA repair pathway choice in S and G2phases by the NHEJ inhibitor CYREN., 2017, 549(7673): 548–552.
[30] Schumacher B, Pothof J, Vijg J, Hoeijmakers JHJ. The central role of DNA damage in the ageing process., 2021, 592(7856): 695–703.
[31] Longhese MP, Bonetti D, Guerini I, Manfrini N, Clerici M. DNA double-strand breaks in meiosis: checking their formation, processing and repair., 2009, 8(9): 1127–1138.
[32] Baudat F, Imai Y, de Massy B. Meiotic recombination in mammals: localization and regulation., 2013, 14(11): 794–806.
[33] MacLennan M, Crichton JH, Playfoot CJ, Adams IR. Oocyte development, meiosis and aneuploidy., 2015, 45: 68–76.
[34] Zhao Y, Feng HW, Zhang YH, Zhang JV, Wang XH, Liu DT, Wang TR, Li RHW, Ng EHY, Yeung WSB, Rodriguez-Wallberg KA, Liu K. Current understandings of core pathways for the activation of mammalian primordial follicles., 2021, 10(6): 1491.
[35] Oktem O, Urman B. Understanding follicle growth., 2010, 25(12): 2944–2954.
[36] Spears N, Lopes F, Stefansdottir A, Rossi V, De Felici M, Anderson RA, Klinger FG. Ovarian damage from chemotherapy and current approaches to its protection., 2019, 25(6): 673–693.
[37] Marci R, Mallozzi M, Di Benedetto L, Schimberni M, Mossa S, Soave I, Palomba S, Caserta D. Radiations and female fertility., 2018, 16(1): 112.
[38] Kerr JB, Hutt KJ, Michalak EM, Cook M, Vandenberg CJ, Liew SH, Bouillet P, Mills A, Scott CL, Findlay JK, Strasser A. DNA damage-induced primordial follicle oocyte apoptosis and loss of fertility require TAp63- mediated induction of Puma and Noxa., 2012, 48(3): 343–352.
[39] Coutandin D, Osterburg C, Srivastav RK, Sumyk M, Kehrloesser S, Gebel J, Tuppi M, Hannewald J, Schäfer B, Salah E, Mathea S, Müller-Kuller U, Doutch J, Grez M, Knapp S, Dötsch V. Quality control in oocytes by p63 is based on a spring-loaded activation mechanism on the molecular and cellular level., 2016, 5: e13909.
[40] Luan Y, Yu SY, Abazarikia A, Dong R, Kim SY. TAp63 determines the fate of oocytes against DNA damage., 2022, 8(51): eade1846.
[41] Green DM, Sklar CA, Boice JD Jr. , Mulvihill JJ, Whitton JA, Stovall M, Yasui Y. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor Study., 2009, 27(14): 2374–2381.
[42] Suh EK, Yang AN, Kettenbach A, Bamberger C, Michaelis AH, Zhu Z, Elvin JA, Bronson RT, Crum CP, McKeon F. p63 protects the female germ line during meiotic arrest., 2006, 444(7119): 624–628.
[43] Livera G, Petre-Lazar B, Guerquin MJ, Trautmann E, Coffigny H, Habert R. p63 null mutation protects mouse oocytes from radio-induced apoptosis., 2008, 135(1): 3–12.
[44] Kim SY, Cordeiro MH, Serna VA, Ebbert K, Butler LM, Sinha S, Mills AA, Woodruff TK, Kurita T. Rescue of platinum-damaged oocytes from programmed cell death through inactivation of the p53 family signaling network., 2013, 20(8): 987–997.
[45] Bolcun-Filas E, Rinaldi VD, White ME, Schimenti JC. Reversal of female infertility by Chk2 ablation reveals the oocyte DNA damage checkpoint pathway., 2014, 343(6170): 533–536.
[46] Adhikari D, Busayavalasa K, Zhang JJ, Hu MW, Risal S, Bayazit MB, Singh M, Diril MK, Kaldis P, Liu K. Inhibitory phosphorylation of Cdk1 mediates prolonged prophase I arrest in female germ cells and is essential for female reproductive lifespan., 2016, 26(11): 1212–1225.
[47] Zhu H, Li A, Yu JH, Xiang CJ, Su SD, Huang L, Fan YJ, Luo Y, Tang WR. The new function of p53 family and its pathway related proteins in female reproduction., 2012, 34(8): 943–949. 朱晖, 李安, 余建华, 向超杰, 苏世达, 黄磊, 范豫杰, 罗瑛, 唐文如. p53家族及其通路相关蛋白调节母性生殖的新功能. 遗传, 2012, 34(8): 943–949.
[48] Huang CZ, Zhao SM, Yang YJ, Guo T, Ke HN, Mi X, Qin YY, Chen ZJ, Zhao SD. TP63 gain-of-function mutations cause premature ovarian insufficiency by inducing oocyte apoptosis., 2023, 133(5): e162315.
[49] Deutsch GB, Zielonka EM, Coutandin D, Weber TA, Schäfer B, Hannewald J, Luh LM, Durst FG, Ibrahim M, Hoffmann J, Niesen FH, Sentürk A, Kunkel H, Brutschy B, Schleiff E, Knapp S, Acker-Palmer A, Grez M, McKeon F, Dötsch V. DNA damage in oocytes induces a switch of the quality control factor TAp63α from dimer to tetramer., 2011, 144(4): 566–576.
[50] Stringer JM, Winship A, Zerafa N, Wakefield M, Hutt K. Oocytes can efficiently repair DNA double-strand breaks to restore genetic integrity and protect offspring health., 2020, 117(21): 11513–11522.
[51] ElInati E, Zielinska AP, McCarthy A, Kubikova N, Maciulyte V, Mahadevaiah S, Sangrithi MN, Ojarikre O, Wells D, Niakan KK, Schuh M, Turner JMA. The BCL-2 pathway preserves mammalian genome integrity by eliminating recombination-defective oocytes., 2020, 11(1): 2598.
[52] Nguyen QN, Zerafa N, Findlay JK, Hickey M, Hutt KJ. DNA repair in primordial follicle oocytes following cisplatin treatment., 2021, 38(6): 1405–1417.
[53] Kujjo LL, Laine T, Pereira RJ, Kagawa W, Kurumizaka H, Yokoyama S, Perez GI. Enhancing survival of mouse oocytes following chemotherapy or aging by targeting Bax and Rad51., 2010, 5(2): e9204.
[54] Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, Dickler M, Robson M, Moy F, Goswami S, Oktay K. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans., 2013, 5(172): 172ra121.
[55] Oktay KH, Bedoschi G, Goldfarb SB, Taylan E, Titus S, Palomaki GE, Cigler T, Robson M, Dickler MN. Increased chemotherapy-induced ovarian reserve loss in women with germline BRCA mutations due to oocyte deoxyribonucleic acid double strand break repair deficiency., 2020, 113(6): 1251–1260. e1.
[56] Kurimasa A, Ouyang H, Dong LJ, Wang S, Li X, Cordon-Cardo C, Chen DJ, Li GC. Catalytic subunit of DNA-dependent protein kinase: impact on lymphocyte development and tumorigenesis., 1999, 96(4): 1403–1408.
[57] Yin H, Suye S, Zhou ZX, Cai HY, Fu C. The reduction of oocytes and disruption of the meiotic prophase I in Fanconi anemia E-deficient mice., 2022, 164(3): 71–82.
[58] Sato E. Intraovarian control of selective follicular growth and induction of oocyte maturation in mammals., 2015, 91(3): 76–91.
[59] Puy V, Barroca V, Messiaen S, Ménard V, Torres C, Devanand C, Moison D, Lewandowski D, Guerquin MJ, Martini E, Frydman N, Livera G. Mouse model of radiation-induced premature ovarian insufficiency reveals compromised oocyte quality: implications for fertility preservation., 2021, 43(5): 799–809.
[60] Zeng FY, Baldwin DA, Schultz RM. Transcript profiling during preimplantation mouse development., 2004, 272(2): 483–496.
[61] Zheng P, Schramm RD, Latham KE. Developmental regulation andculture effects on expression of DNA repair and cell cycle checkpoint control genes in rhesus monkey oocytes and embryos., 2005, 72(6): 1359–1369.
[62] Wang SF, Kou ZH, Jing ZY, Zhang Y, Guo XZ, Dong MQ, Wilmut I, Gao SR. Proteome of mouse oocytes at different developmental stages., 2010, 107(41): 17639–17644.
[63] Jaroudi S, Kakourou G, Cawood S, Doshi A, Ranieri DM, Serhal P, Harper JC, SenGupta SB. Expression profiling of DNA repair genes in human oocytes and blastocysts using microarrays., 2009, 24(10): 2649–2655.
[64] Zhang YY, Yan ZQ, Qin QY, Nisenblat V, Chang HM, Yu Y, Wang TR, Lu CL, Yang M, Yang S, Yao Y, Zhu XH, Xia X, Dang YJ, Ren YX, Yuan P, Li R, Liu P, Guo HY, Han JS, He HJ, Zhang K, Wang YT, Wu Y, Li M, Qiao J, Yan J, Yan LY. Transcriptome landscape of human folliculogenesis reveals oocyte and granulosa cell interactions., 2018, 72(6): 1021–1034. e4.
[65] Stefansdottir A, Johnston ZC, Powles-Glover N, Anderson RA, Adams IR, Spears N. Etoposide damages female germ cells in the developing ovary., 2016, 16(1): 482.
[66] Xiao S, Zhang JY, Liu MJ, Iwahata H, Rogers HB, Woodruff TK. Doxorubicin has dose-dependent toxicity on mouse ovarian follicle development, hormone secretion, and oocyte maturation., 2017, 157(2): 320–329.
[67] Tan JH, Wang HL, Sun XS, Liu Y, Sui HS, Zhang J. Chromatin configurations in the germinal vesicle of mammalian oocytes., 2009, 15(1): 1–9.
[68] Menezo Y Jr. , Russo G, Tosti E, El Mouatassim S, Benkhalifa M. Expression profile of genes coding for DNA repair in human oocytes using pangenomic microarrays, with a special focus on ROS linked decays., 2007, 24(11): 513–520.
[69] Kocabas AM, Crosby J, Ross PJ, Otu HH, Beyhan Z, Can H, Tam WL, Rosa GJM, Halgren RG, Lim B, Fernandez E, Cibelli JB. The transcriptome of human oocytes., 2006, 103(38): 14027–14032.
[70] Lee C, Leem J, Oh JS. Selective utilization of non-homologous end-joining and homologous recombination for DNA repair during meiotic maturation in mouse oocytes., 2022, e13384.
[71] Dong MZ, Ouyang YC, Gao SC, Ma XS, Hou Y, Schatten H, Wang ZB, Sun QY. PPP4C facilitates homologous recombination DNA repair by dephosphorylating PLK1 during early embryo development., 2022, 149(10): dev200351.
[72] Martin JH, Bromfield EG, Aitken RJ, Lord T, Nixon B. Double strand break DNA repair occurs via non-homologous end-joining in mouse MII oocytes., 2018, 8(1): 9685.
[73] Leem J, Bai GY, Kim JS, Oh JS. Melatonin protects mouse oocytes from DNA damage by enhancing nonhomologous end-joining repair., 2019, 67(4): e12603.
[74] Hakem R. DNA-damage repair; the good, the bad, and the ugly., 2008, 27(4): 589–605.
[75] Cheng JM, Li J, Tang JX, Hao XX, Wang ZP, Sun TC, Wang XX, Zhang Y, Chen SR, Liu YX. Merotelic kinetochore attachment in oocyte meiosis II causes sister chromatids segregation errors in aged mice., 2017, 16(15): 1404–1413.
[76] Zhao Y, Wang CX, Yang TM, Li CS, Zhang LH, Du DN, Wang RX, Wang J, Wei M, Ba XQ. Linking oxidative DNA lesion 8-OxoG to tumor development and progression., 2022, 44(6): 466–477. 赵岩, 王晨鑫, 杨天明, 李春爽, 张丽宏, 杜冬妮, 王若曦, 王静, 魏民, 巴雪青. DNA氧化损伤8-羟鸟嘌呤与肿瘤的发生发展. 遗传, 2022, 44(6): 466–477.
[77] Lord T, Aitken RJ. Fertilization stimulates 8-hydroxy- 2'-deoxyguanosine repair and antioxidant activity to prevent mutagenesis in the embryo., 2015, 406(1): 1–13.
[78] Marangos P, Carroll J. Oocytes progress beyond prophase in the presence of DNA damage., 2012, 22(11): 989–994.
[79] Lin F, Ma XS, Wang ZB, Wang ZW, Luo YB, Huang L, Jiang ZZ, Hu MW, Schatten H, Sun QY. Different fates of oocytes with DNA double-strand breaksand., 2014, 13(17): 2674–2680.
[80] Rémillard-Labrosse G, Dean NL, Allais A, Mihajlović AI, Jin SG, Son WY, Chung JT, Pansera M, Henderson S, Mahfoudh A, Steiner N, Agapitou K, Marangos P, Buckett W, Ligeti-Ruiter J, FitzHarris G. Human oocytes harboring damaged DNA can complete meiosis I., 2020, 113(5): 1080–1089. e2.
[81] Coticchio G, Dal Canto M, Guglielmo MC, Albertini DF, Mignini Renzini M, Merola M, Lain M, Sottocornola M, De Ponti E, Fadini R. Double-strand DNA breaks and repair response in human immature oocytes and their relevance to meiotic resumption., 2015, 32(10): 1509–1516.
[82] Li TJ, Liu CY, Zhen XM, Yu Y, Qiao J. Actinomycin D causes oocyte maturation failure by inhibiting chromosome separation and spindle assembly., 2021, 104(1): 94–105.
[83] Solc P, Schultz RM, Motlik J. Prophase I arrest and progression to metaphase I in mouse oocytes: comparison of resumption of meiosis and recovery from G2-arrest in somatic cells., 2010, 16(9): 654–664.
[84] Yamaguchi H, Minopoli G, Demidov ON, Chatterjee DK, Anderson CW, Durell SR, Appella E. Substrate specificity of the human protein phosphatase 2Cdelta, Wip1., 2005, 44(14): 5285–5294.
[85] Leem J, Kim JS, Oh JS. WIP1 phosphatase suppresses the DNA damage response during G2/prophase arrest in mouse oocytes., 2018, 99(4): 798–805.
[86] Subramanian GN, Greaney J, Wei Z, Becherel O, Lavin M, Homer HA. Oocytes mount a noncanonical DNA damage response involving APC-Cdh1-mediated proteolysis., 2020, 219(4): e201907213.
[87] Collins JK, Lane SIR, Merriman JA, Jones KT. DNA damage induces a meiotic arrest in mouse oocytes mediated by the spindle assembly checkpoint., 2015, 6: 8553.
[88] Marangos P, Stevense M, Niaka K, Lagoudaki M, Nabti I, Jessberger R, Carroll J. DNA damage-induced metaphase I arrest is mediated by the spindle assembly checkpoint and maternal age., 2015, 6: 8706.
[89] Leem J, Oh JS. MDC1 is essential for G2/M transition and spindle assembly in mouse oocytes., 2022, 79(4): 200.
[90] Farquhar CM, Bhattacharya S, Repping S, Mastenbroek S, Kamath MS, Marjoribanks J, Boivin J. Female subfertility., 2019, 5(1): 7.
[91] Secomandi L, Borghesan M, Velarde M, Demaria M. The role of cellular senescence in female reproductive aging and the potential for senotherapeutic interventions., 2022, 28(2): 172–189.
[92] Horta F, Ravichandran A, Catt S, Vollenhoven B, Temple-Smith P. Ageing and ovarian stimulation modulate the relative levels of transcript abundance of oocyte DNA repair genes during the germinal vesicle-metaphase II transition in mice., 2021, 38(1): 55–69.
[93] Ruth KS, Day FR, Hussain J, Martínez-Marchal A, Aiken CE, Azad A, Thompson DJ, Knoblochova L, Abe H, Tarry-Adkins JL, Gonzalez JM, Fontanillas P, Claringbould A, Bakker OB, Sulem P, Walters RG, Terao C, Turon S, Horikoshi M, Lin K, Onland-Moret NC, Sankar A, Hertz EPT, Timshel PN, Shukla V, Borup R, Olsen KW, Aguilera P, Ferrer-Roda M, Huang Y, Stankovic S, Timmers P, Ahearn TU, Alizadeh BZ, Naderi E, Andrulis IL, Arnold AM, Aronson KJ, Augustinsson A, Bandinelli S, Barbieri CM, Beaumont RN, Becher H, Beckmann MW, Benonisdottir S, Bergmann S, Bochud M, Boerwinkle E, Bojesen SE, Bolla MK, Boomsma DI, Bowker N, Brody JA, Broer L, Buring JE, Campbell A, Campbell H, Castelao JE, Catamo E, Chanock SJ, Chenevix-Trench G, Ciullo M, Corre T, Couch FJ, Cox A, Crisponi L, Cross SS, Cucca F, Czene K, Smith GD, de Geus E, de Mutsert R, De Vivo I, Demerath EW, Dennis J, Dunning AM, Dwek M, Eriksson M, Esko T, Fasching PA, Faul JD, Ferrucci L, Franceschini N, Frayling TM, Gago-Dominguez M, Mezzavilla M, García-Closas M, Gieger C, Giles GG, Grallert H, Gudbjartsson DF, Gudnason V, Guénel P, Haiman CA, Håkansson N, Hall P, Hayward C, He C, He W, Heiss G, Høffding MK, Hopper JL, Hottenga JJ, Hu F, Hunter D, Ikram MA, Jackson RD, Joaquim MDR, John EM, Joshi PK, Karasik D, Kardia SLR, Kartsonaki C, Karlsson R, Kitahara CM, Kolcic I, Kooperberg C, Kraft P, Kurian AW, Kutalik Z, La Bianca M, LaChance G, Langenberg C, Launer LJ, Laven JSE, Lawlor DA, Le Marchand L, Li JM, Lindblom A, Lindstrom S, Lindstrom T, Linet M, Liu YM, Liu SM, Luan JA, Mägi R, Magnusson PKE, Mangino M, Mannermaa A, Marco B, Marten J, Martin NG, Mbarek H, McKnight B, Medland SE, Meisinger C, Meitinger T, Menni C, Metspalu A, Milani L, Milne RL, Montgomery GW, Mook-Kanamori DO, Mulas A, Mulligan AM, Murray A, Nalls MA, Newman A, Noordam R, Nutile T, Nyholt DR, Olshan AF, Olsson H, Painter JN, Patel AV, Pedersen NL, Perjakova N, Peters A, Peters U, Pharoah PDP, Polasek O, Porcu E, Psaty BM, Rahman I, Rennert G, Rennert HS, Ridker PM, Ring SM, Robino A, Rose LM, Rosendaal FR, Rossouw J, Rudan I, Rueedi R, Ruggiero D, Sala CF, Saloustros E, Sandler DP, Sanna S, Sawyer EJ, Sarnowski C, Schlessinger D, Schmidt MK, Schoemaker MJ, Schraut KE, Scott C, Shekari S, Shrikhande A, Smith AV, Smith BH, Smith JA, Sorice R, Southey MC, Spector TD, Spinelli JJ, Stampfer M, Stöckl D, van Meurs JBJ, Strauch K, Styrkarsdottir U, Swerdlow AJ, Tanaka T, Teras LR, Teumer A, Þorsteinsdottir U, Timpson NJ, Toniolo D, Traglia M, Troester MA, Truong T, Tyrrell J, Uitterlinden AG, Ulivi S, Vachon CM, Vitart V, Völker U, Vollenweider P, Völzke H, Wang Q, Wareham NJ, Weinberg CR, Weir DR, Wilcox AN, van Dijk KW, Willemsen G, Wilson JF, Wolffenbuttel BHR, Wolk A, Wood AR, Zhao W, Zygmunt M, Biobank-based Integrative Omics Study (BIOS) Consortium, eQTLGen Consortium, Biobank Japan Project, China Kadoorie Biobank Collaborative Group, kConFab Investigators, LifeLines Cohort Study, InterAct consortium, 23andMe Research Team, Chen ZM, Li LM, Franke L, Burgess S, Deelen P, Pers TH, Grøndahl ML, Andersen CY, Pujol A, Lopez-Contreras AJ, Daniel JA, Stefansson K, Chang-Claude J, van der Schouw YT, Lunetta KL, Chasman DI, Easton DF, Visser JA, Ozanne SE, Namekawa SH, Solc P, Murabito JM, Ong KK, Hoffmann ER, Murray A, Roig I, Perry JRB. Genetic insights into biological mechanisms governing human ovarian ageing., 2021, 596(7872): 393– 397.
[94] Perry JR, Murray A, Day FR, Ong KK. Molecular insights into the aetiology of female reproductive ageing., 2015, 11(12): 725–734.
[95] Liu CM, Ding LJ, Li JY, Dai JW, Sun HX. Advances in the study of ovarian dysfunction with aging., 2019, 41(9): 816–826. 刘传明, 丁利军, 李佳音, 戴建武, 孙海翔. 衰老导致卵巢功能低下研究进展. 遗传, 2019, 41(9): 816–826.
[96] Yuan LH, Yin P, Yan H, Zhong XF, Ren CX, Li K, Heng BC, Zhang WW, Tong GQ. Single-cell transcriptome analysis of human oocyte ageing., 2021, 25(13): 6289–6303.
[97] He DJ, Wang L, Zhang ZB, Guo K, Li JZ, He XC, Cui QH, Zheng P. Maternal gene Ooep may participate in homologous recombination-mediated DNA double- strand break repair in mouse oocytes., 2018, 39(6): 387–395.
[98] Lin T, Sun L, Lee JE, Kim SY, Jin DI. DNA damage repair is suppressed in porcine aged oocytes., 2021, 63(5): 984–997.
[99] Horta F, Catt S, Ramachandran P, Vollenhoven B, Temple-Smith P. Female ageing affects the DNA repair capacity of oocytes in IVF using a controlled model of sperm DNA damage in mice., 2020, 35(3): 529–544.
[100] Setti AS, Braga D, Provenza RR, Iaconelli A Jr, Borges E Jr. Oocyte ability to repair sperm DNA fragmentation: the impact of maternal age on intracytoplasmic sperm injection outcomes., 2021, 116(1): 123–129.
[101] Horikoshi M, Day FR, Akiyama M, Hirata M, Kamatani Y, Matsuda K, Ishigaki K, Kanai M, Wright H, Toro CA, Ojeda SR, Lomniczi A, Kubo M, Ong KK, Perry JRB. Elucidating the genetic architecture of reproductive ageing in the Japanese population., 2018, 9(1): 1977.
[102] Chiang JL, Shukla P, Pagidas K, Ahmed NS, Karri S, Gunn DD, Hurd WW, Singh KK. Mitochondria in ovarian aging and reproductive longevity., 2020, 63: 101168.
[103] Santos AL, Sinha S. Obesity and aging: molecular mechanisms and therapeutic approaches., 2021, 67: 101268.
[104] Immediata V, Ronchetti C, Spadaro D, Cirillo F, Levi-Setti PE. Oxidative stress and human ovarian response-from somatic ovarian cells to oocytes damage: a clinical comprehensive narrative review., 2022, 11(7): 1335.
[105] Lord T, Nixon B, Jones KT, Aitken RJ. Melatonin prevents postovulatory oocyte aging in the mouse and extends the window for optimal fertilization in vitro., 2013, 88(3): 67.
[106] Zhang H, Li C, Wen DX, Li RY, Lu SH, Xu R, Tang YJ, Sun YD, Zhao XE, Pan MH, Ma BH. Melatonin improves the quality of maternally aged oocytes by maintaining intercellular communication and antioxidant metabolite supply., 2022, 49: 102215.
[107] Al-Shahat A, Hulail MAE, Soliman NMM, Khamis T, Fericean LM, Arisha AH, Moawad RS. Melatonin mitigates cisplatin-induced ovarian dysfunction via altering steroidogenesis, inflammation, apoptosis, oxidative stress, and PTEN/PI3K/Akt/mTOR/AMPK signaling pathway in female rats., 2022, 14(12): 2769.
[108] Jiang Q, Qi X, Ding C, Liu XY, Lei YY, Li SY, Cao ZB. Melatonin rescues dimethoate exposure-induced meiotic and developmental defects of porcine oocytes., 2022, 12(7): 832.
[109] Zhang MQ, ShiYang XY, Zhang YW, Miao YL, Chen Y, Cui ZK, Xiong B. Coenzyme Q10 ameliorates the quality of postovulatory aged oocytes by suppressing DNA damage and apoptosis., 2019, 143: 84–94.
[110] Özcan P, Fıçıcıoğlu C, Kizilkale O, Yesiladali M, Tok OE, Ozkan F, Esrefoglu M. Can Coenzyme Q10 supplementation protect the ovarian reserve against oxidative damage?, 2016, 33(9): 1223–1230.
[111] Niu BY, Liao KX, Zhou YX, Wen T, Quan GL, Pan X, Wu CB. Application of glutathione depletion in cancer therapy: enhanced ROS-based therapy, ferroptosis, and chemotherapy., 2021, 277: 121110.
[112] Liu JM, Liu MY, Ye XY, Liu K, Huang JJ, Wang LL, Ji GZ, Liu N, Tang XD, Baltz JM, Keefe DL, Liu L. Delay in oocyte aging in mice by the antioxidant N-acetyl- L-cysteine (NAC)., 2012, 27(5): 1411– 1420.
[113] Bertoldo MJ, Listijono DR, Ho WJ, Riepsamen AH, Goss DM, Richani D, Jin XL, Mahbub S, Campbell JM, Habibalahi A, Loh WN, Youngson NA, Maniam J, Wong ASA, Selesniemi K, Bustamante S, Li C, Zhao YQ, Marinova MB, Kim LJ, Lau L, Wu RM, Mikolaizak AS, Araki T, Le Couteur DG, Turner N, Morris MJ, Walters KA, Goldys E, O'Neill C, Gilchrist RB, Sinclair DA, Homer HA, Wu LE. NAD+repletion rescues female fertility during reproductive aging., 2020, 30(6): 1670–1681. e7.
[114] Li H, Wang H, Xu JM, Zeng XX, Sun YP, Yang QL. Nicotinamide riboside supplementation ameliorated post-ovulatory oocyte quality decline., 2023, 165(1): 103–111.
[115] Wang LY, Chen YR, Wei JR, Guo FC, Li LY, Han Z, Wang ZZ, Zhu HB, Zhang XL, Li ZY, Dai PX. Administration of nicotinamide mononucleotide improves oocyte quality of obese mice., 2022, 55(11): e13303.
[116] Fleming TP, Watkins AJ, Velazquez MA, Mathers JC, Prentice AM, Stephenson J, Barker M, Saffery R, Yajnik CS, Eckert JJ, Hanson MA, Forrester T, Gluckman PD, Godfrey KM. Origins of lifetime health around the time of conception: causes and consequences., 2018, 391(10132): 1842–1852.
[117] Szostak J, Laurant P. The forgotten face of regular physical exercise: a 'natural' anti-atherogenic activity., 2011, 121(3): 91–106.
[118] Gilbert JS, Banek CT, Bauer AJ, Gingery A, Needham K. Exercise training attenuates placental ischemia-induced hypertension and angiogenic imbalance in the rat., 2012, 60(6): 1545–1551.
[119] Kim S, Kim SW, Han SJ, Lee S, Park HT, Song JY, Kim T. Molecular mechanism and prevention strategy of chemotherapy- and radiotherapy-induced ovarian damage., 2021, 22(14): 7484.
[120] Dolmans MM, Donnez J, Cacciottola L. Fertility preservation: the challenge of freezing and transplanting ovarian tissue., 2021, 27(8): 777–791.
[121] Rinaldi VD, Hsieh K, Munroe R, Bolcun-Filas E, Schimenti JC. Pharmacological inhibition of the DNA damage checkpoint prevents radiation-induced oocyte death., 2017, 206(4): 1823–1828.
[122] Tuppi M, Kehrloesser S, Coutandin DW, Rossi V, Luh LM, Strubel A, Hötte K, Hoffmeister M, Schäfer B, De Oliveira T, Greten F, Stelzer EHK, Knapp S, De Felici M, Behrends C, Klinger FG, Dötsch V. Oocyte DNA damage quality control requires consecutive interplay of CHK2 and CK1 to activate p63., 2018, 25(3): 261–269.
[123] Kim SY, Nair DM, Romero M, Serna VA, Koleske AJ, Woodruff TK, Kurita T. Transient inhibition of p53 homologs protects ovarian function from two distinct apoptotic pathways triggered by anticancer therapies., 2019, 26(3): 502–515.
[124] Kim SY, Cho GJ, Davis JS. Consequences of chemotherapeutic agents on primordial follicles and future clinical applications., 2019, 62(6): 382–390.
[125] Lagunas-Rangel FA. Current role of mammalian sirtuins in DNA repair., 2019, 80: 85–92.
[126] Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu PF, Mostoslavsky G, Franco S, Murphy MM, Mills KD, Patel P, Hsu JT, Hong AL, Ford E, Cheng HL, Kennedy C, Nunez N, Bronson R, Frendewey D, Auerbach W, Valenzuela D, Karow M, Hottiger MO, Hursting S, Barrett JC, Guarente L, Mulligan R, Demple B, Yancopoulos GD, Alt FW. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6., 2006, 124(2): 315–329.
[127] McCord RA, Michishita E, Hong T, Berber E, Boxer LD, Kusumoto R, Guan SH, Shi XB, Gozani O, Burlingame AL, Bohr VA, Chua KF. SIRT6 stabilizes DNA- dependent protein kinase at chromatin for DNA double-strand break repair., 2009, 1(1): 109–121.
[128] Uhl M, Csernok A, Aydin S, Kreienberg R, Wiesmüller L, Gatz SA. Role of SIRT1 in homologous recombination., 2010, 9(4): 383–393.
[129] Mao ZY, Tian X, Van Meter M, Ke ZH, Gorbunova V, Seluanov A. Sirtuin 6 (SIRT6) rescues the decline of homologous recombination repair during replicative senescence., 2012, 109(29): 11800–11805.
[130] Vazquez BN, Thackray JK, Serrano L. Sirtuins and DNA damage repair: SIRT7 comes to play., 2017, 8(2): 107–115.
[131] Han J, Wang HR, Zhang T, Chen ZQ, Zhao T, Lin L, Xia GL, Wang C. Resveratrol attenuates doxorubicin-induced meiotic failure through inhibiting oxidative stress and apoptosis in mouse oocytes., 2020, 12(9): 7717–7728.
[132] Wang F, Tian XZ, Zhang L, He CJ, Ji PY, Li Y, Tan DX, Liu GS. Beneficial effect of resveratrol on bovine oocyte maturation and subsequent embryonic development afterfertilization., 2014, 101(2): 577–586.
[133] Li Y, Wang J, Zhang ZZ, Yi JY, He CJ, Wang F, Tian XZ, Yang MH, Song YK, He PL, Liu GS. Resveratrol compares with melatonin in improvingporcine oocyte maturation under heat stress., 2016, 7: 33.
[134] Yang QY, Hu J, Wang M, Guo N, Yang L, Xi QS, Zhu LX, Jin L. Rapamycin improves the quality and developmental competence ofmatured oocytes in aged mice and humans., 2022, 14(22): 9200–9209.
[135] Yang QY, Xi QS, Wang M, Liu J, Li Z, Hu J, Jin L, Zhu LX. Rapamycin improves the developmental competence of human oocytes by alleviating DNA damage during IVM., 2022, 2022(4): hoac050.
[136] Yang QY, Xi QS, Wang M, Long R, Hu J, Li Z, Ren XL, Zhu LX, Jin L. Rapamycin improves the quality and developmental competence of mice oocytes by promoting DNA damage repair duringmaturation., 2022, 20(1): 67.
Advances in the study of DNA damage and repair in mammalian oocytes
Nan Zhang1, Jue Zhang2, Ge Lin1,2
DNA damage is one of the key factors affecting gametogenesis and embryo development. Oocytes are susceptible to DNA damage induced by various endogenous and exogenous factors (e.g., reactive oxygen species, radiation, chemotherapeutic agents, etc.). Current research has revealed that oocytes at various developmental stages are able to respond to various types of DNA damage, repairing DNA or initiating apoptosis through complex mechanisms. Primordial follicular oocytes are more susceptible to apoptosis induced by DNA damage than oocytes entering the growth stage. DNA damage is less likely to induce arrest of the meiotic maturation process in oocytes, however the developmental capacity of oocytes carrying DNA damage is significantly reduced. In clinical practice, aging, radiation and chemotherapy are common causes of oocyte DNA damage, reduced ovarian reserve and infertility in women. Therefore, various methods that can reduce DNA damage and enhance DNA repair in oocytes have been tried in an attempt to protect oocytes. In this review, we systematically summarize the mechanisms of DNA damage and repair in mammalian oocytes at various developmental stages and discuss their potential clinical value with the aim to provide new strategies for fertility protection.
oocytes; DNA damage; DNA repair; DNA damage response
2023-02-02;
2023-03-23;
2023-03-29
中国博士后科学基金(编号:2021M690983)资助[Supported by the Fellowship of China Postdoctoral Science Foundation (No. 2021M690983)]
张楠,在读硕士研究生,专业方向:生殖医学。E-mail: csudnn@csu.edu.cn
林戈,博士,研究员,研究方向:生殖医学。E-mail: linggf@hotmail.com
10.16288/j.yczz.23-018
(责任编委: 黄俊)
