STIM1在肿瘤发生及转移中的研究进展
2023-05-23严程浩白韦钰张智猛沈俊岭王友军孙建伟
严程浩,白韦钰,张智猛,沈俊岭,王友军,孙建伟
综 述
STIM1在肿瘤发生及转移中的研究进展
严程浩1,白韦钰1,张智猛1,沈俊岭1,王友军2,孙建伟1
1. 云南大学生命科学中心,生命科学学院,省部共建云南生物资源保护与利用国家重点实验室,昆明 650500 2. 北京师范大学生命科学学院,基因资源与分子发展北京重点实验室,北京 100875
基质互作分子1 (stromal interaction molecule 1,STIM1)是细胞钙库操纵性钙内流(store-operated calcium entry,SOCE)通路的关键成员,它定位在内质网膜上,并在多种肿瘤细胞中高表达。异常表达的STIM1能够通过影响侵袭伪足(invadopodia)形成、干扰血管生成、介导炎症反应、改变细胞骨架和细胞动力等方式促进肿瘤发生及转移,然而其具体的调控作用机制仍未完全阐明。本文综述了目前STIM1在不同肿瘤发生及转移中的最新研究进展,总结并探讨了其在肿瘤发生及转移中的调控机制,为将来在肿瘤领域对STIM1的深入研究提供借鉴和参考。
STIM1;钙库操纵性钙内流;肿瘤发生;肿瘤转移
基质互作分子1(stromal interaction molecule 1,STIM1)是一种位于内质网膜上的单次跨膜钙离子结合蛋白。其保守程度很高,最初被定义为一种与血细胞前体相互作用的基质细胞表面分子[1],随后被鉴定为参与激活钙离子流入所必要的一员[2]。STIM1被发现在胶质母细胞瘤[3]、胰腺癌[4]、前列腺癌[5,6]、肝细胞癌[7]、肾透明细胞癌[8]中高表达,并促进癌细胞增殖、迁移、侵袭和凋亡抵抗。同时,功能丧失性突变会导致患者T细胞激活的严重缺陷,伴随着感染易感性增加。因此,STIM1对维持正常免疫系统稳定具有一定的作用。另外,STIM1还能帮助内皮细胞增殖[9]。研究发现,高表达的STIM1能够促进血管生成、肿瘤发生、肿瘤转移[10]以及癌细胞耐药。近年来,关于STIM1在肿瘤发生及转移中的作用机制研究越来越多,本文总结了STIM1在肿瘤发生及转移中的功能,并对其调控机制的研究进展进行了综述。
1 STIM1的生物学功能
1.1 STIM1介导Ca2+内流
细胞内Ca2+水平的变化能够提供普遍和动态的信号,从而调节大多数细胞中的各种生物过程[11,12]。Ca2+参与了细胞的众多生理反应,包括细胞活化、分化和胞吐作用[13,14]。STIM1主要定位于内质网,且在质膜中也有分布[15~18]。静息状态下的内质网如果处于钙储备充足状态,位于其上的Ca2+会与STIM1-EF手形结构域结合。EF手形结构域与SAM结构域具有稳定的相互作用,能够维持STIM1分子内质网腔部分的单体分布[19]。STIM1在内质网中担任Ca2+的传感器,是SOCE的开关。内质网钙库的消耗导致STIM1的构象变化和寡聚,并使其在内质网膜上的分布发生显著变化,从普遍的扩散分布改变为接近质膜(plasma membrane,PM)的离散簇(puncta)[20~22]。激活的STIM1结合并偶联质膜上SOCE通道蛋白Orai1形成内质网钙释放激活钙(calcium release activated calcium channel,CRAC)通道[23,24]。由STIM1和Orai1组成的CRAC通道在内质网与质膜连接区域介导钙离子的内流,并能在Orai1通道口处产生Ca2+微域[23]。内质网钙库Ca2+水平降低后STIM1被激活,并与Orai1以协同的方式在内质网和质膜中形成紧密相对的簇状复合物,从而为SOCE的局部激活提供物理基础。这是理解高度局部化的SOCE信号的下游分子和生理功能的关键。
1.2 STIM1维持内皮细胞增殖
内皮细胞的迁移、增殖、极性、分化和细胞间通信水平上的协调控制对功能性血管形态发生至关重要[25]。Abdullaev等[9]发现,与基因沉默均可抑制内皮细胞的增殖,并导致细胞周期停滞在S期或G2期,表明STIM1参与的SOCE在维持内皮细胞增殖方面具有重要作用。
1.3 STIM1维持免疫系统稳定
Ca2+通过CRAC通道流入细胞内部激活淋巴细胞和免疫反应[26~28]。Picard等[29]发现,缺陷的患者会伴随原发性免疫缺陷,更容易受到病毒和细菌感染,而缺陷的小鼠则更容易患有包括脾肿大、淋巴结病、白细胞器官浸润、皮炎和睑缘炎在内的病症[30]。这些表型归因于活化T细胞蛋白的Ca2+流入异常导致的功能抑制和调节性T细胞发育和功能缺陷。
此外,STIM1介导的SOCE在调节中性粒细胞烟酰胺腺嘌呤二核苷酸磷酸(NADPH)氧化酶的激活和随后的活性氧(ROS)的产生以及肥大细胞的激活、脱颗粒和细胞因子的分泌中发挥了关键作用[31,32]。
Berry等[33]的研究表明,在B细胞中SOCE对于抗凋亡蛋白的转录和上调至关重要。B细胞受体(B cell receptor,BCR)刺激STIM1,STIM2均缺失的B细胞会导致大量细胞死亡。其具体作用机制是,BCR介导的SOCE激活了NFAT和核因子-kappaB (NF-κB)通路,最终聚集在哺乳动物雷帕霉素复合物1(mTORC1)靶点上,激活并诱导c-Myc从而驱动B细胞增殖。
STIM1和Orai1介导的Ca2+内流对T细胞的激活、增殖和代谢至关重要,过度或延长的Ca2+信号可导致细胞死亡[34]。Desvignes等[35]发现,在受到慢性结核分枝杆菌感染的小鼠中,STIM1缺乏的T细胞对T细胞受体(T cell receptor,TCR)刺激下的死亡产生抗性,肺部促凋亡因子FAS (fas cell surfacedeath receptor)、FASL (fas ligand)和NOXA (phorbol-12- myristate-13-acetate-induced protein 1)的表达显著。SOCE是T细胞免疫所需的钙离子进入的主要途径。STIM1、STIM2和Oria1介导的SOCE是中心激活因子,它产生关键的Ca2+微域,这是活化T细胞核因子(nuclear factor of activated T cells,NFAT)核易位和最佳细胞因子基因表达、代谢和增殖所必需的。
1.4 STIM1与脂质信号传递
众所周知,内质网(endoplasmic reticulum,ER)与质膜界面的连接是通过脂质转运实现的,脂质转运的位点则由PI、PI4P、PA、PS、PC和甾醇等各种脂质转运蛋白介导[36]。
内质网与质膜接触位点中的Ca2+与脂质信号之间存在密切的相互作用和反馈回路。Weber-Boyvat等[37]发现,生理条件下,氧甾醇结合蛋白相关蛋白3 (oxysterol-binding protein related proteins 3,ORP3)参与脂质运输和细胞信号传导。ORP3定位于ER-PM界面,与脂质转运和局部粘附动力学有关。它在蛋白激酶C激活时易位到连接点进行连接,并与Ca2+一起产生协同效应。沉默后显著影响ORP3移位到ER-PM多克隆位点(multiple cloning site,MCS)的水平。STIM1在ORP3招募中发挥作用[38],过度表达的STIM1会促进和扩大ER-PM接触区域以增加ORP3与PM的关联,从而影响ORP3发挥作用[39]。
2 STIM1在不同肿瘤中的作用
STIM1广泛表达于人类各种组织,并参与到各种生理和病理过程。TCGA数据分析显示,STIM1在人不同肿瘤如消化系统肿瘤中的胆管癌、胰腺癌,泌尿系统肿瘤中的肾嫌色细胞瘤、肾透明细胞癌、肾乳头状细胞癌,神经系统肿瘤中的嗜铬细胞瘤和副神经瘤等中均高表达(图1),并与肿瘤的病理分型、恶性程度、侵袭性和转移等都有相关性。
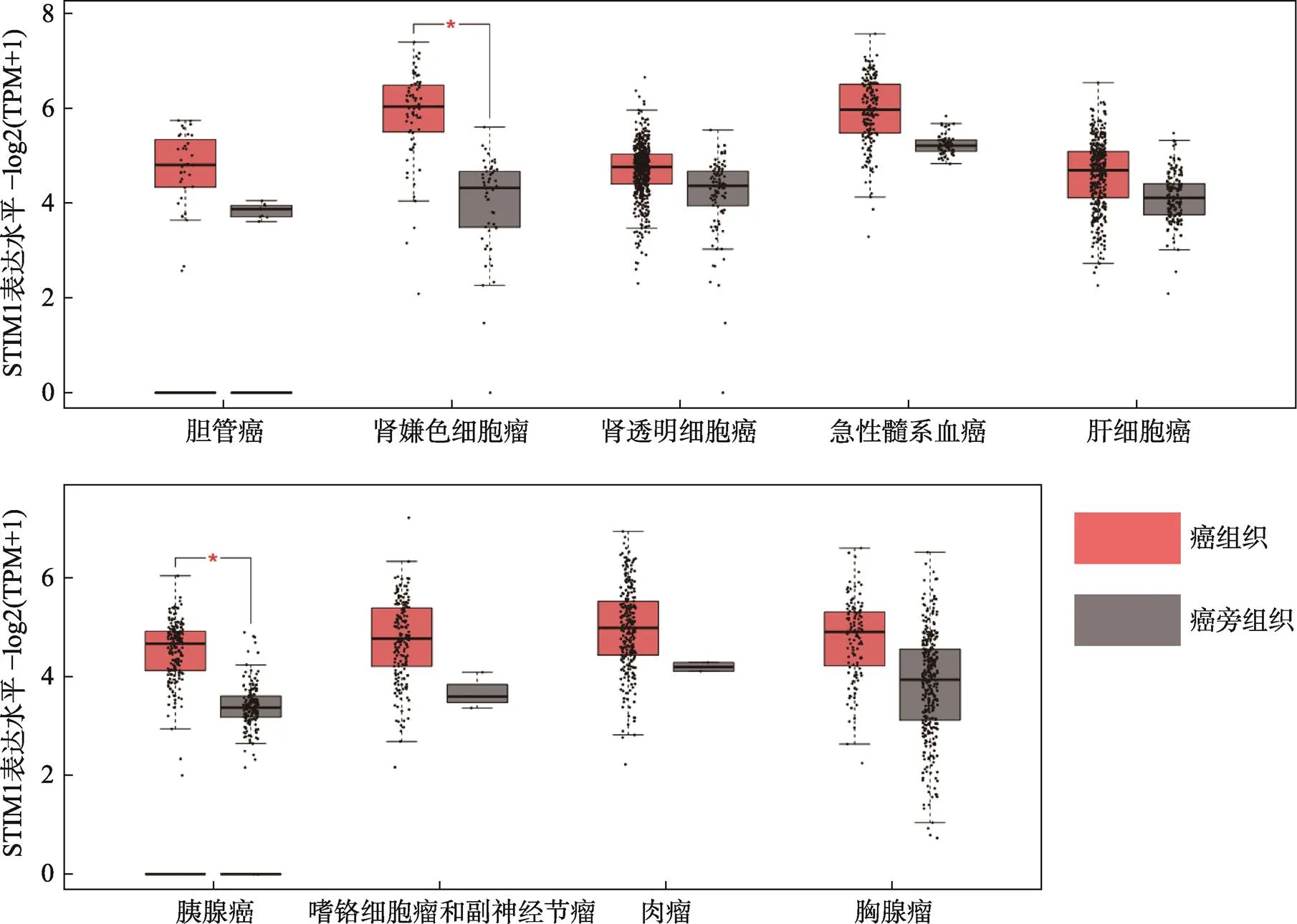
图1 STIM1在不同肿瘤组织与配对正常组织中的表达水平
数据来自于http://gepia2.cancer-pku.cn。*表示<0.05。
2.1 STIM1与肿瘤发生
肿瘤发生是一个复杂的生物学过程,涉及到的影响因素众多,STIM1可从以下几个方面影响肿瘤的发生。
STIM1影响血管生成,从而促进肿瘤发生。STIM1间接参与内皮祖细胞(endothelial progenitor cells,EPCs)从骨髓中的招募,以维持肿瘤血管生成并促进肿瘤发生。Lodola等[40]在肾细胞癌中发现,由STIM1和Orai1介导的SOCE能够通过控制祖细胞增殖和血小管的形成来调节人类EPCs的生长(图2A,①和②)。Ye等[41]发现,EB病毒(epstein-barr virus,EBV)通过激活SOCE促进鼻咽癌(nasopharyngeal carcinoma,NPC)的肿瘤血管生成(图2A,③),并且STIM1在EBV阳性鼻咽癌细胞系中高表达。利用表皮生长因子(epidermal growth factor,EGF)治疗后,在EBV阳性NPC细胞中敲除STIM1可以显著降低Ca2+内流和血管内皮生长因子的产生,同时抑制异种移植物生长和血管生成,表明EBV通过激活STIM1依赖性Ca2+信号来促进EGF诱导的丝裂原活化蛋白激酶(mitogen-activated protein kinase 1,ERK)1/2信号传导(图2A,④),而阻断这种信号传导可能会抑制EBV促进的鼻咽癌血管生成。另有研究表明,STIM1可以与致瘤性和血管生成生长因子启动的信号级联作用,从而启动肿瘤的发生过程[42]。已知表皮生长因子通过磷酸酯酶C (phospholipase C,PLC)/三磷酸肌醇(inositol 1,4,5-trisphosphate,IP3)通路刺激细胞内Ca2+的释放,导致SOCE的激活(图2A,④)。Kokoska等[43]发现,非甾体抗炎药物阻断由EGF诱导的SOCE激活过程,从而干扰细胞增殖,抑制结直肠癌的发生,这独立于其对前列腺素合成的抑制途径。
STIM1参与的细胞内一系列信号串扰(signal crosstalk)能够影响肿瘤发生。Feng等[44]报道了Orai1与分泌途径Ca2+-ATP酶2(secretory pathway Ca2+-ATPases2,SPCA2)相关信号通路,在该信号通路中,Orai1-SPCA2复合物的形成引发了一个能不依赖于钙库并自发激活独立的Ca2+进入通路,该通路调节乳腺癌肿瘤的发生(图2A,⑤)。实际上,STIM1-Orai1作为一种“经典”的信号通路,在雌激素受体阴性乳腺癌细胞生成中占主导地位[45]。SOCE是一个信号复合体,其功能改变是由STIM1在内的数个信号组成单位决定的。异常的SOCE激活会促进肿瘤发生与进展。
STIM1与细胞骨架相关蛋白协同作用,影响肿瘤发生。微管完整性对于STIM1运输到质膜以及与组成SOCE必需的亚单位Orai1的相互作用是必要的。Chen等[46]发现,微管相关组蛋白去乙酰化酶6 (histone deacetylase 6,HDAC6)在宫颈癌细胞和正常宫颈上皮细胞之间对STIM1介导的SOCE的激活进行差异调节(图2A,⑦),而大多数宫颈癌组织过度表达STIM1和Orai1,并伴有低乙酰化的α-微管蛋白,同时,HDAC6-STIM1相关通路能够影响恶性细胞血管的生成。
2.2 STIM1与肿瘤转移
细胞迁移是细胞和组织内稳态的基础,并在许多生理和病理过程中起着关键作用。伤口愈合、免疫监视和血管生成分别需要成纤维细胞、免疫细胞和内皮细胞的迁移[47~50],也有一些病理表型涉及“错误”细胞类型的迁移,这与癌症的进展尤为相关。肿瘤细胞的迁移活动是转移级联反应中的一个关键步骤,它会导致肿瘤细胞在远处器官中的定植[51~53]。细胞运动遵循运动轴以及细胞骨架的极化方向,并受到膜动力学的调节[54~58],这在一定程度上是由迁移细胞的细胞内钙浓度([Ca2+]i)的梯度介导的。STIM/Orai蛋白作为膜蛋白,具有感知和反应肿瘤微环境中已知发生的各种细胞内和细胞外刺激的能力[59]。这些刺激因素包括:(1)缺氧,以及由此产生的氧化应激(图2B,①);(2)ROS(图2B,②);(3)ADP核糖(adenosine diphosphate,ADPr)(图2B,③)。研究发现,STIM1可以介导ROS和Ca2+(作为应力反应信使)之间的耦合(图2B,④)[60]。
转移与侵袭常被视为肿瘤进展的两个标志,STIM1在促肿瘤侵袭方面也有重要作用。近年来,大量研究表明,基因突变有可能是黑色素瘤发生的一大诱因。全基因组分析显示,在黑色素瘤患者的众多突变基因的样本中,B-Raf原癌基因(B-Raf proto-oncogene,BRAF)突变频率最高,占比约为52%[61]。BRAF最常见突变类型为BRAF- V600E[62],小分子抑制剂威罗菲尼(vemurafenib)靶向BRAF-V600E以抑制含有此突变类型的黑色素瘤[63],但长期使用会产生耐药性[64],Shen等[10]发现STIM1在威罗菲尼耐药细胞当中高表达,随后激活下游丙酮酸激酶2 (pyruvate kinase2,PK2)-原癌基因SRC(SRC proto-oncogene)信号轴,从而启动侵袭伪足的形成,导致抗药性产生引起的肿瘤转移(图2B),将STIM1敲除后,侵袭伪足形成显著下降,表明STIM1/Ca2+信号通过影响侵袭伪足的形成调控肿瘤转移。
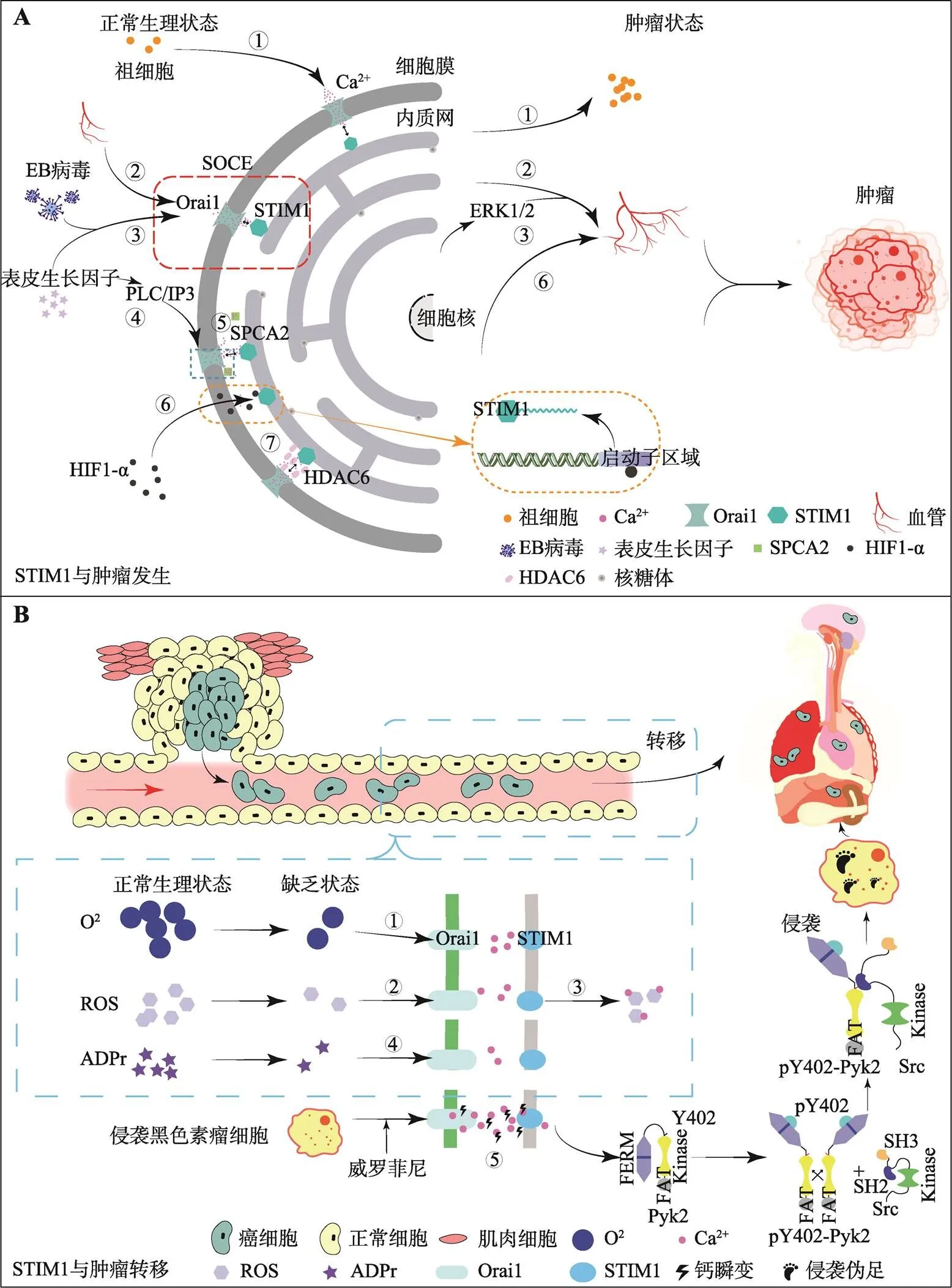
图2 STIM1在肿瘤发生与转移中的作用
A:STIM1与肿瘤发生。①STIM1和Orai1介导的SOCE控制祖细胞增殖;②STIM1和Orai1介导的SOCE影响血小管的形成;③EB病毒通过激活STIM1依赖性Ca2+信号促进EGF诱导的ERK1/2信号传导,促进鼻咽癌中的肿瘤血管生成;④表皮生长因子通过PLC/IP3通路刺激细胞内Ca2+的释放,导致SOCE的激活;⑤Orai1-SPCA2复合物的形成引发了一个不依赖于钙库并自发独立激活的Ca2+进入通路,调节乳腺癌肿瘤的发生;⑥HIF-1α与STIM1启动子结合,并调控其在PANC-1癌细胞中的表达;⑦HDAC6在宫颈癌细胞和正常宫颈上皮细胞之间对STIM1介导的SOCE的激活进行差异调节。B:STIM1与肿瘤转移。①STIM1作为内质网膜蛋白,能够感知和反应肿瘤微环境中的缺氧刺激;②STIM1作为膜蛋白,能够感知和反应肿瘤微环境中的ROS刺激;③STIM1介导ROS和Ca2+(作为应力反应信使)之间的耦合;④STIM1作为膜蛋白,能够感知和反应肿瘤微环境中的ADPr刺激;⑤威罗菲尼耐药细胞当中STIM1高表达,随后激活下游Pyk2-Src信号轴,从而启动侵袭伪足的形成,导致抗药性产生引起的肿瘤转移。
STIM1在肿瘤发生和肿瘤进展中发挥重要的作用(图2),是将来肿瘤研究中的重点和难点。
2.3 消化系统肿瘤
STIM1在多种消化系统肿瘤的患者组织中高表达,并促进肿瘤细胞的侵袭及转移。
胰腺导管腺癌(pancreatic ductal adenocarcinoma,PDAC)患者由于诊断较晚和治疗耐药性,5年生存率仅为7%~9%[65],其一线治疗剂为吉西他滨(gemcitabine),但长期治疗会导致耐药性。Kutschat等[66]发现,吉西他滨耐药细胞显示出相邻的核糖核苷酸还原酶催化亚单位M1(ribonucleotide reductase catalytic subunit M1,RRM1)和基因的共扩增,较高的STIM1依赖性钙内流导致内质网应激反应受损,随后激活NFAT。该工作揭示了STIM1作为变阻器的功能,能介导钙信号参与调节生物过程,控制表观遗传细胞命运的决定。Wang等[67]发现,与正常组织相比,STIM1和缺氧诱导因子-1alpha (hypoxia-inducible factor-1α,HIF-1α)在胰腺癌组织中表达上调。Kaplan-Meier实验显示,HIF-1α和STIM1表达水平升高与无病生存期降低显著相关,HIF-1α在癌组织的表达与STIM1的表达显著正相关。此外,CHIP和荧光素酶检测证实,HIF-1α与STIM1启动子结合,并调控其在PANC-1细胞中的表达(图2A,⑥)。在结肠癌中,STIM1被Liang等[68]发现能够促进肠上皮增加杯状细胞内质网压力和因细胞损失引起的压力,从而减弱细胞维持粘液层的能力,增加微生物暴露风险,导致结肠炎和结直肠癌的发生。Tang等[69]发现,在鳞状食管癌(esophageal squamous cell carcinoma,ESCC)当中,STIM1的高表达与ESCC的晚期肿瘤分级和不良预后相关。siRNA或化学抑制剂对STIM1表达的抑制显著降低了ESCC细胞的活力和迁移能力。来自高通量单克隆抗体芯片、IHC芯片以及相关生存数据和功能分析的证据表明,STIM1在ESCC中是一个不良的预后生物标志物。Xia等[70]关于胃癌的研究指出,较高的STIM1表达与晚期肿瘤的复发和死亡率密切相关,通过降低STIM1的表达水平,能够降低两种胃癌细胞系的增殖、代谢、迁移和侵袭。此外,基因的沉默也能改变异常的细胞周期和上皮-间充质转化(epithelial mesenchymal transition,EMT)的相关标记物水平,提示STIM1促进肿瘤细胞的增殖、代谢、迁移和侵袭,并可作为胃癌的不良预后标志物。
2.4 泌尿系统肿瘤
肾细胞癌(renal cell carcinoma,RCC)是最常见的肾癌类型,占成人所患肿瘤[71,72]的2%~3%,肾细胞癌最常见的亚型是透明细胞肾细胞癌(clear cell renal cell carcinoma,ccRCC)[73]。Monteith等[74]发现,STIM1在ccRCC组织中高表达,STIM1通过常规的SOCE通路以增强ccRCC细胞的运动能力,加快细胞增殖和迁移速度,表明STIM1依赖的信号通路可能成为ccRCC治疗干预的潜在预后标志物和有吸引力的靶点。
2.5 神经系统肿瘤
结节性硬化症(tuberous sclerosis complex,TSC)是一种常染色体显性综合征,其特征是在包括大脑、肾脏、心脏和肺等广泛的器官中伴随着良性肿瘤的生长[75]。Peng等[76]发现,通过过度活跃的mTORC1-STIM1级联反应而增强的SOCE可能有助于TSC相关肿瘤性质向良性倾斜。因此,STIM1激动剂能减弱mTOR抑制剂介导的AKT再活化,从而增强其治疗TSC患者的疗效。Pascual-Caro等[77]利用神经母细胞瘤细胞SH-SY5Y进行研究,发现STIM1是肌醇1,4,5-三磷酸受体3(inositol 1,4,5- trisphosphate receptor 3,ITPR3)基因表达的正调控因子。缺失导致ITPR3转录本和ITPR3蛋白水平显著降低,从而导致线粒体游离Ca2+浓度降低,线粒体耗氧率降低,三磷酸腺苷(adenosine- triphosphate,ATP)合成率降低,加快肿瘤进展。Pascual-Caro等[78]表明,STIM1在SH-SY5Y分化细胞中对细胞存活是绝对必要的,与野生型细胞相比,分化后的-KO细胞线粒体呼吸链复合物I活性显著降低,线粒体内膜去极化,线粒体游离Ca2+浓度降低,衰老水平升高。同时,-KO细胞在去极化时其Ca2+进入增强,说明STIM1在保护SH-SY5Y细胞存活方面具有重要作用。Xie等[79]发现了STIM1的一个选择性剪接变体STIM1β,它在神经胶质瘤组织中异常上调,以扰乱Ca2+信号通路,在功能上,STIM1β促进胶质母细胞瘤细胞的Ca2+流入,从而加快肿瘤细胞的增殖和生长。
2.6 乳腺系统肿瘤
乳腺癌是全球最常见的诊断型癌症,其发病率位居女性恶性肿瘤的首位[80]。Yang等[81]发现,Orai1和STIM1参与介导MDA-MB-231乳腺癌细胞中的库容性钙内流,这对于体外乳腺肿瘤细胞迁移和小鼠肿瘤转移至关重要。在动物模型中,通过RNA干扰或抑制剂减弱SOCE,在转移性强的人乳腺癌细胞内减少Orai1或STIM1的表达均能够减少肿瘤转移,证明了Orai1和STIM1在肿瘤转移中发挥作用。Pan等[82]发现,-KO可以通过直接抑制miR-145的表达,减少外泌体的产生,从而抑制胰岛素受体底物1(insulin receptor substrate 1,IRS1)表达,以阻止异常的血管生成。Yang等[83]研究表明,乳腺癌患者中STIM1表达明显高于邻近非肿瘤组织。肿瘤大小、淋巴结转移和雌激素受体阴性水平与STIM1过表达呈正相关。Cheng等[84]指出,在MDA-MB-231细胞中过表达STIM1能够将TGF-β诱导的细胞周期阻滞和细胞增殖抑制恢复到与对照几乎一致的水平,表明STIM1在TGF-β诱导的细胞增殖中发挥了关键作用。Chen等[85]研究发现,沉默抑制了活性局灶粘连激酶(focal adhesion kinase,FAK)和TLN1(talin1)在局灶粘连处的募集和关联,表明整合素信号通路的力转导被阻断。表皮生长因子诱导的肌球蛋白II调节轻链的磷酸化被STIM1的下调所消除,这说明肌动球蛋白的形成依赖于STIM1介导的Ca2+进入。更重要的是,STIM1能够调控细胞收缩力的产生,这些结果突出了STIM1依赖的Ca2+信号通路通过调节肌动球蛋白重组和增强的收缩力来控制细胞迁移的独特作用。Huang等[86]发现的基因多态性与乳腺癌存在相关性。的风险G等位基因促进了STIM1的高表达,的两个无义替代突变rs3750996 G和rs2304891 A与乳腺癌的进展相关。此外,Grady等[87]研究发现,Ca2+信号通路的正常生理活动在癌症中经常被吸收和重塑,在受影响的细胞群中产生一个强大的致癌驱动。细胞内Ca2+水平的增加和降低都有可能增加细胞的恶性潜能,一些Ca2+信号通路的活性已被证明会影响化疗反应,这表明结合传统化疗与Ca2+靶向药物的协同方法也能改善患者的预后水平,因此靶向调控相关Ca2+通路代表了精确医学和乳腺癌症治疗的一种新方法。
2.7 其他系统肿瘤
STIM1在皮肤癌当中也有重要的促瘤作用。紫外线照射的最直接后果是诱导嘧啶二聚体形成,这可导致DNA损伤和/或突变[88],紫外线诱导的胆固醇生物合成对于SOCE抑制和细胞的转移至关重要。Wong等[89]对黑色素瘤细胞进行单细胞测序,发现STIM1在黑色素瘤中的表达以细胞特异性的方式影响细胞生理。具体来说,恶性细胞来源的STIM1共表达基因表达被抑制,研究首次将黑色素瘤组织中恶性STIM1的表达与免疫调节联系起来。STIM1与C-X-C基序趋化因子配体12(C-X-C motif chemokine ligand 12,CXCL12)共表达,而CXCL12可与CD8+T细胞、CD4+T调节细胞、CD4+T辅助细胞、NK细胞和巨噬细胞上的趋化因子受体C-X-C基序趋化因子受体4(C-X-C motif chemokine receptor 4,CXCR4)结合,触发下游免疫信号通路。另外,Sun等[90,91]发现,STIM1介导的Ca2+振荡信号通过激活Src促进了侵袭伪足前驱体的组装,而Ca2+振荡的破坏则抑制了侵袭伪足的形成,表明STIM1参与调控侵袭伪足的蛋白水解活性,驱动黑色素瘤的转移。
与非转移性肺癌组织相比,STIM1在转移性肺癌组织中表达显著增加,Wang等[92]在A549细胞中发现,沉默可抑制A549细胞在体内的转移。STIM1不仅在癌细胞中有表达,在基质细胞当中也能检测到它的存在,提示STIM1与细胞状态的转化有关。
在血癌当中,Lominy等[93]发现,缺陷的小鼠体内白血病浸润器官的坏死炎症反应显著减少,且一些与炎症相关的信号通路下调。研究表明,白血病的引起依赖于异常STIM1介导的Ca2+流入而造成的白血病浸润器官的炎症。
此外,STIM1在甲状腺癌[94]、前列腺癌[95,96]、骨肉瘤[97]中均高表达,并在肿瘤的发生发展及浸润转移中发挥重要作用。
3 STIM1在不同肿瘤中的调控
STIM1在癌症进展当中能够发挥不同作用,不仅可以作为核心因子直接参与肿瘤的侵袭或转移,也可以作为调节因子对肿瘤的进展进行间接调控。
3.1 STIM1的上游调控途径
除了已发现的SOCE特异调控剂外,一些生物信号或通路也参与调控STIM1的表达,进而影响疾病的发生。
Ritchie等[98]发现,肾母细胞瘤基因1(wilms tumor suppressor 1,WT1)和早期生长反应因子1 (early growth response 1,EGR1)在急性髓系白血病、肾母细胞瘤、乳腺癌、胶质母细胞瘤和前列腺癌中能够调控STIM1表达;最近,Lee等[99]在乳腺癌当中发现孕酮受体膜组分1 (progesterone receptor membrane component 1,PGRMC1)促进SOCE,并作为STIM1的功能相互作用因子,促进STIM1构象转换,加快肿瘤进展;此外,Faris等[100]在转移性结直肠癌的淋巴细胞中发现二酰甘油激酶(diacylglycerol kinase)负调控STIM1,干扰SOCE自身强度,以间接影响癌症进展。
另外,microRNA也能实现对STIM1的调控。Zhang等[101]发现,一种与EMT相关的miR-185介导的转录后调控能够左右STIM1的水平,miR-185-STIM1轴促进结直肠癌转移。另一种miR-195也被发现能够调控STIM1,并在调控损伤后细胞的迁移中起着重要作用[102]。miR‑541‑3p被证实直接调控STIM1表达,从而实现对细胞活力和迁移的刺激[103]。与上述几个microRNA相反,miR-4725-3p通过直接靶向STIM1的30个非翻译区来抑制胶质瘤细胞的侵袭[104];miR-223靶向STIM1,抑制乳腺癌生物学行为[105]。综上所述,STIM1可以通过miRNA实现对癌症的进展的多元化的调控。
3.2 STIM1参与肿瘤进展的下游调控
STIM1作为介导SOCE的关键因子,在肿瘤的发生和转移当中担任核心角色。Wang等[106]发现,STIM1的异位表达促进了结直肠癌细胞的运动性。STIM1通过增加环氧合酶-2(cyclooxygenase-2,COX-2)的表达和前列腺素E2(production of prostaglandin E2,PGE2)的产生来促进结直肠癌细胞的迁移。Chen等[107]的研究表明,沉默显著改变了podosome的动态,缩短了podosome的维持时间,并减弱了细胞的侵袭性。在缺失(−/−)小鼠胚胎纤维细胞中将cDNA进行短暂表达,促进了podosome的形成,表明STIM1介导的SOCE激活直接调控podosome的形成。另有研究表明,下调STIM1的表达可显著抑制细胞增殖,并使肺癌细胞周期阻滞在G2/M期和S期[108]。此外,Algariri等[109]在急性髓细胞白血病-M5细胞系中通过研究发现STIM1正调控NADPH氧化酶-2(NADPH oxidase-2,NOX2)和蛋白激酶C(protein kinase C,PKC),改变细胞内ROS水平,以影响细胞内环境。
STIM1除了直接影响肿瘤迁移和相关进展,也能通过改变细胞骨架或细胞黏附实现同样的目的。Lin等[110]发现,构成型活性的STIM1显著增加了Ca2+内流、钙蛋白酶活性和FA蛋白的周转,从而阻碍细胞的迁移;研究表明,癌细胞需要适量的Ca2+调节钙蛋白酶活性来控制局灶粘连的组装和拆卸。超载的Ca2+导致异常的钙蛋白酶活性,不利于癌症转移。
3.3 STIM1与其他途径相互作用调控癌症进展
在STIM1单独不能参与调控癌症进展时,一些与STIM1相互作用分子或途径可以帮助其实现目标。Li等[111]发现,STIM1与缺氧肝癌细胞中HIF-1α相互作用。HIF-1α直接控制STIM1的转录,并参与对SOCE的调控。在宫颈癌中,表皮生长因子EGF被发现可以显著促进STIM1和Orai1在细胞膜膜旁区域的相互作用,以诱导Ca2+内流[112]。
另外,Sun等[113]在入侵的黑色素瘤细胞中,观察了由STIM1-Orai1复合物介导的钙瞬变现象。在静息条件下,钙瞬变优先发生在单个侵袭小体和细胞外周附近的部位。异常的钙瞬变提供了较高的Ca2+微域以局部激活Ca2+/钙调素依赖的Pyk2,从而启动细胞侵袭所需的SOCE-Pyk2-Src信号级联。敲除显著降低了钙瞬变频率,降低了整体SOCE水平,减弱了侵袭小体的形成。上述结果表明,STIM1与Orai1介导的SOCE时空动态在协调侵袭信号形成等复杂细胞行为中具有关键作用。
4 结语与展望
STIM1在多数肿瘤中高表达,通过促侵袭伪足生成、促血管生成、介导炎症反应、改变细胞骨架和细胞动力等多个生物进程,促进肿瘤细胞向周围组织浸润及转移。既往研究中有关靶向STIM1抑制肿瘤进展分为以下几个方向:STIM1能够通过Ca2+构象的改变以实现自抑制[114];也能通过miRNA实现功能抑制。此外,传统SOCE的抑制剂可对STIM1起作用,如羧基胺三唑,可抑制血管生成[115~118],而2-氨基乙基二苯基硼酸盐(2-APB)和SKF-96365,都能对癌细胞产生类似的作用[119~122],然而其特异性需要实验进一步来评估。
除前面综述的STIM1与肿瘤发生发展的相关分子机制外,还有许多可供研究的方向与思路:首先,SOCE作为细胞内Ca2+信号的传导者之一,对维持胞内钙信号稳态至关重要。鉴于STIM1在SOCE当中的重要作用,探究与STIM1相关信号调控通路十分必要。其次,胞外基质金属蛋白酶(matrix metallopeptidase,MMPs)家族是一类锌依赖性内肽酶,能够降解大多数细胞外基质。其成员之一MT1-MMP定位在细胞膜上,并在多种肿瘤中高表达[123]。研究发现SOCE阻断可能通过抑制MT1-MMP囊泡的质膜插入而降低了MT1-MMP的膜表达[90],因此研究细胞外基质重构与STIM1/SOCE信号间联系也具有重要意义。另外,自噬调控失调和肿瘤的发生发展密切相关,Ca2+在基础自噬和诱导自噬中起着重要的作用。研究STIM1在自噬介导的肿瘤发生发展中的作用将会为肿瘤的治疗提供策略和靶点[124]。
鉴于STIM1在多种肿瘤高表达并促进肿瘤的恶化,开发SOCE抑制剂,靶向STIM1的治疗将会为肿瘤治疗提供新的方向。
[1] Kurosaki T, Baba Y. Ca2+signaling and STIM1., 2010, 103(1): 51–58.
[2] Oritani K, Kincade PW. Identification of stromal cell products that interact with pre-B cells., 1996, 134(3): 771–782.
[3] Motiani RK, Hyzinski-García MC, Zhang XX, Henkel MM, Abdullaev IF, Kuo YH, Matrougui K, Mongin AA, Trebak M. STIM1 and Orai1 mediate CRAC channel activity and are essential for human glioblastoma invasion., 2013, 465(9): 1249–1260.
[4] Kondratska K, Kondratskyi A, Yassine M, Lemonnier L, Lepage G, Morabito A, Skryma R, Prevarskaya N. Orai1 and STIM1 mediate SOCE and contribute to apoptotic resistance of pancreatic adenocarcinoma., 2014, 1843(10): 2263–2269.
[5] Flourakis M, Lehen'kyi V, Beck B, Raphaël M, Vandenberghe M, Abeele FV, Roudbaraki M, Lepage G, Mauroy B, Romanin C, Shuba Y, Skryma R, Prevarskaya N. Orai1 contributes to the establishment of an apoptosis-resistant phenotype in prostate cancer cells., 2010, 1(9): e75.
[6] Dubois C, Vanden Abeele F, Lehen'kyi V, Gkika D, Guarmit B, Lepage G, Slomianny C, Borowiec AS, Bidaux G, Benahmed M, Shuba Y, Prevarskaya N. Remodeling of channel-forming ORAI proteins determines an oncogenic switch in prostate cancer., 2014, 26(1): 19–32.
[7] Yang N, Tang Y, Wang F, Zhang HB, Xu D, Shen YF, Sun SH, Yang GS. Blockade of store-operated Ca2+entry inhibits hepatocarcinoma cell migration and invasion by regulating focal adhesion turnover., 2013, 330(2): 163–169.
[8] Kim JH, Lkhagvadorj S, Lee MR, Hwang KH, Chung HC, Jung JH, Cha SK, Eom M. Orai1 and STIM1 are critical for cell migration and proliferation of clear cell renal cell carcinoma., 2014, 448(1): 76–82.
[9] Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation., 2008, 103(11): 1289–1299.
[10] Shen JL, Yang JL, Sang L, Sun R, Bai WY, Wang C, Sun Y, Sun JW. PYK2 mediates the BRAF inhibitor (vermurafenib)-induced invadopodia formation and metastasis in melanomas., 2021, 19(8): 1211–1223.
[11] Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling., 2000, 1(1): 11–21.
[12] Lewis RS. Calcium signaling mechanisms in T lymphocytes., 2001, 19: 497–521.
[13] Feske S. Calcium signalling in lymphocyte activation and disease., 2007, 7(9): 690–702.
[14] Scharenberg AM, Humphries LA, Rawlings DJ. Calcium signalling and cell-fate choice in B cells., 2007, 7(10): 778–789.
[15] Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Meyer T. STIM is a Ca2+sensor essential for Ca2+-store-depletion-triggered Ca2+influx., 2005, 15(13): 1235–1241.
[16] Manji SS, Parker NJ, Williams RT, van Stekelenburg L, Pearson RB, Dziadek M, Smith PJ. STIM1: a novel phosphoprotein located at the cell surface., 2000, 1481(1): 147–155.
[17] Williams RT, Senior PV, Van Stekelenburg L, Layton JE, Smith PJ, Dziadek MA. Stromal interaction molecule 1 (STIM1), a transmembrane protein with growth suppressor activity, contains an extracellular SAM domain modified by N-linked glycosylation., 2002, 1596(1): 131–137.
[18] Spassova MA, Soboloff J, He LP, Xu W, Dziadek MA, Gill DL. STIM1 has a plasma membrane role in the activation of store-operated Ca2+channels., 2006, 103(11): 4040–4045.
[19] Nelson HA, Roe MW. Molecular physiology and pathophysiology of stromal interaction molecules., 2018, 243(5): 451–472.
[20] Baba Y, Hayashi K, Fujii Y, Mizushima A, Watarai H, Wakamori M, Numaga T, Mori Y, Iino M, Hikida M, Kurosaki T. Coupling of STIM1 to store-operated Ca2+entry through its constitutive and inducible movement in the endoplasmic reticulum., 2006, 103(45): 16704–16709.
[21] Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane., 2006, 174(6): 803–813.
[22] Putney JW. Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here)., 2007, 42(2): 103–110.
[23] Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions., 2006, 174(6): 815–825.
[24] Baba Y, Kurosaki T. Physiological function and molecular basis of STIM1-mediated calcium entry in immune cells., 2009, 231(1): 174–188.
[25] Herbert SP, Stainier DYR. Molecular control of endothelial cell behaviour during blood vessel morphogenesis., 2011, 12(9): 551–564.
[26] Oh-hora M, Rao A. Calcium signaling in lymphocytes., 2008, 20(3): 250–258.
[27] Lewis RS. The molecular choreography of a store- operated calcium channel., 2007, 446(7133): 284–287.
[28] Partiseti M, Le Deist F, Hivroz C, Fischer A, Korn H, Choquet D. The calcium current activated by T cell receptor and store depletion in human lymphocytes is absent in a primary immunodeficiency., 1994, 269(51): 32327–32335.
[29] Picard C, McCarl CA, Papolos A, Khalil S, Lüthy K, Hivroz C, LeDeist F, Rieux-Laucat F, Rechavi G, Rao A, Fischer A, Feske S. STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity., 2009, 360(19): 1971–1980.
[30] Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance., 2008, 9(4): 432–443.
[31] Demaurex N, Saul S. The role of STIM proteins in neutrophil functions., 2018, 596(14): 2699–2708.
[32] Vig M, DeHaven WI, Bird GS, Billingsley JM, Wang HY, Rao PE, Hutchings AB, Jouvin MH, Putney JW, Kinet JP. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels., 2008, 9(1): 89–96.
[33] Berry CT, Liu XH, Myles A, Nandi S, Chen YH, Hershberg U, Brodsky IE, Cancro MP, Lengner CJ, May MJ, Freedman BD. BCR-induced Ca2+signals dynamically tune survival, metabolic reprogramming, and proliferation of naive B cells., 2020, 31(2): 107474.
[34] Trebak M, Kinet JP. Calcium signalling in T cells., 2019, 19(3): 154–169.
[35] Desvignes L, Weidinger C, Shaw P, Vaeth M, Ribierre T, Liu MH, Fergus T, Kozhaya L, McVoy L, Unutmaz D, Ernst JD, Feske S. STIM1 controls T cell-mediated immune regulation and inflammation in chronic infection., 2015, 125(6): 2347–2362.
[36] Chang CL, Chen YJ, Liou J. ER-plasma membrane junctions: Why and how do we study them?, 2017, 1864(9): 1494–1506.
[37] Weber-Boyvat M, Kentala H, Lilja J, Vihervaara T, Hanninen R, Zhou Y, Peränen J, Nyman TA, Ivaska J, Olkkonen VM. OSBP-related protein 3 (ORP3) coupling with VAMP-associated protein A regulates R-Ras activity., 2015, 331(2): 278–291.
[38] Machaca K. Ca2+signaling and lipid transfer 'pas a deux' at ER-PM contact sites orchestrate cell migration., 2020, 89: 102226.
[39] Tong JS, Tan LC, Im YJ. Structure of human ORP3 ORD reveals conservation of a key function and ligand specificity in OSBP-related proteins., 2021, 16(4): e0248781.
[40] Lodola F, Laforenza U, Bonetti E, Lim D, Dragoni S, Bottino C, Ong HL, Guerra G, Ganini C, Massa M, Manzoni M, Ambudkar IS, Genazzani AA, Rosti V, Pedrazzoli P, Tanzi F, Moccia F, Porta C. Store-operated Ca2+entry is remodelled and controlsangiogenesis in endothelial progenitor cells isolated from tumoral patients., 2012, 7(9): e42541.
[41] Ye JX, Wei JZ, Luo Y, Deng YY, Que T, Zhang XJ, Liu F, Zhang JY, Luo XL. Epstein-barr virus promotes tumor angiogenesis by activating STIM1-dependent Ca2+signaling in nasopharyngeal carcinoma., 2021, 10(10): 1275.
[42] Pan Z, Ma JJ. Open Sesame: treasure in store- operated calcium entry pathway for cancer therapy., 2015, 58(1): 48–53.
[43] Kokoska ER, Smith GS, Miller TA. Nonsteroidal anti-inflammatory drugs attenuate proliferation of colonic carcinoma cells by blocking epidermal growth factor-induced Ca++mobilization., 2000, 4(2): 150–161.
[44] Feng MY, Grice DM, Faddy HM, Nguyen N, Leitch S, Wang YY, Muend S, Kenny PA, Sukumar S, Roberts- Thomson SJ, Monteith GR, Rao R. Store-independent activation of Orai1 by SPCA2 in mammary tumors., 2010, 143(1): 84–98.
[45] Ay AS, Benzerdjeb N, Sevestre H, Ahidouch A, Ouadid-Ahidouch H. Orai3 constitutes a native store-operated calcium entry that regulates non small cell lung adenocarcinoma cell proliferation., 2013, 8(9): e72889.
[46] Chen YT, Chen YF, Chiu WT, Liu KY, Liu YL, Chang JY, Chang HC, Shen MR. Microtubule-associated histone deacetylase 6 supports the calcium store sensor STIM1 in mediating malignant cell behaviors., 2013, 73(14): 4500–4509.
[47] Stupack DG, Cheresh DA. Integrins and angiogenesis., 2004, 64: 207–238.
[48] Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly., 2005, 15(11): 599–607.
[49] Friedl P, Weigelin B. Interstitial leukocyte migration and immune function., 2008, 9(9): 960–969.
[50] Silva MT. When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system., 2010, 87(1): 93–106.
[51] Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation., 2011, 144(5): 646–674.
[52] Nabi IR. The polarization of the motile cell., 1999, 112(Pt 12): 1803–1811.
[53] Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments., 2003, 112(4): 453–465.
[54] Anderson TW, Vaughan AN, Cramer LP. Retrograde flow and myosin II activity within the leading cell edge deliver F-actin to the lamella to seed the formation of graded polarity actomyosin II filament bundles in migrating fibroblasts., 2008, 19(11): 5006–5018.
[55] Le Clainche C, Carlier MF. Regulation of actin assembly associated with protrusion and adhesion in cell migration., 2008, 88(2): 489–513.
[56] Keren K. Cell motility: the integrating role of the plasma membrane., 2011, 40(9): 1013–1027.
[57] Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors., 2005, 17(5): 559–564.
[58] Gupta GP, Massagué J. Cancer metastasis: building a framework., 2006, 127(4): 679–695.
[59] Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions., 2006, 5: 14.
[60] Nielsen N, Lindemann O, Schwab A. TRP channels and STIM/ORAI proteins: sensors and effectors of cancer and stroma cell migration., 2014, 171(24): 5524–5540.
[61] Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma., 2017, 14(8): 463–482.
[62] Cantwell-Dorris ER, O'Leary JJ, Sheils OM. BRAFV600E: implications for carcinogenesis and molecular therapy., 2011, 10(3): 385–394.
[63] Kim A, Cohen MS. The discovery of vemurafenib for the treatment of BRAF-mutated metastatic melanoma., 2016, 11(9): 907–916.
[64] Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P, Kehoe SM, Johannessen CM, Macconaill LE, Hahn WC, Meyerson M, Garraway LA. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling., 2011, 29(22): 3085–3096.
[65] Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020., 2020, 70(1): 7–30.
[66] Kutschat AP, Hamdan FH, Wang X, Wixom AQ, Najafova Z, Gibhardt CS, Kopp W, Gaedcke J, Ströbel P, Ellenrieder V, Bogeski I, Hessmann E, Johnsen SA. STIM1 mediates calcium-dependent epigenetic reprogramming in pancreatic cancer., 2021, 81(11): 2943–2955.
[67] Wang J, Shen JL, Zhao KL, Hu JM, Dong JX, Sun JW. STIM1 overexpression in hypoxia microenvironment contributes to pancreatic carcinoma progression., 2019, 16(1): 100–108.
[68] Liang XJ, Xie JS, Liu H, Zhao RJ, Zhang W, Wang HD, Pan HM, Zhou YB, Han WD. STIM1 deficiency in intestinal epithelium attenuates colonic inflammation and tumorigenesis by reducing ER stress of goblet cells., 2022, 14(1): 193–217.
[69] Tang J, Ye SF, Wang MQ, Li J, Meng X, Liu F. Stromal interaction molecule 1 promotes tumor growth in Esophageal squamous cell carcinoma., 2020, 112(3): 2146–2153.
[70] Xia JL, Wang HQ, Huang HX, Sun L, Dong ST, Huang N, Shi M, Bin JP, Liao YL, Liao WJ. Elevated Orai1 and STIM1 expressions upregulate MACC1 expression to promote tumor cell proliferation, metabolism, migration, and invasion in human gastric cancer., 2016, 381(1): 31–40.
[71] Bausch B, Jilg C, Gläsker S, Vortmeyer A, Lützen N, Anton A, Eng C, Neumann HPH. Renal cancer in von Hippel-Lindau disease and related syndromes., 2013, 9(9): 529–538.
[72] Cohen HT, McGovern FJ. Renal-cell carcinoma., 2005, 353(23): 2477–2490.
[73] Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease., 2003, 30(4): 843–852.
[74] Monteith GR, McAndrew D, Faddy HM, Roberts- Thomson SJ. Calcium and cancer: targeting Ca2+transport., 2007, 7(7): 519–530.
[75] Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex., 2006, 355(13): 1345–1356.
[76] Peng H, Liu J, Sun Q, Chen R, Wang Y, Duan J, Li C, Li B, Jing Y, Chen X, Mao Q, Xu KF, Walker CL, Li J, Wang J, Zhang H. mTORC1 enhancement of STIM1-mediated store-operated Ca2+entry constrains tuberous sclerosis complex-related tumor development., 2013, 32(39): 4702–4711.
[77] Pascual-Caro C, Orantos-Aguilera Y, Sanchez-Lopez I, de Juan-Sanz J, Parys JB, Area-Gomez E, Pozo-Guisado E, Martin-Romero FJ. STIM1 deficiency leads to specific down-regulation of ITPR3 in SH-SY5Y cells., 2020, 21(18): 6598.
[78] Pascual-Caro C, Berrocal M, Lopez-Guerrero AM, Alvarez-Barrientos A, Pozo-Guisado E, Gutierrez- Merino C, Mata AM, Martin-Romero FJ. STIM1 deficiency is linked to Alzheimer's disease and triggers cell death in SH-SY5Y cells by upregulation of L-type voltage-operated Ca2+entry., 2018, 96(10): 1061–1079.
[79] Xie JS, Ma GL, Zhou LJ, He L, Zhang Z, Tan P, Huang ZX, Fang SH, Wang TL, Lee YT, Wen SF, Siwko S, Wang LQ, Liu JD, Du YC, Zhang NX, Liu XX, Han L, Huang Y, Wang R, Wang YJ, Zhou YB, Han WD. Identification of a STIM1 splicing variant that promotes glioblastoma growth., 2022, 9(11): e2103940.
[80] Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012., 2015, 65(2): 87–108.
[81] Yang SY, Zhang JJ, Huang XY. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis., 2009, 15(2): 124–134.
[82] Pan SL, Zhao XX, Shao C, Fu BJ, Huang YY, Zhang N, Dou XJ, Zhang Z, Qiu YL, Wang R, Jin MH, Kong DX. STIM1 promotes angiogenesis by reducing exosomal miR-145 in breast cancer MDA-MB-231 cells., 2021, 12(1): 38.
[83] Yang YF, Jiang ZS, Wang B, Chang LL, Liu J, Zhang LN, Gu L. Expression of STIM1 is associated with tumor aggressiveness and poor prognosis in breast cancer., 2017, 213(9): 1043–1047.
[84] Cheng HY, Wang SQ, Feng RQ. STIM1 plays an important role in TGF-β-induced suppression of breast cancer cell proliferation., 2016, 7(13): 16866–16878.
[85] Chen YT, Chen YF, Chiu WT, Wang YK, Chang HC, Shen MR. The ER Ca2+sensor STIM1 regulates actomyosin contractility of migratory cells., 2013, 126(Pt 5): 1260–1267.
[86] Huang CC, Lin MR, Yang YC, Hsu YW, Wong HSC, Chang WC. Germline genetic association between stromal interaction molecule 1 (STIM1) and clinical outcomes in breast cancer patients., 2020, 10(4): 287.
[87] O'Grady S, Morgan MP. Calcium transport and signalling in breast cancer: functional and prognostic significance., 2021, 72: 19–26.
[88] Gross S, Hooper R, Tomar D, Armstead AP, Shanas N, Mallu P, Joshi H, Ray S, Chong PLG, Astsaturov I, Farma JM, Cai KQ, Chitrala KN, Elrod JW, Zaidi MR, Soboloff J. Suppression of Ca2+signaling enhances melanoma progression., 2022, 41(19): e110046.
[89] Wong HSC, Chang WC. Single-cell melanoma transcriptomes depicting functional versatility and clinical implications of STIM1 in the tumor microenvironment., 2021, 11(11): 5092–5106.
[90] Sun JW, Lu FJ, He HF, Shen JL, Messina J, Mathew R, Wang DP, Sarnaik AA, Chang WC, Kim M, Cheng HP, Yang SY. STIM1- and Orai1-mediated Ca2+oscillation orchestrates invadopodium formation and melanoma invasion., 2014, 207(4): 535–548.
[91] Sun JW, Lin SC, Keeley T, Yang SY. Disseminating melanoma cells surf on calcium waves., 2015, 2(4): e1002714.
[92] Wang YD, Wang HY, Pan T, Li L, Li JM, Yang HY. STIM1 silencing inhibits the migration and invasion of A549 cells., 2017, 16(3): 3283–3289.
[93] Saint Fleur-Lominy S, Maus M, Vaeth M, Lange I, Zee I, Suh D, Liu C, Wu XJ, Tikhonova A, Aifantis I, Feske S. STIM1 and STIM2 mediate cancer-induced inflammation in T cell acute lymphoblastic leukemia., 2018, 24(11): 3045–3060.e5.
[94] Asghar MY, Lassila T, Paatero I, Nguyen VD, Kronqvist P, Zhang JX, Slita A, Löf C, Zhou Y, Rosenholm J, Törnquist K. Stromal interaction molecule 1 (STIM1) knock down attenuates invasion and proliferation and enhances the expression of thyroid-specific proteins in human follicular thyroid cancer cells., 2021, 78(15): 5827–5846.
[95] Xu YX, Zhang S, Niu HY, Ye YJ, Hu F, Chen S, Li XF, Luo XH, Jiang S, Liu YH, Chen YN, Li JY, Xiang R, Li N. STIM1 accelerates cell senescence in a remodeled microenvironment but enhances the epithelial-to- mesenchymal transition in prostate cancer., 2015, 5: 11754.
[96] Zhou YB, Gu P, Li J, Li F, Zhu J, Gao P, Zang YC, Wang YC, Shan YX, Yang DR. Suppression of STIM1 inhibits the migration and invasion of human prostate cancer cells and is associated with PI3K/Akt signaling inactivation., 2017, 38(5): 2629–2636.
[97] Zang J, Zuo DQ, Shogren KL, Gustafson CT, Zhou ZF, Thompson MA, Guo RW, Prakash YS, Lu LC, Guo W, Maran A, Yaszemski MJ. STIM1 expression is associated with osteosarcoma cell survival., 2019, 31(1): 203–211.
[98] Ritchie MF, Zhou YD, Soboloff J. WT1/EGR1- mediated control of STIM1 expression and function in cancer cells., 2011, 16(7): 2402–2415.
[99] Lee SK, Kweon YC, Lee AR, Lee YY, Park CY. Metastasis enhancer PGRMC1 boosts store-operated Ca2+entry by uncoiling Ca2+sensor STIM1 for focal adhesion turnover and actomyosin formation., 2022, 38(3): 110281.
[100] Faris P, Rumolo A, Tapella L, Tanzi M, Metallo A, Conca F, Negri S, Lefkimmiatis K, Pedrazzoli P, Lim D, Montagna D, Moccia F. Store-operated Ca2+entry is up-regulated in tumour-infiltrating lymphocytes from metastatic colorectal cancer patients., 2022, 14(14): 3312.
[101] Zhang Z, Liu X, Feng B, Liu N, Wu Q, Han Y, Nie Y, Wu K, Shi Y, Fan D. STIM1, a direct target of microRNA-185, promotes tumor metastasis and is associated with poor prognosis in colorectal cancer., 2016, 35(46): 6043.
[102] Zhuang R, Rao JN, Zou TT, Liu L, Xiao L, Cao S, Hansraj NZ, Gorospe M, Wang JY. miR-195 competes with HuR to modulate stim1 mRNA stability and regulate cell migration., 2013, 41(16): 7905–7919.
[103] Lv ZY, Yi DL, Zhang C, Xie YJ, Huang H, Fan ZC, Liu X. miR-541-3p inhibits the viability and migration of vascular smooth muscle cells via targeting STIM1., 2021, 23(5): 312.
[104] Ho KH, Chang CK, Chen PH, Wang YJ, Chang WC, Chen KC. miR-4725-3p targeting stromal interacting molecule 1 signaling is involved in xanthohumol inhibition of glioma cell invasion., 2018, 146(3): 269–288.
[105] Yang YF, Jiang ZS, Ma N, Wang B, Liu J, Zhang LN, Gu L. MicroRNA-223 targeting STIM1 inhibits the biological behavior of breast cancer., 2018, 45(2): 856–866.
[106] Wang JY, Sun J, Huang MY, Wang YS, Hou MF, Sun Y, He H, Krishna N, Chiu SJ, Lin S, Yang S, Chang WC. STIM1 overexpression promotes colorectal cancer progression, cell motility and COX-2 expression., 2015, 34(33): 4358–4367.
[107] Chen YW, Lai CS, Chen YF, Chiu WT, Chen HC, Shen MR. STIM1-dependent Ca2+signaling regulates podosome formation to facilitate cancer cell invasion., 2017, 7(1): 11523.
[108] Ge CL, Zeng BZ, Li RL, Li Z, Fu QF, Wang WW, Wang ZY, Dong SW, Lai ZC, Wang Y, Xue YB, Guo JY, Di TN, Song X. Knockdown of STIM1 expression inhibits non-small-cell lung cancer cell proliferationand in nude mouse xenografts., 2019, 10(1): 425–436.
[109] Algariri ES, Mydin RBSMN, Moses EJ, Okekpa SI, Rahim NAA, Yusoff NM. Knockdown of stromal interaction molecule 1 (STIM1) suppresses acute myeloblastic leukemia-M5 cell line survival through inhibition of reactive oxygen species activities., 2023, 40(1): 11–17.
[110] Lin YS, Lin YH, Nguyen Thi M, Hsiao SC, Chiu WT. STIM1 controls the focal adhesion dynamics and cell migration by regulating SOCE in osteosarcoma., 2021, 23(1): 162.
[111] Li YS, Guo B, Xie QC, Ye DY, Zhang DX, Zhu Y, Chen HX, Zhu B. STIM1 mediates hypoxia-driven hepatocarcinogenesis via interaction with HIF-1., 2015, 12(3): 388–395.
[112] Chen YF, Chiu WT, Chen YT, Lin PY, Huang HJ, Chou CY, Chang HC, Tang MJ, Shen MR. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis., 2011, 108(37): 15225–15230.
[113] Lu FJ, Sun JW, Zheng QX, Li JH, Hu YZ, Yu P, He HF, Zhao Y, Wang XH, Yang SY, Cheng HP. Imaging elemental events of store-operated Ca2+entry in invading cancer cells with plasmalemmal targeted sensors., 2019, 132(6): jcs224923.
[114] van Dorp S, Qiu RY, Choi UB, Wu MM, Yen M, Kirmiz M, Brunger AT, Lewis RS. Conformational dynamics of auto-inhibition in the ER calcium sensor STIM1., 2021, 10: e6619.
[115] Guo L, Li ZS, Wang HL, Ye CY, Zhang DC. Carboxyamido-triazole inhibits proliferation of human breast cancer cells via G(2)/M cell cycle arrest and apoptosis., 2006, 538(1–3): 15–22.
[116] Perabo FGE, Demant AW, Wirger A, Schmidt DH, Sitia M, Wardelmann E, Müller SC, Kohn EC. Carboxyamido-triazole (CAI) reverses the balance between proliferation and apoptosis in a rat bladder cancer model., 2005, 25(2A): 725–729.
[117] Ge S, Rempel SA, Divine G, Mikkelsen T. Carboxyamido-triazole induces apoptosis in bovine aortic endothelial and human glioma cells., 2000, 6(4): 1248–1254.
[118] Mignen O, Brink C, Enfissi A, Nadkarni A, Shuttleworth TJ, Giovannucci DR, Capiod T. Carboxyamidotriazole- induced inhibition of mitochondrial calcium import blocks capacitative calcium entry and cell proliferation in HEK-293 cells., 2005, 118(Pt 23): 5615–5623.
[119] Enfissi A, Prigent S, Colosetti P, Capiod T. The blocking of capacitative calcium entry by 2-aminoethyl diphenylborate (2-APB) and carboxyamidotriazole (CAI) inhibits proliferation in Hep G2 and Huh-7 human hepatoma cells., 2004, 36(6): 459–467.
[120] Padar S, Bose DD, Livesey JC, Thomas DW. 2-Aminoethoxydiphenyl borate perturbs hormone- sensitive calcium stores and blocks store-operated calcium influx pathways independent of cytoskeletal disruption in human A549 lung cancer cells., 2005, 69(8): 1177–1186.
[121] Kazerounian S, Pitari GM, Shah FJ, Frick GS, Madesh M, Ruiz-Stewart I, Schulz S, Hajnóczky G, Waldman SA. Proliferative signaling by store-operated calcium channels opposes colon cancer cell cytostasis induced by bacterial enterotoxins., 2005, 314(3): 1013–1022.
[122] Koslowski M, Sahin U, Dhaene K, Huber C, Türeci O. MS4A12 is a colon-selective store-operated calcium channel promoting malignant cell processes., 2008, 68(9): 3458–3466.
[123] Hao QG, Sun FG, Yan CH, Sun JW. Progress on the role and mechanism of MT1-MMP in tumor metastasis., 2022, 44(9): 745–755.郝庆刚, 孙凤桂, 严程浩, 孙建伟. MT1-MMP在肿瘤转移中的研究进展. 遗传, 2022, 44(9): 745–755.
[124] Sukumaran P, Nascimento Da Conceicao V, Sun YY, Ahamad N, Saraiva LR, Selvaraj S, Singh BB. Calcium signaling regulates autophagy and apoptosis., 2021, 10(8): 2125.
The roles and mechanism of STIM1 in tumorigenesis and metastasis
Chenghao Yan1, Weiyu Bai1, Zhimeng Zhang1, Junling Shen1, Youjun Wang2, Jianwei Sun1
STIM1 (stromal interaction molecule 1) is one of the key components of the store operated Ca2+entry channel (SOCE), which is located on the endoplasmic reticulum membrane and highly expressed in most kinds of tumors. STIM1 promotes tumorigenesis and metastasis by modulating the formation of invadopodia, promoting angiogenesis, mediating inflammatory response, altering the cytoskeleton and cell dynamics. However, the roles and mechanism of STIM1 in different tumors have not been fully elucidated. In this review, we summarize the latest progress and mechanisms of STIM1 in tumorigenesis and metastasis, thereby providing insights and references for the study on STIM1 in the field of cancer biology in the future.
STIM1; SOCE; tumorigenesis; metastasis
2023-02-18;
2023-04-03;
2023-04-17
国家自然科学基金项目(编号: 82273460,32260167)和云南大学研究生科研创新项目(编号: 2021Z088,2023Y0222)资助[Supported by the National Natural Science Foundation of China (Nos. 82273460,32260167),and Yunnan University Graduate Research Innovation Project (Nos. 2021Z088, 2023Y0222)]
严程浩,在读硕士研究生,专业方向:肿瘤转移与发生机制。E-mail: ych9702@126.com
白韦钰,在读博士研究生,专业方向:肿瘤转移与发生机制。E-mail: weiyubai@mail.ynu.edu.cn
严程浩和白韦钰并列第一作者。
孙建伟,博士,教授,研究方向:肿瘤发生与转移机制研究。E-mail: jwsun@ynu.edu.cn
王友军,博士,研究方向:钙信号转导机制及其生理病理作用。E-mail: wyoujun@bnu.edu.cn
10.16288/j.yczz.23-035
(责任编委: 宋旭)
