延龄草总皂苷通过调控GRP78/IRE1α/TRAF2/JNK信号通路抑制内质网应激而减轻PSCI大鼠海马神经元损伤*
2023-03-10王刚杨丽君杨丹段壬泽赵方毓陈显兵
王刚, 杨丽君, 杨丹, 段壬泽, 赵方毓, 陈显兵
延龄草总皂苷通过调控GRP78/IRE1α/TRAF2/JNK信号通路抑制内质网应激而减轻PSCI大鼠海马神经元损伤*
王刚, 杨丽君, 杨丹, 段壬泽, 赵方毓, 陈显兵△
(湖北民族大学附属民大医院病理科,湖北民族大学医学部,湖北 恩施 445000)
探讨延龄草总皂苷(TST)对卒中后认知障碍(PSCI)大鼠海马神经元的保护作用及分子机制。将采用改良Zea Longa线栓法造模成功的大鼠随机分为模型(model)组、TST(100 mg/kg)组和盐酸多奈哌齐(DON; 0.45 mg/kg)组,另设假手术(sham)组,每组10只,连续给药4周。采用Morris水迷宫实验检测大鼠学习记忆能力;TTC染色检测大鼠脑梗死体积变化,HE、Nissl和TUNEL染色观察大鼠海马组织神经元病理变化;免疫组化及Western blot检测葡萄糖调节蛋白78(GRP78)、肌醇需求酶1α(IRE1α)、肿瘤坏死因子受体相关因子2(TRAF2)、磷酸化c-Jun氨基末端激酶(p-JNK)、胱天蛋白酶12(caspase-12)、Bax和Bcl-2的蛋白水平。与sham组相比,model组大鼠逃避潜伏期显著延长,穿越平台次数及目标象限停留时间显著减少(<0.01);大鼠脑梗死体积显著增大,神经元尼氏小体数量显著减少,凋亡细胞显著增多;海马组织中GRP78、IRE1α、TRAF2、p-JNK、caspase-12和Bax蛋白水平显著升高,Bcl-2蛋白水平显著降低(<0.01)。与model组比较,TST组及DON组大鼠逃避潜伏期显著缩短,穿越平台次数及目标象限停留时间显著增加(<0.01);大鼠脑梗死体积显著缩小,神经元尼氏小体数量显著增多,凋亡细胞显著减少;GRP78、IRE1α、TRAF2、p-JNK、caspase-12和Bax蛋白水平显著降低,Bcl-2蛋白水平显著升高(<0.01)。TST对PSCI大鼠海马神经元具有保护作用,其机制可能与减轻内质网应激、减少神经元凋亡并抑制GRP78/IRE1α/TRAF2/JNK信号通路有关。
延龄草总皂苷;卒中后认知障碍;内质网应激;细胞凋亡;GRP78/IRE1α/TRAF2/JNK信号通路
卒中后认知障碍(post-stroke cognitive impairment, PSCI)是指由卒中引起的从轻度认知障碍到痴呆的一系列综合征,是世界范围内卒中后发病率和死亡率的主要来源。PSCI包括两种不同程度的认知损害,即卒中后认知障碍非痴呆(post-stroke cognitive impairment with no dementia, PSCIND)和卒中后痴呆(post-stroke dementia, PSD)。研究表明,19.3%的卒中患者在中风后10年内发展为痴呆症[1]。因此,针对PSCI早期干预治疗,阻止PSCI向PSD发展,是亟待解决的问题。
内质网应激(endoplasmic reticulum stress, ERS)是指细胞受到各种应激因素的影响,使内质网腔内出现错误折叠和未折叠蛋白质积聚、Ca2+平衡紊乱,引起细胞内稳态失衡的状态[2-3]。而未折叠蛋白反应(unfolded protein response, UPR)是指ER应激发生时,细胞为促进蛋白质正确折叠,或降解错误折叠蛋白质而产生的应激反应[4]。PSCI的发病机制复杂,有缺血再灌注损伤、氧化应激、炎症反应、自噬等机制[5-6]。研究显示,ERS在PSCI发展过程中起重要作用,适当的ERS有利于去除错误折叠的蛋白质,对疾病的病理过程起到保护作用[7]。但是,在严重或长期内质网应激的病理条件下,内质网的生物学功能受损,引发海马神经元凋亡,从而导致认知功能下降[8-9]。目前,能够引起细胞凋亡的信号转导途径共有三种:死亡受体信号途径、线粒体途径以及内质网应激途径[10-11]。而在内质网应激介导的凋亡途径中,JNK和caspase-12的激活起着重要作用[12-13]。
延龄草(Maxim, TTM)又名头顶一颗珠,为湖北省恩施地区珍贵的土家药材,该药味甘、性平,有小毒。前期研究发现,其提取物TTM总皂苷(total saponins from TTM, TST)具有抗氧化、延缓衰老和神经保护作用,但其作用效果和分子机制仍有待深入研究[14-15]。因此,本项工作采用改良线栓法行大脑中动脉栓塞术制备PSCI大鼠模型,进一步探讨其对PSCI大鼠学习记忆能力及海马神经元的保护机制。
材料和方法
1 实验动物
SPF级雄性SD大鼠60只,2月龄,体质量(200±20) g,由三峡大学实验动物中心提供,许可证号:SCXK(鄂)2017-0012。动物饲养在本校清洁级动物房,温度控制在23~25 ℃,相对湿度控制在50%~60%,常规适应性喂养1周后开始实验。
2 药品、试剂与仪器
头顶一颗珠采自恩施本地,经专家鉴定为百合科延龄草属植物延龄草的干燥根茎。洁净药材后,经烘干粉碎置于圆底烧瓶内,加入适量75%乙醇浸泡萃取。将提取后的药液过滤并浓缩干燥,得延龄草提取物,加入双蒸水将提取物完全溶解,采用饱和正丁醇再次萃取,减压浓缩干燥,检测提取物中总皂苷含量为14.72 mg/g生药。盐酸多奈哌齐(donepezil hydrochloride, DON)片(批号:0000001782,浙江华海药业股份有限公司)。
TTC染液(批号:G3005,Solarbio);TUNEL凋亡试剂盒(批号:E-CK-A321,武汉伊莱瑞特生物科技股份有限公司);免疫组化试剂盒(批号:2201269710A,福州迈新生物技术开发有限公司);葡萄糖调节蛋白78(glucose-regulated protein 78, GRP78)抗体、肌醇需求酶1α(inositol-requiring enzyme 1α, IRE1α)抗体、HRP标记的山羊抗兔IgG和HRP标记的山羊抗小鼠IgG(批号分别为ab21685、ab37073、ab6721和ab6789;Abcam);肿瘤坏死因子受体相关因子2(tumor necrosis factor receptor-associated factor 2, TRAF2)、磷酸化c-Jun氨基末端激酶(phosphorylated c-Jun N-terminal kinase, p-JNK)和胱天蛋白酶12(caspase-12)抗体(批号分别为:ML02846、WL01813、WL03268,万类生物技术有限公司);Bcl-2和Bax抗体(批号分别为A19693和A19684,ABclonal);β-actin抗体(批号:66009-1-Ig,武汉三鹰生物技术有限公司)。
1510型酶标仪(Thermo Scientific);水迷宫系统(Top Scan 2.00);Western Blot电泳及转膜全套装置(Bio-Rad)。
3 主要方法
3.1大脑中动脉闭塞(middle cerebral artery occlusion, MCAO)大鼠模型的制备60只SD大鼠随机分为造模组(50只)和假手术(sham)组(10只)。造模组参照文献[16]采用改良Zea Longa线栓法制备MCAO大鼠模型;sham组仅切开皮肤,钝性分离肌肉及筋膜,分离出左侧颈总动脉后即缝合切口。手术后将动物置于放有清洁垫料的饲养盒内并维持大鼠体温,待大鼠苏醒后进行行为学评分,1~3分为造模成功。其中造模组50只大鼠术后共死亡13只,sham组无死亡,剩余存活造模组大鼠进行神经功能评分,纳入评分标准的造模大鼠一共30只。
3.2MCAO模型的评价与动物的分组干预按照Zea Longa 5分法对造模后1、3、7、14和28 d大鼠神经功能进行评分,并将30只造模成功大鼠随机分为模型(model)组(10只)、TST组(10只)和DON组(10只),连同上述sham组(10只)大鼠进行实验。根据动物与人体间等效剂量确定各组大鼠的给药量,TST组给予100 mg/kg TST进行灌胃,DON组给予0.45 mg/kg DON进行灌胃,sham及model组给予等体积的双蒸水灌胃,各组均连续灌胃28 d。
3.3Morris水迷宫评价大鼠学习记忆能力给药结束当天对大鼠进行定位航行实验,共持续5 d,提前将实验大鼠搬进水迷宫实验室以适应新环境,系统自动将圆形水面分为4个象限,求生平台放在第3象限略低于水面0.6~1.0 cm处,大鼠面向池壁分别沿第1、2、4象限放入,系统自动记录60 s内大鼠寻找平台所需时间,即逃避潜伏期;第6天休息;第7天上午进行正式定位航行实验,将大鼠沿第1象限放入求得寻找平台所需时间,下午进行空间探索实验,将平台撤去,大鼠沿第1象限放入,记录60 s内大鼠穿越平台次数及目标象限停留时间比,并进行统计分析。
3.4脑组织灌注及海马病理形态学观察给药7 d后每组随机挑选出3只大鼠进行TTC染色,以10%水合氯醛按3 mL/kg腹腔注射麻醉大鼠,将大脑完整剥取出后,-20 ℃冰箱冷冻20 min,沿冠状位切1 mm厚片,加入适量TTC染液覆盖脑组织,放入37 ℃恒温箱中20 min,期间注意翻面,拍照分析;给药28 d后每组取3只大鼠进行灌注固定,大鼠麻醉后,经心脏灌注固定,断头取全脑,沿大脑视交叉处做4~5 mm冠状切片,将其置于4%多聚甲醛中固定48 h。常规脱水、石蜡包埋后,行4 μm石蜡切片,进行HE、Nissl和TUNEL染色,切片扫描并观察脑组织形态改变情况。
3.5免疫组化测定GRP78、IRE1α、TRAF2、p-JNK和caspase-12蛋白在海马CA1区的表达定位脑组织被制成3 μm石蜡切片后,置于75 ℃摊片机中烘烤2 h,二甲苯脱蜡3次,每次10 min,无水乙醇浸泡3次,每次5 min,纯水冲洗2 min,置于柠檬酸钠配成的修复液中,水浴修复20 min。自然冷却室温后,组化笔圈起组织,PBS冲洗3次,每次3 min。严格按照免疫组化试剂盒说明操作,滴加A液(内源性过氧化物酶阻断剂)反应10 min;PBS冲洗3次,除去PBS,滴加B液(非特异染色阻断剂)反应10 min,滴加稀释好的GRP78抗体(1∶1 000)、IRE1α抗体(1∶500)、TRAF2抗体(1∶200)、p-JNK抗体(1∶200)和caspase-12抗体(1∶200),室温孵育1 h;PBS冲洗3次,除去PBS,滴加C液(生物素标记的羊抗兔IgG聚合物),反应10 min;PBS冲洗3次,除去PBS,滴加D液(辣根过氧化物酶)反应10 min;PBS冲洗3次,除去PBS,滴加DAB显色液避光显色2 min,PBS冲洗3次,置于苏木素中复染1 min,水洗1 min,分化30 s,水洗1 min,返蓝15 s,水洗1 min,无水乙醇2 min,二甲苯2 min,中性树胶湿封,切片扫描分析。
3.6Western blot检测海马中相关蛋白表达大鼠麻醉后,断头取脑,冰上剥取左侧海马并标记好放入-80 ℃备用;称取50 mg组织放入EP管中,加入裂解液和PMSF混合液(100∶1)500 μL,冰上剪碎、研磨、超声、裂解,得到混悬液,10 080×离心15 min,吸取上清液,配平组织蛋白浓度。制备12% SDS-PAGE凝胶,依次进行电泳、转膜、封闭、洗膜;加入GRP78抗体(1∶1 000)、IRE1α抗体(1∶1 000)、TRAF2抗体(1∶1 000)、p-JNK抗体(1∶500)、caspase-12抗体(1∶1 000)、Bax抗体(1∶1 000)和Bcl-2抗体(1∶1 000),4 ℃冰箱孵育过夜;次日洗膜,Ⅱ抗室温孵育1 h;洗膜,滴加ECL显色液进行曝光处理,采用GIS 1D图像分析软件进行灰度值计算。
4 统计学处理
采用统计软件SPSS 22.0进行数据分析处理,实验数据以均数±标准差(mean±SD)表示。多组间比较采用单因素方差分析(one-way ANOVA)。以<0.05为差异具有统计学意义。
结果
1 各组大鼠神经功能评分的比较
如图1所示,术后model组中的大鼠神经功能评分在前阶段呈现出升高的趋势,3 d后出现降低的趋势,TST组与DON组在给药两周后神经功能恢复显著。与sham组比较,model组大鼠术后神经功能评分显著升高(<0.01);与model组比较,TST组及DON组神经功能评分显著降低(<0.01);TST组与DON组比较无统计学意义。
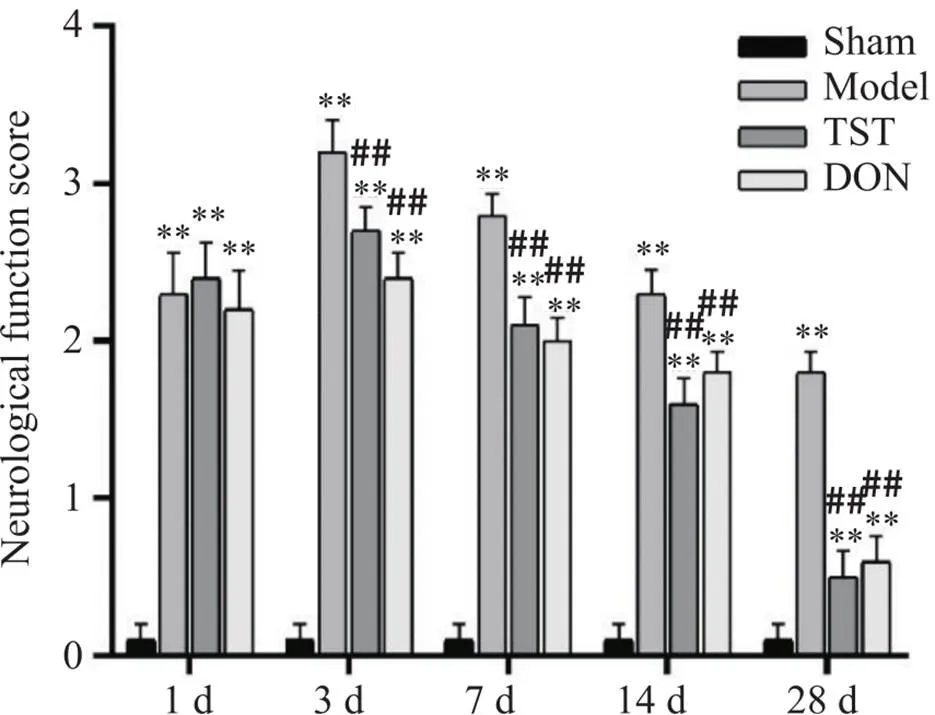
Figure 1. Comparison of neurological function scores of the rats in each group. Mean±SD. n=7. **P<0.01 vs sham group; ##P<0.01 vs model group.
2 TST对PSCI大鼠学习记忆能力的影响
如图2所示,sham组与给药组大鼠路线清晰较短且表现出一定的目标趋向性,model组大鼠路线杂乱且长目标趋向混乱。与sham组相比,model组大鼠逃避潜伏期时间显著增加(<0.01),穿越平台次数(<0.05)及目标象限停留时间显著减少(<0.01);与model组相比,TST组和DON组大鼠逃避潜伏期时间显著减少(<0.01),穿越平台次数(<0.05)及目标象限停留时间显著增加(<0.01),见图3。
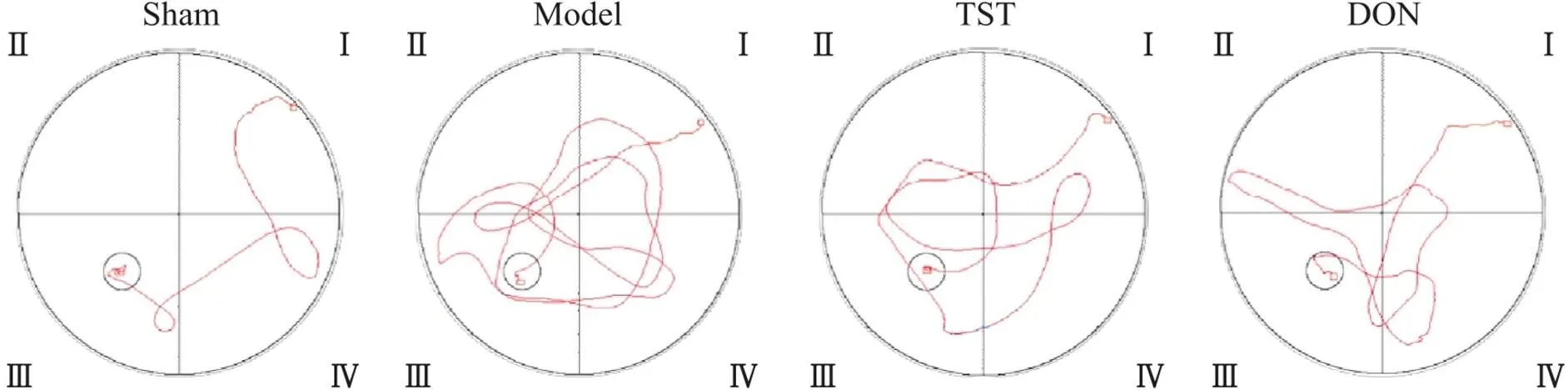
Figure 2. Morris water maze swimming trajectories of the rats in each group.
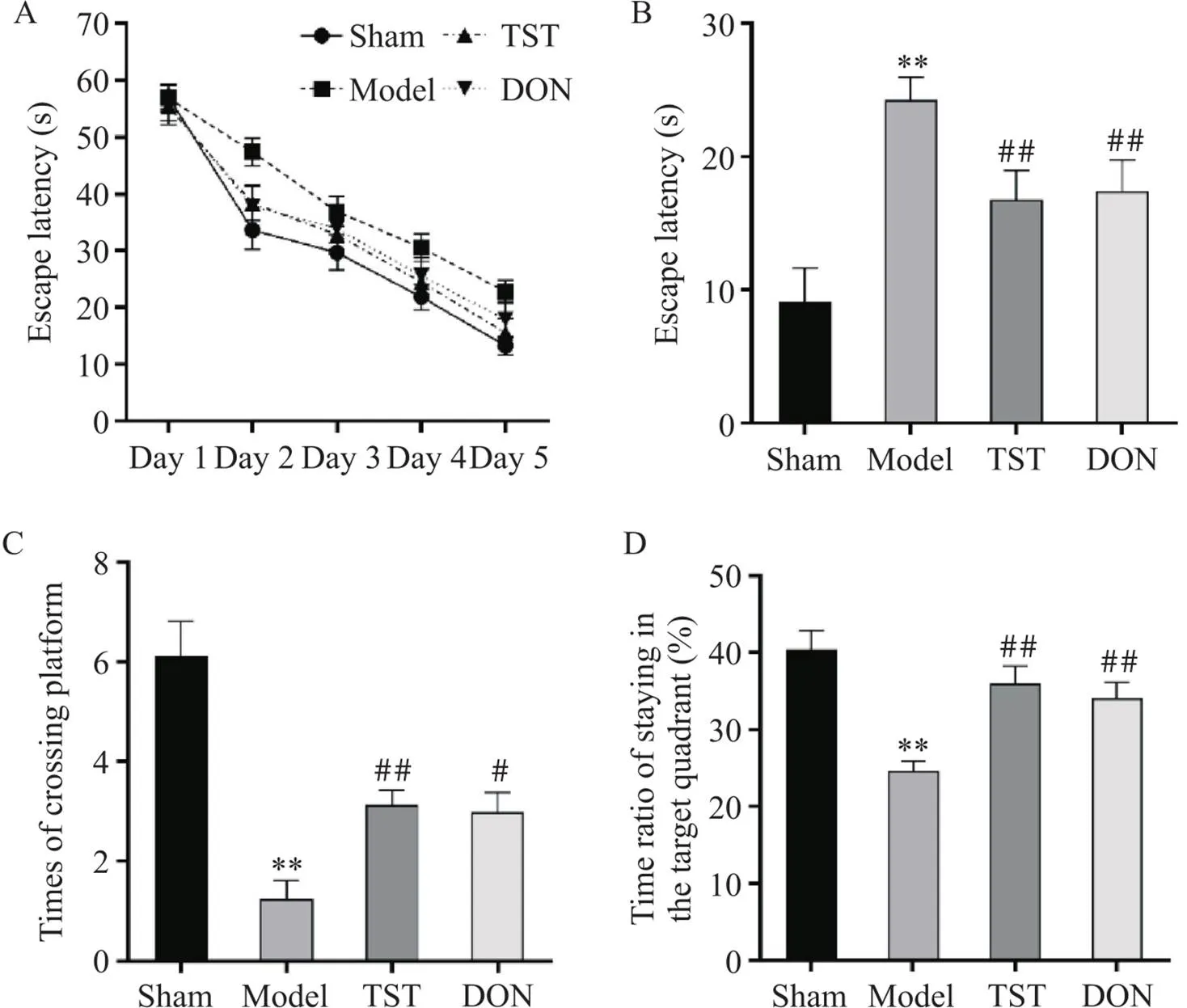
Figure 3. The results of Morris water maze experiment in the rats of each group. A: escape latencies in rats for 5 consecutive days; B: bar chart of escape latency of the rats in each group; C: the times of the rat crossing the platform within 60 s; D: the ratio of the time that the rats stay in the target quadrant. Mean±SD. n=7. **P<0.01 vs sham group; #P<0.05, ##P<0.01 vs model group.
3 TST对各组大鼠脑梗死体积的影响
如图4所示,假手术组大鼠脑组织呈均匀红色,无缺血梗死灶,其它各组大鼠脑组织均有不同程度的缺血梗死灶出现。与sham组相比,model组大鼠左侧脑组织白色缺血梗死灶显著增大,与model组相比,TST组及DON组大鼠左侧脑组织白色缺血梗死灶显著减小。
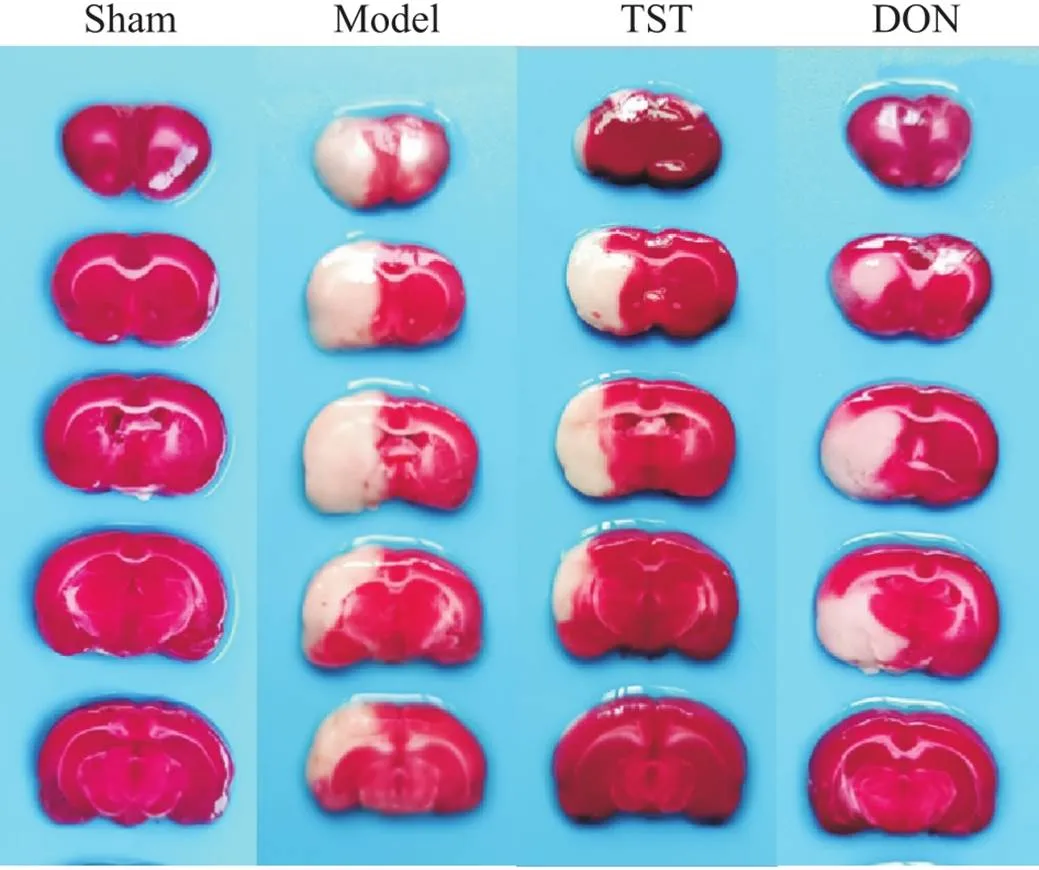
Figure 4. The effect of TST on the volume of cerebral infarction in the rats of each group. In sham group, the brain tissue was uniformly red, with no ischemic infarction; in model group, the left brain tissue had obvious white ischemic infarction; the left infarction in TST group and DON group decreased to varying degrees.
4 TST对PSCI大鼠海马组织病理变化的影响
如图5所示,sham组大鼠脑组织着色均匀,海马CA1区神经元饱满丰富、排列整齐、数量较多,胞质内有丰富的尼氏体。皮质区细胞排列紧密,细胞核着色清晰,未见明显的坏死细胞及空泡化现象;与sham组相比,model组大鼠脑组织着色不均,皮质区域着色较浅,海马CA1区神经元排列紊乱,胞质中尼氏体明显减少,出现核固缩现象。皮质区出现大量坏死细胞及空泡化现象;与model组相比,TST组及DON组大鼠脑组织着色较为均匀,海马CA1区神经元较饱满,胞质内尼氏体明显增多,空泡化现象减少。皮质区着色较深,细胞空泡化现象及坏死细胞明显减少。
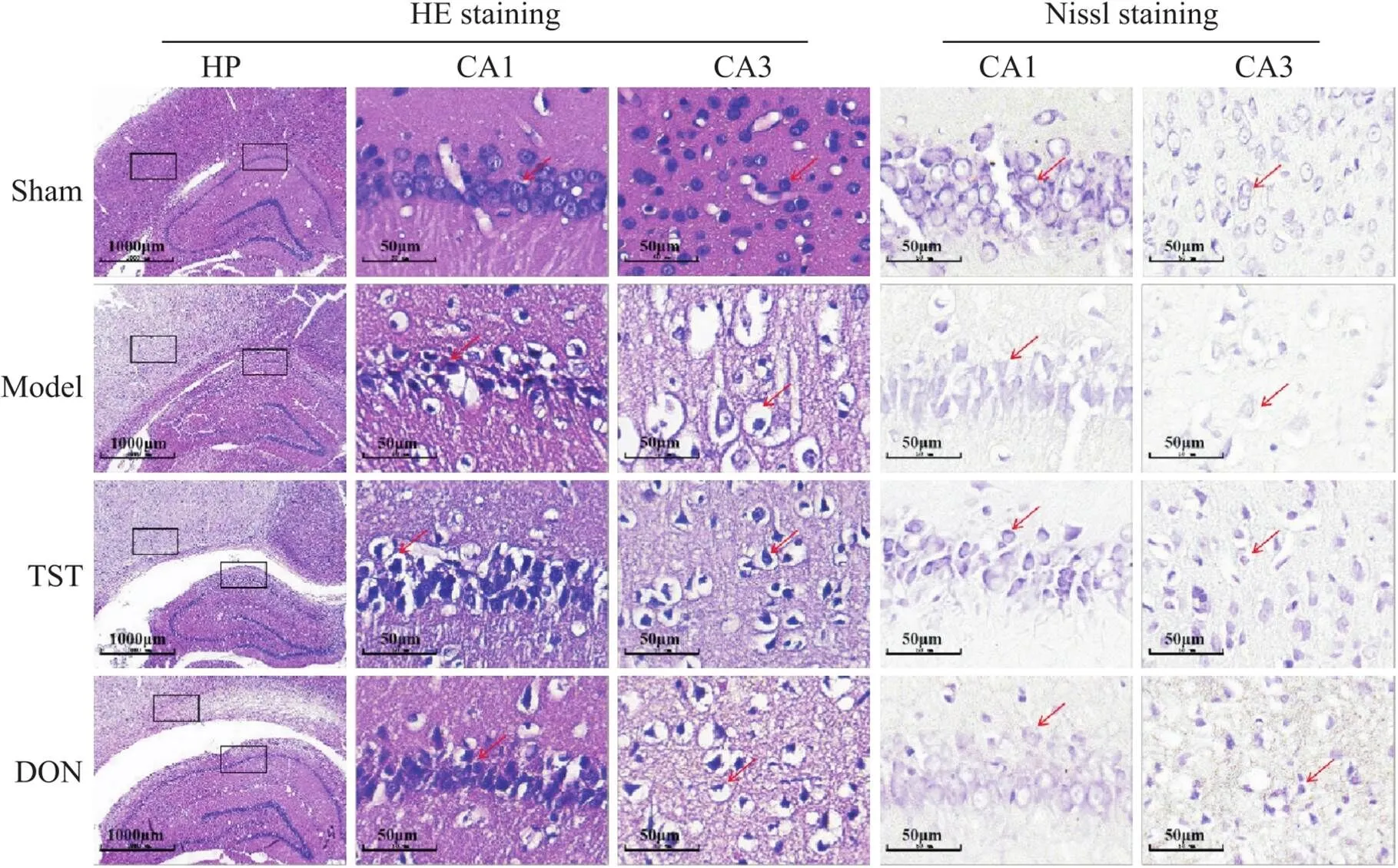
Figure 5. The effect of TST on the pathological changes of brain tissue in PSCI rats (HE staining, scale bar=1 000 µm in HP, scale bar=50 µm CA1 and CA3; Nissl staining, scale bar=50 µm in CA1 and CA3). In sham group, the nerve cells were neatly arranged, complete and abundant, and there were abundant Nissl bodies in the cytoplasm. In model group, part of the cerebral cortex showed a loose mesh structure, the arrangement of nerve cells was disordered, the cells shrank, the cell number decreased, and the Nissl bodies in the cytoplasm decreased significantly. In TST group and DON group, the atrophic morphology of nerve cells was improved, the cell number increased, and the Nissl bodies in the cytoplasm increased significantly.
5 TST对PSCI大鼠海马神经元凋亡的影响
如图6所示,图中显蓝色为细胞核,绿色为凋亡标记神经元。与sham组相比,model组大鼠海马CA1区绿色荧光强度明显增强,阳性细胞数量明显增多。与model组相比,TST组及DON组大鼠海马CA1区绿色荧光强度明显减弱,阳性细胞数量明显减少。
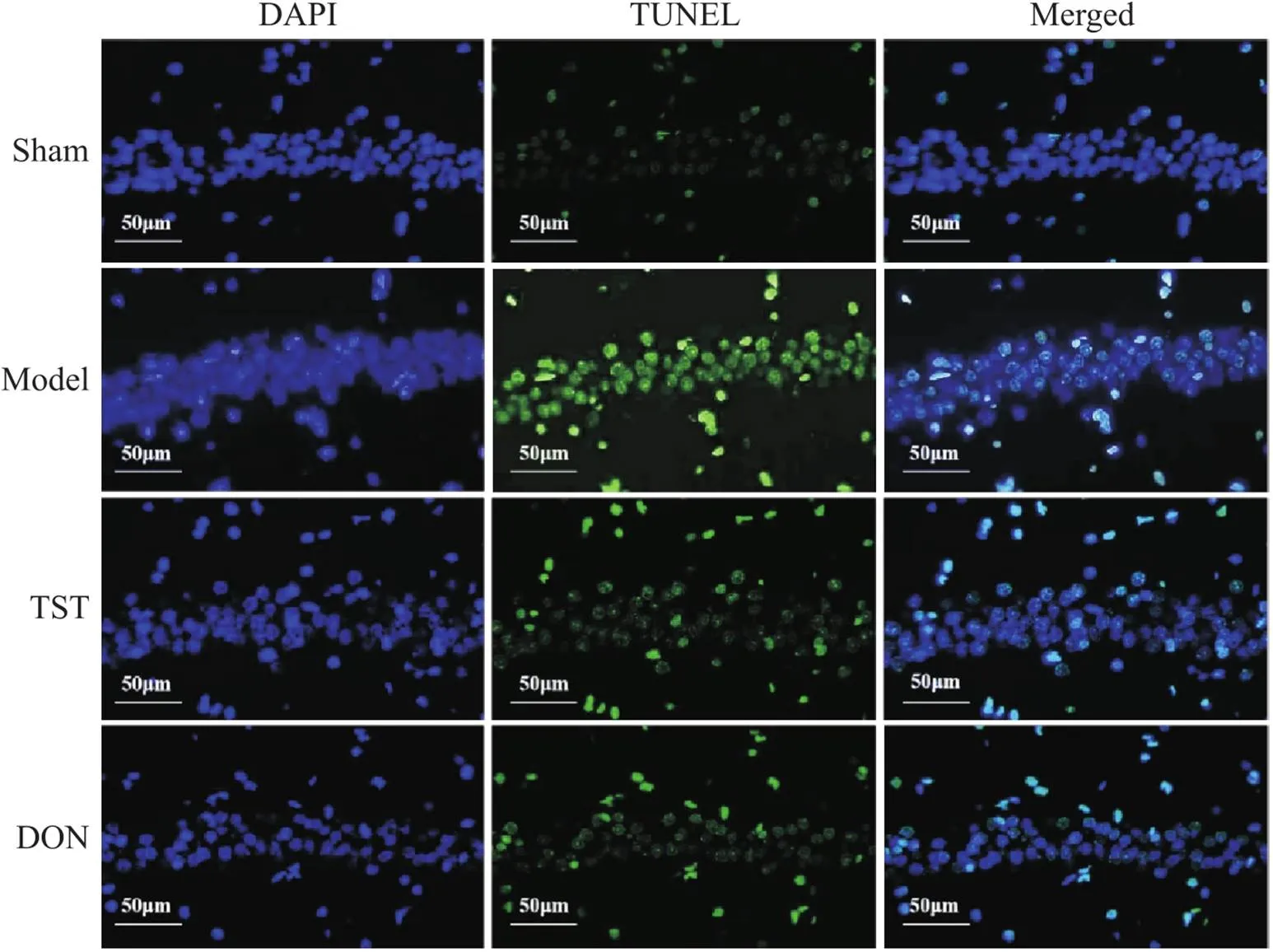
Figure 6. The effect of TST on the apoptosis of hippocampal neurons in PSCI rats (scale bar=50 µm). In sham group, the green fluorescence intensity in the CA1 region of the hippocampus was weak, and the number of apoptotic cells was small. In model group, the green fluorescence intensity in the CA1 region of the hippocampus was significantly enhanced, and the number of apoptotic cells increased. The green fluorescence intensity in hippocampal CA1 area of TST group and DON group was significantly weakened, and the number of positive cells was significantly reduced.
6 TST对大鼠海马CA1区GRP78、IRE1α、TRAF2、p-JNK和caspase-12蛋白定位表达的影响
如图7所示,扫描图像400倍放大后显示,除IRE1α为细胞核表达外,其余蛋白均为胞质表达。与sham组相比,model组大鼠海马CA1区GRP78、IRE1α、TRAF2、p-JNK和caspase-12的阳性细胞数量显著增多;与model组相比,TST组及DON组GRP78、IRE1α、TRAF2、p-JNK和caspase-12的阳性细胞数量显著减少。
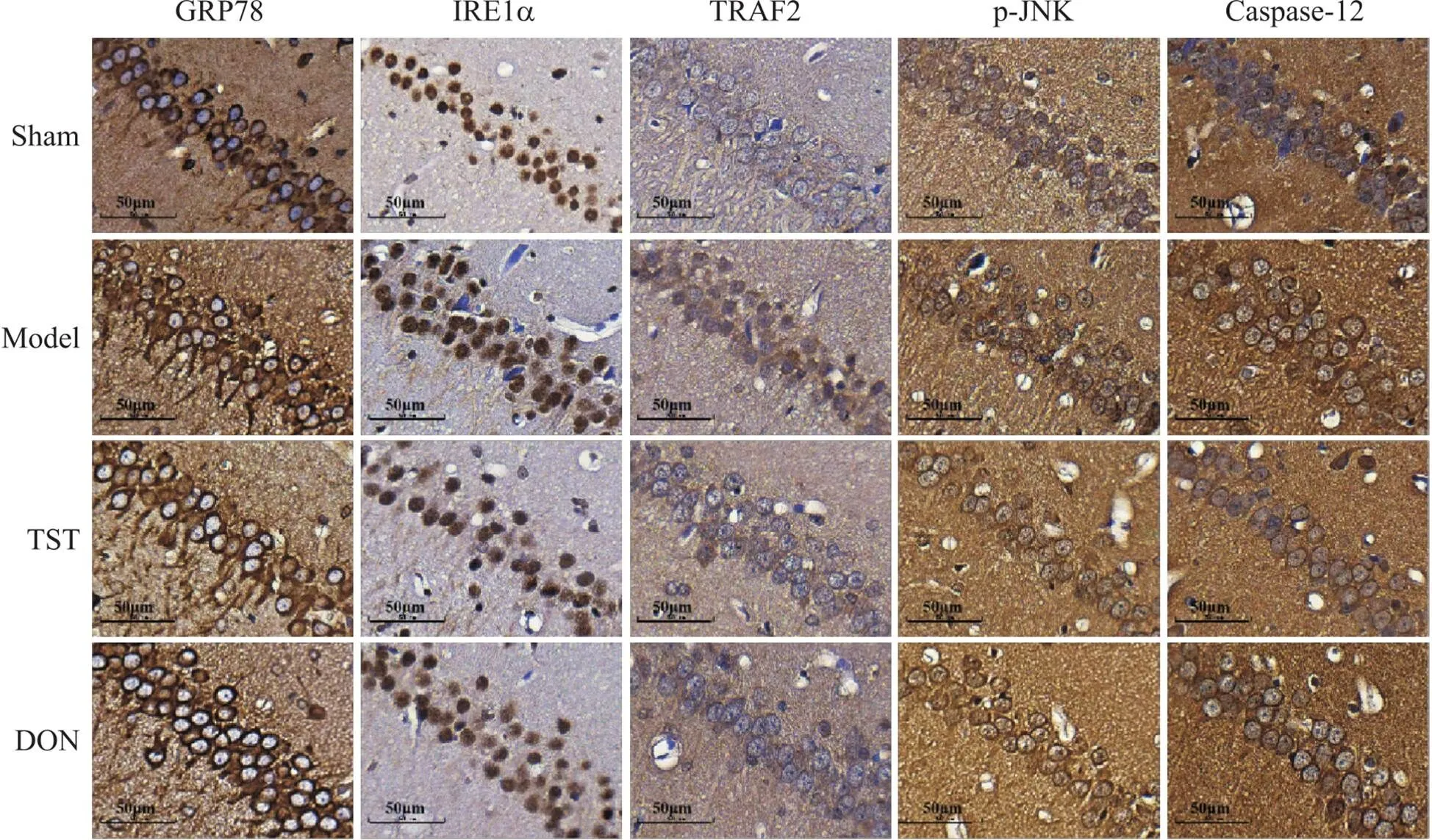
Figure 7. The effect of TST on the protein expression and localization of GRP78, IRE1α, TRAF2, p-JNK and caspase-12 in the CA1 region of the rat hippocampus (scale bar=50 µm). In sham group, the numbers of positive cells for GRP78, IRE1α, TRAF2, p-JNK and caspase-12 in the CA1 region of the hippocampus were less. In model group, the numbers of positive cells of GRP78, IRE1α, TRAF2, p-JNK and caspase-12 in the CA1 region of the hippocampus increased significantly. In TST group and DON group, the numbers of positive cells for GRP78, IRE1α, TRAF2, p-JNK and caspase-12 were significantly reduced compared with model group.
7 TST对大鼠海马组织GRP78、IRE1α、TRAF2、p-JNK、caspase-12、Bax和Bcl-2蛋白表达的影响
如图8所示,与sham组相比,model组大鼠海马组织GRP78、IRE1α、TRAF2、p-JNK、caspase-12和Bax的蛋白表达水平显著升高,Bcl-2的蛋白表达水平显著降低(<0.01);与model组相比,TST组及DON组大鼠海马组织GRP78、IRE1α、TRAF2、p-JNK、caspase-12和Bax的蛋白表达水平显著降低,Bcl-2的蛋白表达水平显著升高(<0.01)。
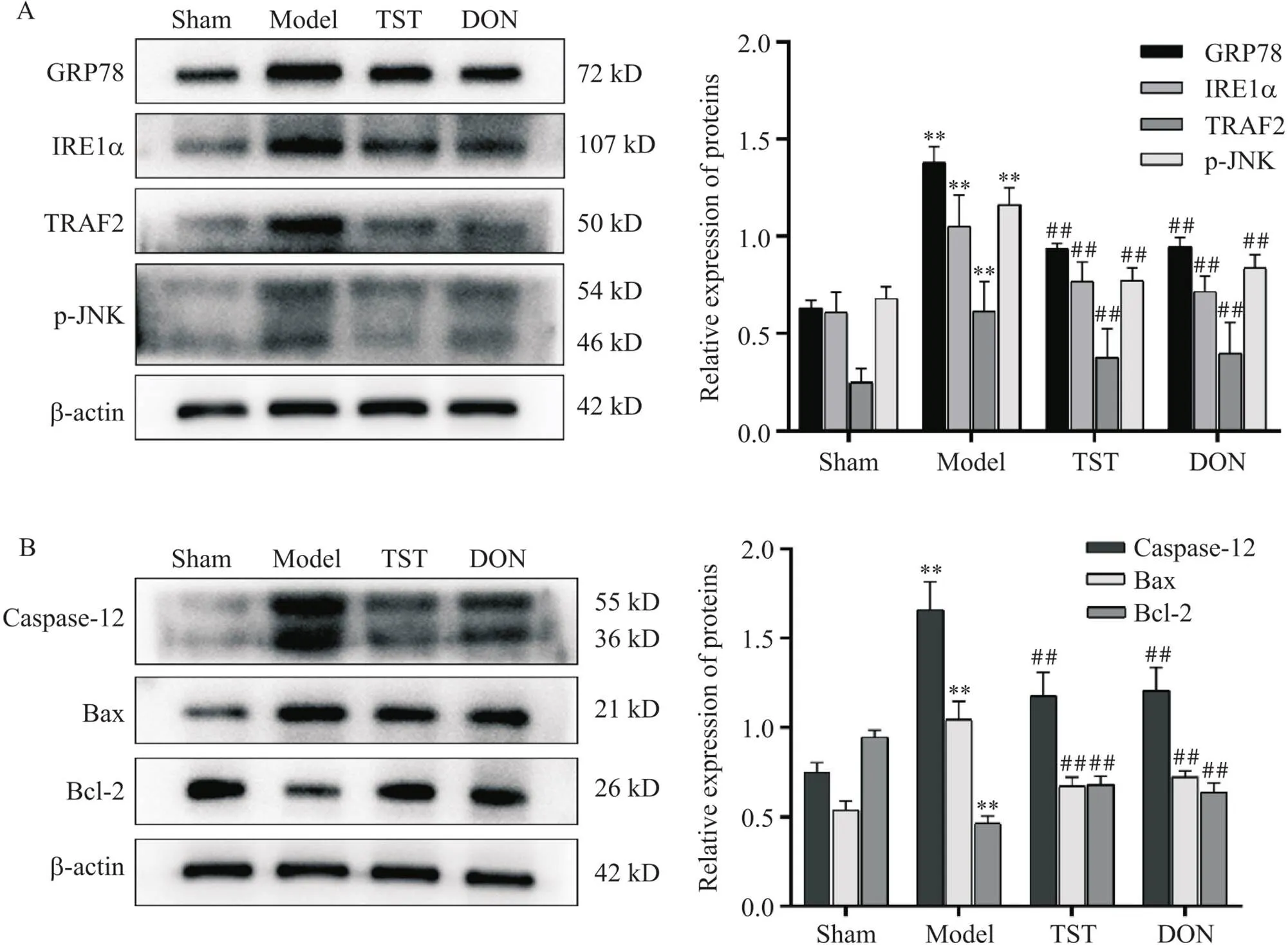
Figure 8. The effect of TST on the protein levels of GRP78, IRE1α, TRAF2, p-JNK, caspase-12, Bax and Bcl-2 in rat hippocampus. A: the protein levels of GRP78, IRE1α, TRAF2 and p-JNK; B: the protein levels of caspase-12, Bax and Bcl-2. Mean±SD. n=4. **P<0.01 vs sham group; ##P<0.01 vs model group.
讨论
缺血性脑卒中是一种急性脑血管疾病,其特征是大脑局部的血流突然中断,导致局部产生缺血缺氧状态,认知功能损害是其并发症之一。研究显示,ERS是导致神经元损伤的关键因素之一,也被认为是缺血性脑损伤干预的潜在治疗靶点。脑缺血后,蛋白质的错误折叠和过度积累,以及缺血再灌注引起的ROS过度产生,会触发ERS并增加神经元凋亡,从而导致认知功能的下降[17-18]。本研究采用水迷宫对各组大鼠的学习记忆能力进行评价,结果显示,模型大鼠逃避潜伏期延长,寻找平台的路径杂乱且缺乏目标趋向性;在给予TST药物干预后,大鼠逃避潜伏期及寻找平台路径缩短,目标趋向明确,这表明TST对于缺血性脑卒中引起的认知功能障碍具有明显的改善作用。
内质网是最大的管状网状细胞器,在细胞生理过程中发挥重要作用,主要负责蛋白质的合成和折叠、钙的储存和释放、脂质的合成和分配等功能[19]。研究表明,ERS在脑缺血病理生理学中起着至关重要的作用,轻微的ERS有助于提高细胞耐受性并恢复细胞稳态;然而,过度或长期ERS会导致细胞的凋亡[20, 21]。本研究显示,经改良线栓法造模后的大鼠出现肢体及认知功能损害,在经TTC染色后病灶侧出现缺血变白区域,初步判定大脑局部出现缺血缺氧的病理性损伤。进一步采用HE及尼氏染色对大鼠左侧半脑缺氧状态下的病理微观变化进行分析,结果显示,model组大鼠皮质及海马CA1区神经元坏死凋亡现象最为显著,神经元出现排列疏松、核固缩及空泡化现象,且尼氏体数量显著减少。在给予TST治疗后,神经元空泡化及核固缩现象显著减轻,这说明TST对于缺血缺氧造成的神经元丢失及损伤具有抑制作用。TUNEL染色显示,model组海马CA1区绿色荧光强度明显增强,阳性细胞明显增多,而给予TST治疗后其荧光强度明显减弱,阳性细胞数明显减少,表明TST在一定程度上能够抑制神经元的凋亡,对神经元起到一定保护作用。
研究表明,GRP78/IRE1α/TRAF2/JNK通路已被广泛应用在调节内质网应激引起的神经细胞凋亡中,并且发挥重要作用[22-23]。当细胞受到轻微应激时,ERS激活UPR使GRP78与IRE1α发生解离,此时IRE1α在内质网膜上发生二聚化和自磷酸化从而激活JNK,活化后的JNK可以下调抗凋亡蛋白Bcl-2来增强Bax依赖性凋亡[24]。然而,在长期或严重的应激下,IRE1α无法缓解ERS时,UPR会激活凋亡信号通路,此时激活后的IRE1α募集TRAF2,TRAF2可以募集和激活凋亡信号调节蛋白1,最终激活JNK和caspase-12通路来促进细胞凋亡,以维持内环境的稳定[25-27]。本研究显示,model组大鼠海马CA1区内质网相关蛋白棕色阳性表达明显,给予TST及DON治疗后海马CA1区棕色阳性表达减弱;Western blot结果显示,TST能够下调海马组织中GRP78、IRE1α、TRAF2、p-JNK、caspase-12和Bax的蛋白表达,上调Bcl-2的蛋白表达,这表明TST可能对GRP78/IRE1α/TRAF2/JNK信号通路起到抑制作用,从而对PSCI大鼠海马神经元起到一定保护作用。
综上所述,延龄草总皂苷可以改善PSCI大鼠的学习记忆能力,并能改善由内质网应激引起的海马神经元损伤,其机制可能与其抑制GAP78/IRE1α/TRAF2/JNK信号通路,上调Bcl-2的蛋白表达,下调caspase-12及Bax的蛋白表达有关。
[1] Ivan CS, Seshadri S, Beiser A, et al. Dementia after stroke: the Framingham study[J]. Stroke, 2004, 35(6):1264-1268.
[2] Jo F, Jo H, Hilzendeger AM, et al. Brain endoplasmic reticulum stress mechanistically distinguishes the saline-intake and hypertensive response to deoxycorticosterone acetate-salt[J]. Hypertension, 2015, 65(6):1341-1348.
[3] Zhang XY, Yang SM, Zhang HP, et al. Endoplasmic reticulum stress mediates the arsenic trioxide-induced apoptosis in human hepatocellular carcinoma cells[J]. Int J Biochem Cell Biol, 2015, 68:158-165.
[4] Smith HL, Mallucci GR. The unfolded protein response: mechanisms and therapy of neurodegeneration[J]. Brain, 2016, 139(Pt 8):2113-2121.
[5] Hetz C, Saxena S. ER stress and the unfolded protein response in neurodegeneration[J]. Nat Rev Neurol, 2017, 13(8):477-491.
[6]陈丽敏, 白艳杰, 王岩, 等. 线粒体质量控制失调介导卒中后认知障碍的研究进展[J]. 中国病理生理杂志, 2022, 38(7):1320-1327.
Chen LM, Bai YJ, Wang Y, et al. Progress in mitochondrial quality control disorder-mediated cognitive impairment after stroke[J]. Chin J Pathophysiol, 2022, 38(7):1320-1327.
[7] Mohammed Thangameeran SI, Tsai ST, Hung HY, et al. A role for endoplasmic reticulum stress in intracerebral hemorrhage[J]. Cells, 2020, 9(3):750.
[8] Xin Q, Ji B, Cheng B, et al. Endoplasmic reticulum stress in cerebral ischemia[J]. Neurochem Int, 2014, 68:18-27.
[9] Chen L, Xia YF, Shen SF, et al. Syntaxin 17 inhibits ischemic neuronal injury by resuming autophagy flux and ameliorating endoplasmic reticulum stress[J]. Free Radic Biol Med, 2020, 160:319-333.
[10] Ten V, Galkin A. Mechanism of mitochondrial complex I damage in brain ischemia/reperfusion injury: a hypothesis[J]. Mol Cell Neurosci, 2019, 100:103408.
[11] Datta A, Sarmah D, Mounica L, et al. Cell death pathways in ischemic stroke and targeted pharmacotherapy[J]. Transl Stroke Res, 2020, 11(6):1185-1202.
[12] Di Sano F, Ferraro E, Tufi R, et al. Endoplasmic reticulum stress induces apoptosis by an apoptosome-dependent but caspase 12-independent mechanism[J]. J Biol Chem, 2006, 281(5):2693-2700.
[13] Szegezdi E, Logue SE, Gorman AM, et al. Mediators of endoplasmic reticulum stress-induced apoptosis[J]. EMBO Rep, 2006, 7(9):880-885.
[14] Wang L, Du J, Zhao F, et al. Trillium tschonoskii maxim saponin mitigates D-galactose-induced brain aging of rats through rescuing dysfunctional autophagy mediated by Rheb-mTOR signal pathway[J]. Biomed Pharmacother, 2018, 98:516-522.
[15] Chen XB, Wang ZL, Yang QY, et al. Diosgenin glucoside protects against spinal cord injury by regulating autophagy and alleviating apoptosis[J]. Int J Mol Sci, 2018, 19(8):2274.
[16] Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats[J]. Stroke, 1989, 20(1):84-91.
[17] Huang M, Li ZX, Chen J, et al. Extracts of bauhinia championiialleviate acute neuronal injury after ischemic reperfusion by improving endoplasmic reticulum stress-mediated neuronal apoptosis[J]. Curr Med Sci, 2022, 42(3):483-490.
[18] Xin Q, Ji B, Cheng B, et al. Endoplasmic reticulum stress in cerebral ischemia[J]. Neurochem Int, 2014, 68:18-27.
[19] Addinsall AB, Wright CR, Andrikopoulos S, et al. Emerging roles of endoplasmic reticulum-resident selenoproteins in the regulation of cellular stress responses and the implications for metabolic disease[J]. Biochem J, 2018, 475(6):1037-1057.
[20] Wang L, Liu Y, Zhang X, et al. Endoplasmic reticulum stress and the unfolded protein response in cerebral ischemia/reperfusion injury[J]. Front Cell Neurosci, 2022, 16:864426.
[21] 兰卓, 王欢, 何夕松, 等. 柚皮素对心肌缺血/再灌注损伤大鼠PI3K/AKT信号通路和内质网应激及其相关凋亡通路的影响[J]. 中国病理生理杂志, 2021, 37(1):41-47.
Lan Z, Wang H, He XS, et al. Effects of naringenin on PI3K/AKT signaling pathway and endoplasmic reticulum stress and its related apoptotic pathways in rats with myocardial ischemia/reperfusion injury[J]. Chin J Pathophysiol, 2021, 37(1):41-47.
[22] Xu B, Xu J, Cai N, et al. Roflumilast prevents ischemic stroke-induced neuronal damage by restricting GSK3β-mediated oxidative stress and IRE1α/TRAF2/JNK pathway[J]. Free Radic Biol Med, 2021, 163:281-296.
[23] Ji M, Niu S, Guo J, et al. Silencing RNF13 alleviates Parkinson's disease-like problems in mouse models by regulating the endoplasmic reticulum stress-mediated IRE1α- TRAF2-ASK1-JNK pathway[J]. J Mol Neurosci, 2020, 70(12):1977-1986.
[24] Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis[J]. Proc Natl Acad Sci U S A, 2003, 100(5):2432-2437.
[25] Hetz C, Papa FR. The unfolded protein response and cell fate control[J]. Mol Cell, 2018, 69(2):169-181.
[26] Wang F, Weng H, Quon MJ, et al. Dominant negative FADD dissipates the proapoptotic signalosome of the unfolded protein response in diabetic embryopathy[J]. Am J Physiol Endocrinol Metab, 2015, 309(10):E861-E873.
[27] Schönthal AH. Pharmacological targeting of endoplasmic reticulum stress signaling in cancer[J]. Biochem Pharmacol, 2013, 85(5):653-666.
Total saponins fromMaxim attenuate hippocampal neuronal injury in PSCI rats by inhibiting endoplasmic reticulum stress via regulating GRP78/IRE1α/TRAF2/JNK signaling pathway
WANG Gang, YANG Lijun, YANG Dan, DUAN Renze, ZHAO Fangyu, CHEN Xianbing△
(,,,445000,)
To investigate the protective effect of total saponins fromMaxim (TST) on hippocampal neurons in rats with post-stroke cognitive impairment (PSCI), and to explore the molecular mechanism.The rat PSCI model was successfully established by the modified Zea Longa suture method, and the rats were randomly divided into model group, TST (100 mg/kg) group, and donepezil hydrochloride (DON; 0.45 mg/kg) group. Another normal rats served as sham group. There were 10 animals in each group, and they were given continuous administration for 4 weeks. Morris water maze was used to detect the learning and memory ability of rats, TTC staining was used to detect the volume change of cerebral infarction in rats, and HE, Nissl and TUNEL staining was used to observe the pathological changes of neurons in the hippocampus of rats. The protein levels of glucose-regulated protein 78 (GRP78), inositol-requiring enzyme 1α (IRE1α), tumor necrosis factor receptor-associated factor 2 (TRAF2), phosphorylated c-Jun N-terminal kinase (p-JNK), caspase-12, Bax and Bcl-2 were detected by immunohistochemistry and Western blot.Compared with sham group, the escape latency of the rats in model group was significantly prolonged, and the number of platform crossings and the residence time in the target quadrant were significantly decreased (<0.01). The volume of cerebral infarction in rats was significantly increased, the number of neuronal Nissl bodies was significantly decreased, and the number of apoptotic cells was significantly increased. The protein levels of GRP78, IRE1α, TRAF2, p-JNK, caspase-12 and Bax in the hippocampus were significantly increased, and the expression of Bcl-2 was significantly decreased (<0.01). Compared with model group, the escape latency of the rats in TST group and DON group was significantly shortened, and the times of crossing the platform and the residence time of the target quadrant were significantly increased (<0.01). The cerebral infarction volume of the rats was significantly reduced, and the number of neuronal Nissl bodies was significantly increased. Apoptotic cells were significantly reduced. The protein levels of GRP78, IRE1α, TRAF2, p-JNK, caspase-12 and Bax were significantly decreased, and the protein expression of Bcl-2 was significantly increased (<0.01).TST has a protective effect on hippocampal neurons in PSCI rats, and its mechanism may be related to reducing endoplasmic reticulum stress and neuronal apoptosis and inhibiting GRP78/IRE1α/TRAF2/JNK signaling pathway.
total saponins fromMaxim; post-stroke cognitive impairment; endoplasmic reticulum stress; apoptosis; GRP78/IRE1α/TRAF2/JNK signaling pathway
R74; R363.2
A
10.3969/j.issn.1000-4718.2023.02.006
1000-4718(2023)02-0241-09
2022-09-30
2022-12-16
[基金项目]国家自然科学基金资助项目(No. 82260821);湖北民族大学博士启动基金项目(No. MD2020B014)
Tel: 13517134626; E-mail: chenxianbing7612@163.com
(责任编辑:林白霜,罗森)
