CIRBP对小鼠血管平滑肌细胞增殖及迁移的调控及机制研究*
2023-03-10赵嘉琪杨大春王强孙雄山杨永健
赵嘉琪, 杨大春, 王强, 孙雄山△, 杨永健,△
CIRBP对小鼠血管平滑肌细胞增殖及迁移的调控及机制研究*
赵嘉琪1, 杨大春2, 王强2, 孙雄山2△, 杨永健1,2△
(1西南医科大学临床医学院,四川 泸州 646000;2西部战区总医院心内科,四川 成都 610083)
探讨冷诱导RNA结合蛋白(CIRBP)对小鼠血管平滑肌细胞(VSMCs)增殖及迁移过程的调控及机制。将雄性C57BL/6J小鼠分为假手术对照组、假手术干扰组、颈总动脉损伤对照组和颈总动脉损伤干扰组,每组5只。造模后按照组别分别用阴性对照腺病毒(AD-NC)和沉默腺病毒(AD-I)转染,28 d后观察CIRBP表达及血管内膜的增生情况。将小鼠VSMCs分为对照组和腺病毒沉默组,分别用AD-NC和AD-转染细胞48 h,然后加入激活雷帕霉素靶蛋白复合物1(mTORC1)活性的胰岛素。通过RT-qPCR检测CIRBP的mRNA表达,Western blot检测CIRBP、磷酸化核糖体蛋白S6(p-S6Ser235/236)和磷酸化4E结合蛋白1(p-4EBP1Thr37/46)蛋白水平变化,Ki67免疫荧光染色和CCK-8实验检测细胞增殖,划痕实验检测细胞迁移,HE染色检测颈动脉内膜增生程度。沉默后,VSMCs的活力下降,Ki67阳性细胞比率降低,细胞迁移速度减慢,同时mTORC1活性下降。加入胰岛素恢复mTORC1活性后,细胞活力、Ki67阳性细胞率和细胞迁移速度下降幅度减弱。损伤小鼠颈总动脉内皮后CIRBP表达增加,体内干扰小鼠CIRBP表达后,小鼠血管内皮损伤后内膜增生程度减轻。CIRBP通过mTORC1途径加强小鼠VSMCs增殖及迁移,促进小鼠血管损伤后血管内膜增生。
血管平滑肌细胞;细胞增殖;细胞迁移;冷诱导RNA结合蛋白;哺乳动物雷帕霉素靶蛋白复合物1
在我国,患心血管病的人数在迅速增加,其中动脉粥样硬化性心血管病的患病率与死亡率逐年上升,并且有年轻化的趋势[1-2]。经皮冠脉介入治疗(percutaneous coronary intervention, PCI)是主要解决方法,但术后血管再狭窄严重制约PCI的预后。血管内皮细胞受到机械损伤后,经过内皮剥落,血小板活化,促炎细胞因子和趋化因子的释放以及血管平滑肌细胞移动到内皮下等过程,最终使血管内膜增生,引起血管内再狭窄[3]。当前血管平滑肌细胞增殖的分子调控机制主要包括Ras/丝裂原活化蛋白激酶、Janus激酶/信号转导及转录激活因子等[4],针对血管内再狭窄的治疗手段进步显著,但术后血管再狭窄的发生率仍有5%~10%[5],提示仍有未知的分子机制需要探索。
冷诱导RNA结合蛋白(cold-inducible RNA-binding protein, CIRBP)是一种冷休克相关蛋白,属于RNA结合蛋白家族,在冷应激等条件下可以促进翻译[6]。CIRBP能调控器官与组织细胞的生长、增殖和分化[7]、维持端粒酶活性[8]、抑制细胞凋亡[9]、促进炎症细胞因子的激活与释放[10]。多个研究表明CIRBP在心力衰竭和血管损伤等心血管疾病中有重要意义[10-14],当血管受到各种损伤和病理因素刺激时,血管平滑肌细胞会从分化状态转变为去分化状态,增殖异常活跃,并进一步向内膜迁移,导致内膜增生及参与动脉粥样硬化、肺动脉高压等心血管疾病的进展[15]。既往研究显示CIRBP在乳腺癌、黑色素瘤、垂体腺瘤和胰腺导管腺癌等肿瘤细胞中发挥促进肿瘤细胞增殖作用[16-19]。但是CIRBP对血管平滑肌细胞增殖、迁移和血管内膜增生引起血管内再狭窄的调控目前并不清楚。本研究通过机械损伤小鼠颈总动脉内皮的方法构建血管再狭窄模型,用腺病毒感染方法干扰CIRBP表达,探究CIRBP在小鼠VSMCs增殖、迁移和血管内膜增生中的作用机制,为PCI术后防治血管再狭窄提供参考资料。
材料和方法
1 动物
20只约7周龄SPF级的雄性C57BL/6J小鼠,重约25g(成都药康生物科技有限公司),许可证编号:SCXK(川)2020-034。小鼠先适应性培养1周,白天适当光照12 h,恒温通风,喂以水和食物。
2 细胞和主要试剂
小鼠主动脉平滑肌细胞系MOVAS(深圳市豪地华拓生物科技有限公司);RT-qPCR实验试剂盒(TaKaRa);RT-qPCR实验所用的引物(成都擎科伟业生物技术有限公司);DMEM高糖培养液、0.25%胰蛋白酶及青霉素+链霉素双抗(HyClone);胎牛血清(Gibco);磷酸盐缓冲液(phosphate-buffered saline, PBS)、5%牛血清白蛋白(bovine serum albumin, BSA)和CIRBP抗体(武汉三鹰生物科技有限公司);4E结合蛋白1(4E-binding protein 1, 4EBP1)抗体、磷酸化4EBP1(phosphorylated 4EBP1, p-4EBP1Thr37/46)抗体、GAPDH单克隆抗体、核糖体蛋白S6(ribosomal protein S6, S6)抗体、磷酸化S6(phosphorylated S6, p-S6Ser235/236)抗体和山羊抗兔Ⅱ抗(Cell Signaling Technology);4',6-二脒基2苯基吲哚(4′,6-diamidino-2-phenylindole, DAPI)和Ki-67抗体(Bioss);重组人胰岛素(Solarbio);CIRBP腺病毒(上海吉凯基因有限公司);ECL发光液(Millipore);细胞活力及毒性检测(CCK-8)试剂盒(Biosharp);苏木精-伊红染色试剂(北京索莱宝科技有限公司)。
3 主要方法
3.1VSMCs分组及干预用含1%双抗和10%胎牛血清的DMEM高糖培养液培养VSMCs,将培养皿放在5% CO2、37 ℃培养箱中。隔2~3 d更换一次培养液,当细胞增殖到90%左右用0.25%胰蛋白酶消化生长状态良好的细胞,并且按照1∶2~1∶4传代培养,选择对数生长期细胞进行实验。为观察CIRBP对细胞增殖和迁移的影响,根据预实验结果,将腺病毒复感染指数(MOI)定为100,按照分组分别向细胞中加入阴性对照腺病毒(AD-NC)和沉默腺病毒(AD-I)(105247-1),继续培养6~8 h,随后用PBS冲洗2遍,换成完全培养液继续共孵育48 h。为进一步探究CIRBP是否通过mTORC1调节VSMCs增殖及迁移,在沉默表达后,向相应的组别中加入人重组胰岛素(5 mg/L)孵育24 h。
3.2RT-qPCR测定CIRBP的mRNA表达用RNA提取试剂盒提取细胞总RNA,用基因相对定量法标准化各组RNA浓度,接着反转录为cDNA然后扩增反应。内参照β-actin(NM_007393)的上游引物序列为5′-CGTGAAAAGATGACCCAGATCA-3′,下游引物序列为5′-TGGTACGACCAGAGGCATACAG-3′;CIRBP(NM_007705)的上游引物序列为5′-AGCTCGGGAGGGTCCTACAG-3′,下游引物序列为5′‑GAGGGCTTTTACTCGTTGTGTGT-3′[20]。
3.3Western blot检测细胞CIRBP蛋白表达及反映mTORC1活性的底物S6和4EBP1的磷酸化水平用全蛋白提取试剂盒提取VSMCs蛋白,用BSA试剂盒测定蛋白浓度。取适量的蛋白样品进行电泳,然后把蛋白转至相应孔径的聚偏二氟乙烯膜,用牛血清白蛋白或者5%脱脂奶粉室温条件下封闭1 h,再加入对应的抗体(CIRBP、4EBP1、p-4EBP1Thr37/46、S6、p-S6Ser235/236和GAPDH,稀释比:1∶1 000)4 ℃孵育过夜。接着用缓冲液洗涤,然后加入山羊抗兔Ⅱ抗(稀释比:1∶1 000),孵育1 h后,用缓冲液洗涤,接着加入ECL化学发光液显色,最后通过ImageJ分析条带。
3.4CCK-8检测细胞活力用完全培养基将细胞配成含有2×108/L的细胞悬液,铺板到96孔板中,每个孔加100 μL,每组设置3个复孔。当细胞增殖到80%左右时每孔加入10 μL CCK-8溶液,放到细胞培养箱内继续孵育2 h,用酶标仪检测450 nm处的吸光度(),比较不同组间的细胞活力。
3.5Ki-67免疫荧光染色检测细胞增殖将爬片放入6孔板中,加入配制好的细胞悬液,进行干预后,倒掉培养液用PBS清洗3次,用5%BSA封闭30 min后加入Ⅰ抗Ki-67(稀释比:1∶500),放入湿盒中4 ℃孵育过夜,再次用PBS清洗3次后加入荧光Ⅱ抗(稀释比:1∶500),避光条件下室温孵育1 h,PBS再次清洗后加DAPI染液避光室温孵育10 min,用PBS清洗甩干后,用抗荧光淬灭剂封片,显微镜采集图像,记录Ki-67阳性细胞率。
3.6划痕实验检测细胞迁移速度使用记号笔在6孔板背面大约每隔1 cm均匀划一条竖线,每个孔划3条竖线。向6孔板中加入一定数量的细胞,按照分组进行干预,待细胞增殖到90%左右用无菌的规格为100 μL吸头垂直于竖线划一道痕,随后用PBS洗涤细胞3遍,立刻拍摄时间点为0 h的细胞,接着加入无血清培养液,放入细胞培养箱中培养,分别在12 h及24 h两个时点用光学显微镜拍照,以划痕的宽窄变化快慢测量细胞迁移率。
3.7小鼠分组及颈总动脉内皮损伤模型的建立 用0.3%戊巴比妥钠(30 mg/kg)麻醉已经称了重的小鼠,然后将麻醉好的小鼠固定于手术台面上,把颈部毛发脱去后予以消毒处理,接着在颈正中做约1 cm的纵行切口,分离左颈总动脉,用粗糙导丝来回摩擦血管内皮后,迅速取出导丝,结扎导丝穿刺点,缝合消毒切口[21]。将小鼠分为损伤对照组、损伤转染组、假手术对照组和假手术转染组,每组5只,损伤对照组和假手术对照组转染阴性对照腺病毒AD-NC,损伤转染组和假手术转染组转染腺病毒AD-I,在术后7、14和21 d通过尾静脉继续追加注射腺病毒;28 d后取出小鼠颈总动脉,标记按组分装。
3.8HE染色将各组小鼠的血管取材,4%多聚甲醛固定,石蜡包埋、切片、染色、封片,拍照记录,观察对比血管结构及内膜增生程度。
4 统计学处理
采用GraphPad Prism 8.3.0软件对数据进行绘图,采用SPSS 21软件分析数据。用均数±标准差(mean±SD)表示定量资料。选用独立样本检验进行两组间比较,选用单因素方差分析进行多组间比较。以<0.05为差异有统计学意义。
结果
1 对照组与腺病毒沉默组细胞中CIRBP表达比较
通过RT-qPCR实验得到,与对照组相比,腺病毒沉默组中CIRBP的mRNA水平显著下降(<0.01),同时Western blot实验显示,和对照组比较,腺病毒沉默组中的CIRBP蛋白表达亦显著降低(<0.05),见图1。
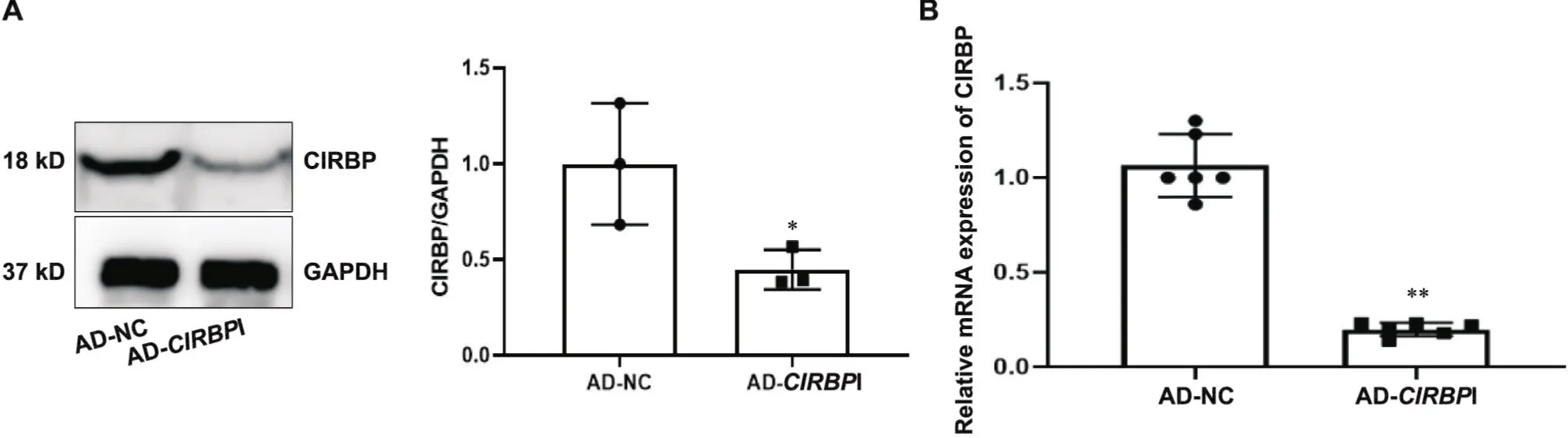
Figure 1. The protein and mRNA levels of CIRBP were decreased in VSMCs transfected by AD-CIRBPI. A: the protein level of CIRBP was explored by Western blot; B: the mRNA expression of CIRBP was explored by RT-qPCR. Mean±SD. n=3. *P<0.05, **P<0.01 vs AD-NC group.
2 干扰CIRBP的表达后小鼠VSMCs增殖和迁移情况
划痕实验结果显示,与对照组相比,干扰表达后细胞划痕愈合速度减慢(<0.05),见图2A。Ki-67免疫荧光染色显示,和对照组相比,干扰表达显著降低Ki-67阳性细胞率(<0.05),见图2B。
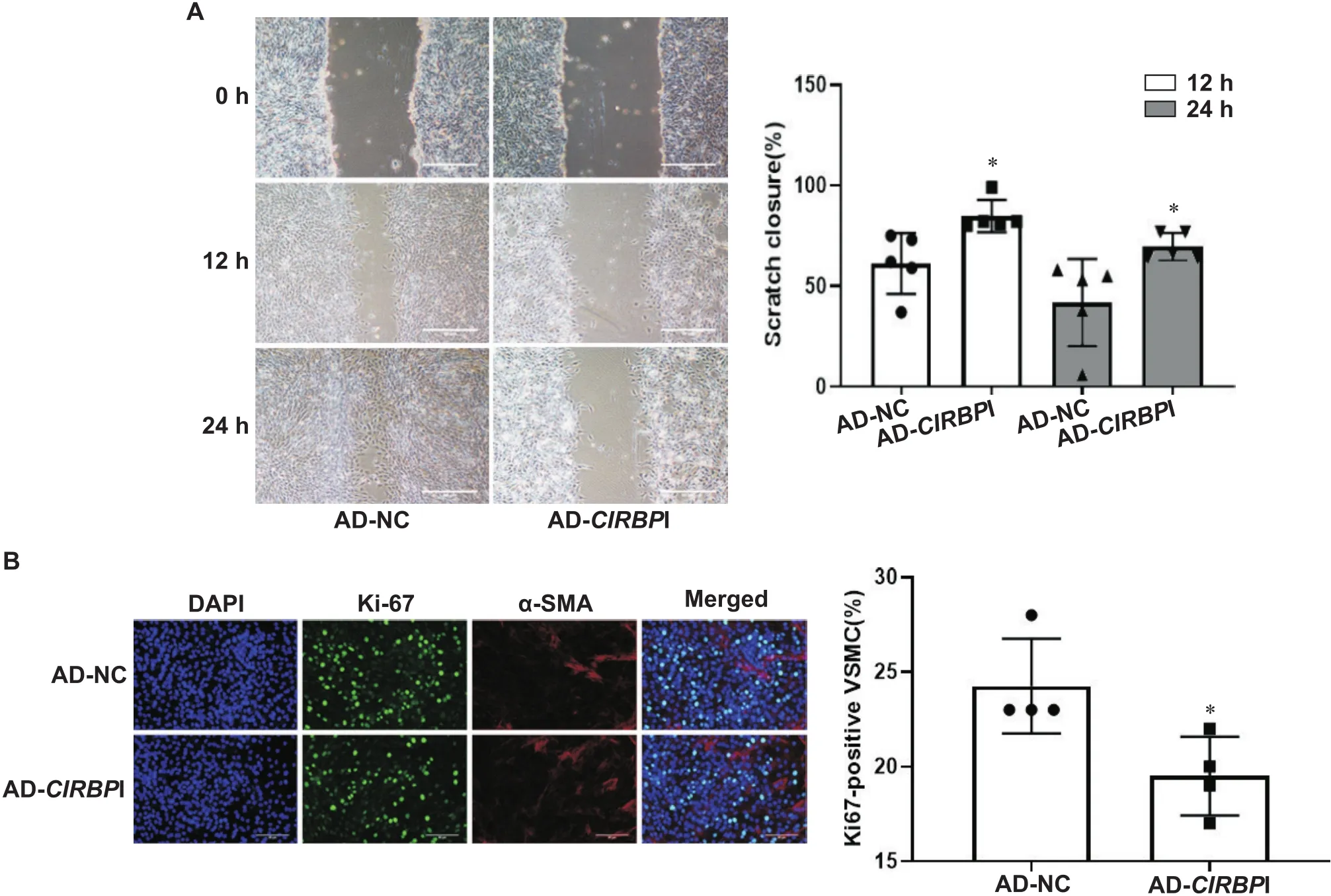
Figure 2. Silencing of CIRBP suppressed migration and proliferation of mouse VSMCs. A: the migration of the cells was explored by scratch test; B: the proliferation of VSMCs was explored by Ki-67 staining (α-SMA: α-smooth muscle actin). Scale bar=50 μm. Mean± SD. n=5. *P<0.05 vs AD-NC group.
CCK-8实验检测显示,干扰表达之后在450 nm处的吸光度显著下降(<0.05),见表1。

表1 各组细胞活力比较
*<0.05AD-NC group.
3 干扰CIRBP的表达后,mTORC1的变化及小鼠VSMCs增殖和迁移情况
经过Western blot实验,结果显示和对照组比较,干扰表达后,VSMCs中mTORC1底物p-4EBP1Thr37/46和p-S6Ser235/236蛋白水平显著降低(<0.05或<0.01),见图3。

Figure 3. The activity of mTORC1 was reduced in VSMCs transfected with AD-CIRBPI. The protein levels of p-4EBP1Thr37/46, 4EBP1, p-S6Ser235/236 and S6 were explored by Western blot. Mean±SD. n=3. *P<0.05, **P<0.01 vs AD-NC group.
然而向VSMCs相应组别中加入胰岛素后mTORC1底物p-4EBP1Thr37/46和p-S6Ser235/236蛋白表达显著增高(<0.01),此时Ki-67阳性细胞率(0.01)和细胞划痕愈合速度(<0.05或<0.01)下降程度减慢,见图4。
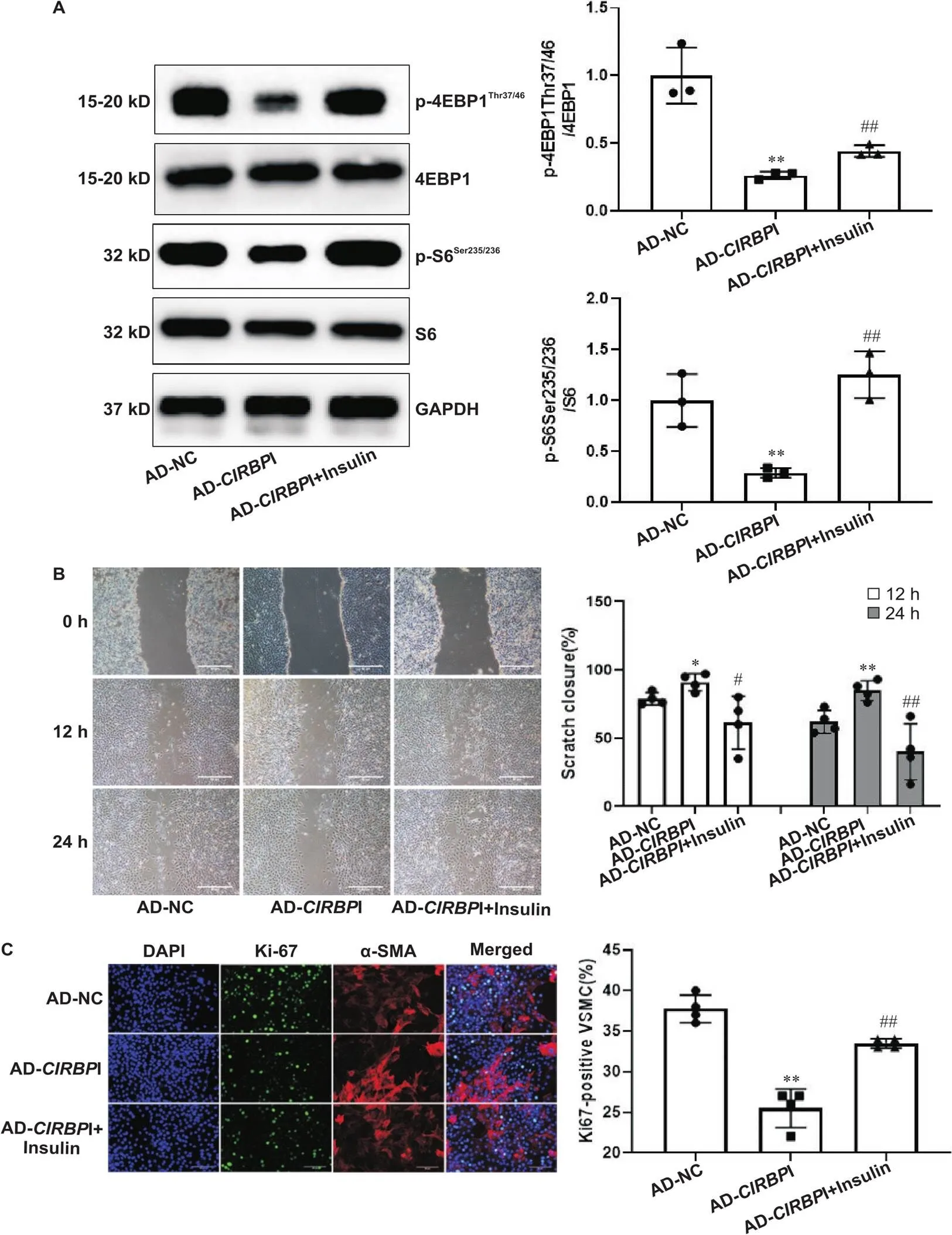
Figure 4. The inhibitory effects of CIRBP silencing on migration and proliferation were attenuated after restoring the activity of mTORC1 by insulin. A: the protein levels of p-4EBP1Thr37/46 and p-S6Ser235/236 were explored by Western blot after restoring the activity of mTORC1 by insulin (n=3); B: the migration of the cells was explored by scratch test after restoring the activity of mTORC1 by insulin (n=5); C: the proliferation of the cells was explored by Ki-67 staining after restoring the activity of mTORC1 by insulin (n=5; α-SMA: α-smooth muscle actin). Scale bar=50 μm. Mean±SD. *P<0.05, **P<0.01 vs AD-NC group; #P<0.05, ##P<0.01 vs AD-CIRBPI group.
4 小鼠血管内皮损伤后各组CIRBP表达及血管内膜变化
进一步行Western blot实验显示,和假手术组对比,颈动脉内皮损伤组CIRBP表达显著升高(<0.05),见图5A,血管内膜增厚,血管管腔狭窄;在颈动脉内皮损伤的基础上再干扰CIRBP表达,结果显示内膜增生及血管管腔再狭窄程度减轻,见图5B。
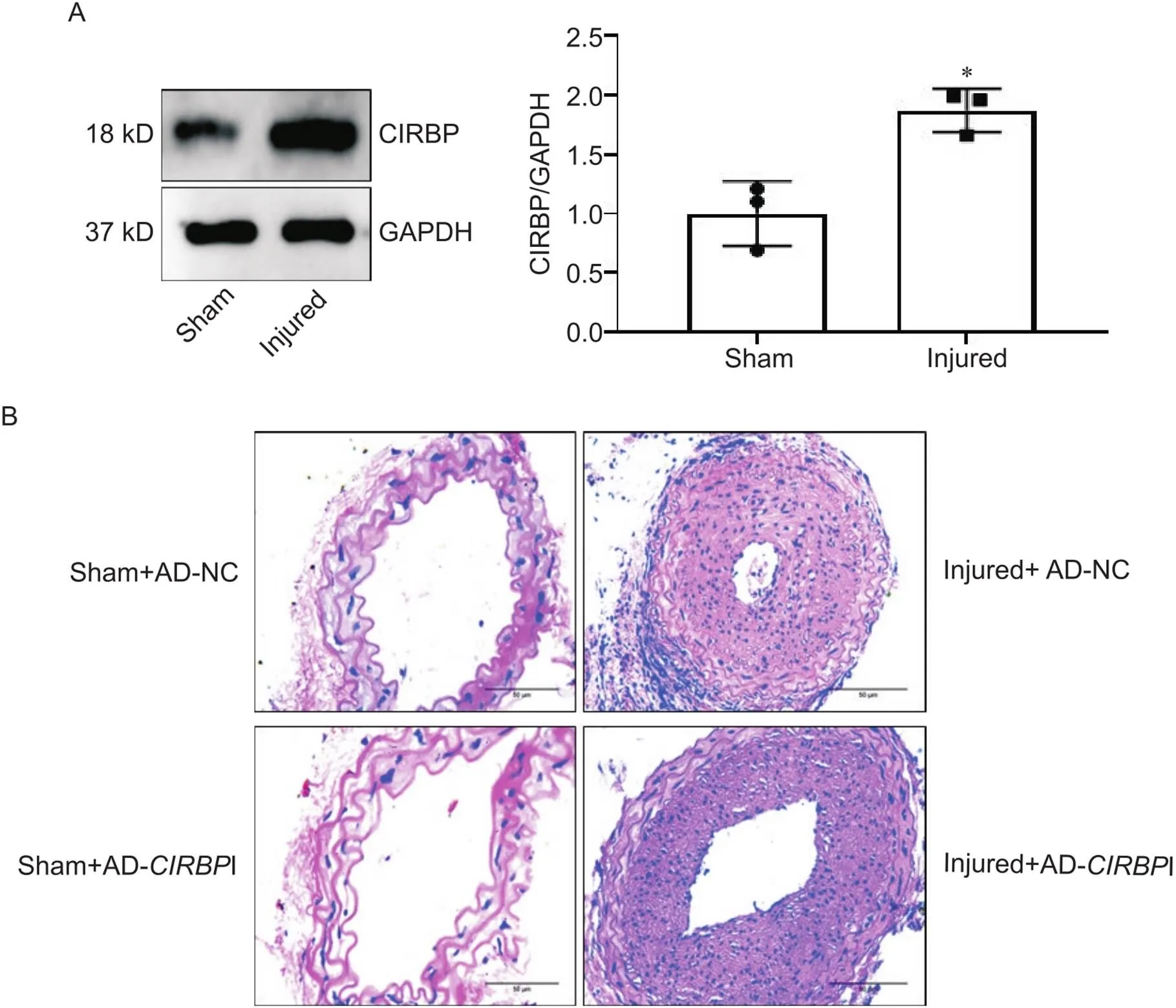
Figure 5. The expression of CIRBP and pathological changes of carotid artery in mice of different groups. A: the protein level of CIRBP was explored by Western blot; B: pathological changes of carotid artery were determined by HE staining (scale bar=50 μm). Mean±SD. n=3. *P<0.05 vs sham group.
讨论
冠状动脉粥样硬化性心脏病是全世界发病率和死亡率的主要病因,PCI可让一部分有阻塞性冠状动脉疾病的患者获益[22],然而手术引起的血管内再狭窄并发症依旧无法完全避免,整个病理过程一方面可能与新生内膜破裂、薄帽纤维弹性瘤形成、非闭塞性血栓形成等有关[23];另一方面,血管内皮细胞受损后一氧化氮、前列环素等减少,组织因子和活性氧等表达增加,同时血管壁内细胞释放促炎因子等促进了血管平滑肌细胞表型转换,位于中膜的血管平滑肌细胞被激活,增殖并迁移到内皮下引起内膜增生也是重要的病理基础[3],是预防血管内再狭窄的关键之一。血小板衍生生长因子BB(platelet-derived growth factor-BB, PDGF-BB)是VSMCs表型转化过程重要的刺激因子[15],Sun等[24]的研究表明用20 μg/L的PDGF-BB处理血管平滑肌细胞之后会形成增殖型VSMCs,本实验通过干扰表达,探讨CIRBP对小鼠VSMCs增殖、迁移、血管内膜增生的调控及有关机制。
CIRBP能和多种细胞内信号分子相互作用发挥生物学效应,如与能编码昼夜节律调节器蛋白的RNA结合,促进翻译[25];结合TLR4及其附属蛋白MD2复合物引发炎症反应[14];结合TLR4诱导内质网应激等[26]。在心力衰竭患者和心肌梗死小鼠心脏中CIRBP表达降低,导致心肌细胞凋亡增多[27]。Yang等[10]的研究结果显示CIRBP通过刺激小鼠肺血管内皮细胞中的Nlrp3炎症小体组装和激活、caspase-1的激活以及促进IL-1β的释放,导致内皮功能障碍,而Nlrp3炎症通路与新生血管内膜增生密切联系[28]。以上研究结果表明CIRBP在心血管病理调控中有重要意义,但在机械损伤血管内皮后内膜增生中的作用及机制并未提及。本研究观察到增殖型VSMCs中CIRBP表达增加,腺病毒干扰表达后,可以抑制VSMCs的增殖和迁移,说明CIRBP正相调控VSMCs的增殖和迁移过程,然而调控的具体分子机制尚不清楚。
mTOR是丝/苏氨酸激酶,在控制细胞代谢、生长和增殖等信号网络中具有核心作用[29]。mTORC1是mTOR发挥效应的多蛋白复合体之一,参与调节蛋白质和脂质合成、细胞生长、增殖和自噬等[30]。CIRBP是否通过mTORC1调控VSMCs增殖和迁移尚不清楚。接着我们通过体外实验进一步研究了CIRBP和mTORC1的关系,干扰表达后,mTORC1下游的两个核心底物S6和4EBP1的磷酸化水平均降低,表明了CIRBP正向调控mTORC1活性。然后根据分组加入胰岛素[31],观察到干扰表达后S6和4EBP1的磷酸化水平发生逆转,说明胰岛素此时已激活mTORC1活性。当mTORC1活性被恢复之后,通过一系列功能学实验显示干扰所致VSMCs增殖和迁移的抑制效应被削弱,由此我们证明CIRBP可能通过mTORC1途径发挥促进小鼠VSMCs增殖及迁移的作用。
我们通过建立小鼠血管再狭窄的模型进一步探究CIRBP在血管内皮损伤后内膜增生中的作用。实验结果显示损伤组CIRBP蛋白表达高于假手术组,提示血管内皮损伤后CIRBP表达量显著增加,说明其在血管内膜增生中可能发挥某种调控作用。用腺病毒转染小鼠干扰CIRBP表达后,CIRBP表达降低后血管内膜增生及血管腔狭窄程度减轻,提示CIRBP促进小鼠血管内皮损伤后内膜增生。
综合以上实验证明,CIRBP可能通过激活mTORC1途径加强小鼠VSMCs增殖和迁移,加重血管内皮损伤后血管再狭窄,为防治PCI术后血管再狭窄及改善经皮冠脉介入治疗的预后提供了参考资料,但对于分子机制还需深入研究。
[1] Zhao D, Liu J, Wang M, et al. Epidemiology of cardiovascular disease in China: current features and implications[J]. Nat Rev Cardiol, 2019, 16(4):203-212.
[2] Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals[J]. Nat Rev Cardiol, 2018, 15(4):230-240.
[3] Pashova A, Work LM, Nicklin SA. The role of extracellular vesicles in neointima formation post vascular injury[J]. Cell Signal, 2020, 76:109783.
[4] Wang D, Uhrin P, Mocan A, et al. Vascular smooth muscle cell proliferation as a therapeutic target. Part 1: molecular targets and pathways[J]. Biotechnol Adv, 2018, 36(6):1586-1607.
[5] Pleva L, Kukla P, Hlinomaz O. Treatment of coronary in-stent restenosis: a systematic review[J]. J Geriatr Cardiol, 2018, 15(2):173-184.
[6] Al-Fageeh MB, Smales CM. Cold-inducible RNA binding protein (CIRP) expression is modulated by alternative mRNAs[J]. RNA, 2009, 15(6):1164-1176.
[7]刘和宇, 夏伟. 冷诱导RNA结合蛋白的生物学功能与信号通路[J]. 中国生物化学与分子生物学报, 2018, 34(7):697-705.
Liu HY, Xia W. Biological function and signal pathway of CIRBP[J]. Chin J Biochem Mol Biol, 2018, 34(7):697-705.
[8] Zhang Y, Wu Y, Mao P, et al. Cold-inducible RNA-binding protein CIRP/hnRNP A18 regulates telomerase activity in a temperature-dependent manner[J]. Nucleic Acids Res, 2016, 44(2):761-775.
[9] Zhang H, Xue J, Zhang Z, et al. Cold-inducible RNA-binding protein inhibits neuron apoptosis through the suppression of mitochondrial apoptosis[J]. Brain Res, 2015, 1622:474-483.
[10] Yang W, Sharma A, Wang Z, et al. Cold-inducible RNA-binding protein causes endothelial dysfunction via activation of Nlrp3 inflammasome[J]. Sci Rep, 2016, 6:26571
[11] Long T, Jing R, Kuang F, et al. CIRBP protects H9C2 cells against myocardial ischemia through inhibition of NF-κB pathway[J]. Braz J Med Biol Res, 2017, 50(4):e5861.
[12] Liu Y, Xing J, Li Y, et al. Chronic hypoxia-induced Cirbp hypermethylation attenuates hypothermic cardioprotection via down-regulation of ubiquinone biosynthesis[J]. Sci Transl Med, 2019, 11(489):eaat8406
[13] Liu C, Cheng X, Xing J, et al. CIRBP-OGFR axis safeguards against cardiomyocyte apoptosis and cardiotoxicity induced by chemotherapy[J]. Int J Bio. Sci, 2022, 18(7):2882-2897.
[14] Qiang X, Yang W, Wu R, et al. Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis[J]. Nat Med, 2013, 19(11):1489-1495.
[15] Zhang D, Cao Y, Liu D, et al. The etiology and molecular mechanism underlying smooth muscle phenotype switching in intimal hyperplasia of vein graft and the regulatory role of microRNAs[J]. Front Cardiovasc Med, 2022, 9:935054.
[16] Guo X, Wu Y, Hartley RS. Cold-inducible RNA-binding protein contributes to human antigen R and cyclin E1 deregulation in breast cancer[J]. Mol Carcinog, 2010, 49(2):130-140.
[17] Chang ET, Palak PR, Yang Q, et al.Heterogenous ribonucleoprotein A18 (hnRNP A18) promotes tumor growth by increasing protein translation of selected transcripts in cancer cells[J]. Oncotarget, 2016, 7(9):10578-10593.
[18] Jian F, Chen Y, Ning G, et al. Cold inducible RNA binding protein upregulation in pituitary corticotroph adenoma induces corticotroph cell proliferation via Erk signaling pathway[J]. Oncotarget, 2016, 7(8):9175-9187.
[19] Chen X, Xie H, Wang X, et al. CIRBP knockdown attenuates tumourigenesis and improves the chemosensitivity of pancreatic cancer via the downregulation of DYRK1B[J]. Front Cell Dev Biol, 2021, 9:667551.
[20] Zhou M, Yang W, Ji Y, et al. Cold-inducible RNA-binding protein mediates neuroinflammation in cerebral ischemia[J]. Biochim Biophys Acta Gen Subj, 2014, 1840(7):2253-2261.
[21] Sun X, Li S, Gan X, et al. NF2 deficiency accelerates neointima hyperplasia following vascular injury via promoting YAP-TEAD1 interaction in vascular smooth muscle cells[J]. Aging (Albany NY), 2020, 12(10):9726-9744.
[22] Hirai T, Grantham JA. Indications and patient selection for percutaneous coronary intervention of chronic total occlusions[J]. Interv Cardiol Clin, 2021, 10(1):1-5.
[23] Nicolais C, Lakhter V, Virk HUH, et al. Therapeutic options for in-stent restenosis[J]. Curr Cardiol Rep, 2018, 20(2):7.
[24] Sun X, Zhao W, Wang Q, et al. Inhibition of VRK1 suppresses proliferation and migration of vascular smooth muscle cells and intima hyperplasia after injury via mTORC1/β-catenin axis[J]. BMB Rep, 2022, 55(5):244-249.
[25] Morf J, Rey G, Schneider K, et al. Cold-inducible RNA-binding protein modulates circadian gene expression posttranscriptionally[J]. Science, 2012, 338(6105):379-383.
[26] Khan MM, Yang W, Brenner M, et al. Cold-inducible RNA-binding protein (CIRP) causes sepsis-associated acute lung injury via induction of endoplasmic reticulum stress[J]. Sci Rep, 2017, 7(1):41363.
[27] Chen M, Fu H, Zhang J, et al. CIRP downregulation renders cardiac cells prone to apoptosis in heart failure[J]. Biochem Biophys Res Commun, 2019, 517(4):545-550.
[28] Wei W, Li X, Xu M. Inhibition of vascular neointima hyperplasia by FGF21 associated with FGFR1/Syk/NLRP3 inflammasome pathway in diabetic mice[J]. Atherosclerosis, 2019, 289:132-142.
[29] Liu G, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease[J]. Nat Rev Mol Cell Biol, 2020, 21(4):183-203.
[30] Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease[J]. Cell, 2017, 169(2):361-371.
[31] Yin S, Liu L, Gan W. The roles of post-translational modifications on mTOR signaling[J]. Int J Mol Sci, 2021, 22(4):1784.
Regulatory effect of cold-inducible RNA-binding protein on proliferation and migration of mouse vascular smooth muscle cells and its mechanism
ZHAO Jiaqi1, YANG Dachun2, WANG Qiang2, SUN Xiongshan2△, YANG Yongjian1,2△
(1,,646000,;2,,610083,)
To investigate the regulatory effect of cold-inducible RNA binding protein (CIRBP) on the proliferation and migration of mouse vascular smooth muscle cells (VSMCs), and to explore its mechanism.Male C57BL/6J mice were divided into 4 groups: sham+adenovirus-negative control (AD-NC) group, sham+adenovirus-inhibitor (AD-I) group, carotid vascular injury+AD-NC group, and carotid vascular injury+AD-I group, with 5 mice in each group. After the injury model was established, the mice were transfected with AD-NC or AD-I. The CIRBP expression and vascular intimal hyperplasia were measured at 28 d after injury. Mouse VSMCs were divided into AD-NC and AD-I group. The cells of these two groups were transfected with AD-NC and AD-I for 48 h, respectively. The activity of mammalian target of rapamycin complex 1 (mTORC1) was restored by insulin. The mRNA expression of CIRBP was explored by RT-qPCR. The levels of CIRBP, phosphorylated ribosomal protein S6 (p-S6Ser235/236) and phosphorylated 4E-binding protein 1 (p-4EBP1Thr37/46) were detected by Western blot. The cell proliferation was explored by Ki67 immunofluorescence staining and CCK-8 assay. The cell migration was determined by scratch test. The carotid intimal hyperplasia was determined by HE staining.The viability of VSMCs, the ratio of Ki67-positive cells, wound-healing rate and the activity of mTORC1 were decreased after silencing of. However, the inhibitory effects ofsilencingon cell viability, ratio of Ki67-positive cells and wound-healing rate were attenuated after restoring the activity of mTORC1 by insulin. The expression of CIRBP was increased after mouse common carotid vascular injury. The vascular intimal hyperplasia was attenuated after interfering with the expression of.CIRBP promotes the proliferation and migration of mouse VSMCs, and vascular intimal hyperplasia after mouse common carotid vascular injury through the mTORC1 pathway.
vascular smooth muscle cells; cell proliferation; cell migration; cold-inducible RNA-binding protein; mammalian target of rapamycin complex 1
R543.5; R363.2+1
A
10.3969/j.issn.1000-4718.2023.02.002
1000-4718(2023)02-0204-08
2022-09-16
2022-11-04
[基金项目]国家自然科学基金资助项目(No.82070289);四川省自然科学基金资助项目(No.2022NSFSC0820)
杨永健 Tel: 028-86571250; E-mail: yyj10001@126.com; 孙雄山 Tel: 028-86571255; E-mail: shan19910927@sina.com
(责任编辑:宋延君,李淑媛)
