针刺调控CIRI大鼠缺血侧海马组织差异表达circRNAs的功能研究*
2023-03-10江姗姗唐红汪红娟吕倩忆谢灿明王瑶陈楚淘田浩梅
江姗姗, 唐红, 汪红娟, 吕倩忆, 谢灿明, 王瑶, 陈楚淘, 田浩梅△
针刺调控CIRI大鼠缺血侧海马组织差异表达circRNAs的功能研究*
江姗姗1, 唐红1, 汪红娟1, 吕倩忆2, 谢灿明1, 王瑶1, 陈楚淘1, 田浩梅1△
(1湖南中医药大学针灸推拿与康复学院,湖南 长沙 410208;2成都市第二人民医院,四川 成都 610021)
探讨针刺对脑缺血再灌注损伤(CIRI)大鼠脑组织的保护作用,观察针刺对CIRI大鼠缺血侧海马组织环状RNAs(circRNAs)差异表达的影响,并对其进行基因本体(GO)分析。6~8周龄SD大鼠54只,运用随机数字法随机分为造模组和假手术组(sham组),造模成功后再随机分为模型组(model组)和针刺组(AC组),每组18只。采用改良Longa线栓法制备大脑中动脉闭塞再灌注(MCAO/R)模型,激光散斑成像仪监测造模前、MCAO手术后及再灌注后脑血流量,假手术组只剥离血管,不插入线栓;干预期间模型组和假手术组只捆绑不针刺,针刺组捆绑+针刺。采用改良加西亚(Garcia)评分法对神经功能进行评定,TTC染色法检测脑梗死面积,Western blot法检测神经元核抗原(NeuN)的表达,尼氏染色法观察缺血侧海马组织神经元损伤程度,基因芯片微阵列分析筛选出缺血侧海马组织差异表达的circRNAs,并对模型组/假手术组、针刺组/模型组共同差异表达circRNAs的来源基因进行GO分析。与假手术组比较,模型组大鼠脑梗死面积比显著升高(<0.01),Garcia神经功能评分、NeuN表达量和海马CA1区尼氏染色阳性细胞数显著降低(<0.05或<0.01);与模型组比较,针刺组脑梗死面积比显著降低(<0.01),神经功能评分、NeuN表达量和尼氏染色阳性细胞数显著升高(<0.05或<0.01)。芯片筛选结果显示,与假手术组比较,模型组上调的circRNAs个数为288,下调个数为315;与模型组比较,针刺组上调的差异表达circRNAs个数为33,下调个数为18(FC>1.25,<0.05);其中模型组/假手术组、针刺组/模型组共同差异表达的circRNAs个数为23个;GO分析显示共同差异表达circRNAs的来源基因功能涉及神经系统发育,神经元的产生、发育、分化及投射,头部、大脑及海马的发育,突触的形成、发育、延伸及运输等。CIRI大鼠缺血侧海马组织circRNAs在造模后及针刺干预后均存在差异表达。针刺能显著改善CIRI大鼠的神经功能和脑梗死面积,减轻海马组织神经元损伤,其机制可能与针刺调控缺血侧海马组织多种circRNAs的差异表达及激发其促进神经元发育分化、抗神经损伤等功能有关。
针刺;脑缺血再灌注损伤;神经元损伤;环状RNA;差异表达
脑缺血再灌注损伤(cerebral ischemia reperfusion injury, CIRI)是指脑组织经历一段时间缺血后,恢复缺血区的血液灌注,但在某些情况下缺血后恢复血流并不能恢复组织功能,反而使组织损伤及功能障碍更加严重[1],这种情况可诱发兴奋毒性、氧化应激损伤、炎症反应等而发生一系列的神经细胞中毒事件,而加重脑组织损伤[2-3]。因此进一步探究与脑缺血再灌注损伤有关的病理机制,找到有效的靶向治疗方法是治疗脑缺血再灌注损伤的关键。
环状RNA(circularRNA, circRNA)是一类内源性非编码RNA,其通过特殊的剪接机制形成共价键结合的闭合环状结构,circRNA在真核生物中广泛表达,具有高度保守性、组织特异性和时空特异性,因其特殊的闭合环状结构,没有游离的尾部启动降解,circRNA对核酸外切酶具有抗性,不易被核酸外切酶降解,较线性RNA稳定[4-5]。Mehta等[6]报道了局灶性脑缺血后小鼠脑内circRNAs表达谱的分析结果显示,这些circRNAs涉及细胞通讯,生物调节,代谢过程以及与蛋白质等结合的生物和分子功能,参与并影响包括细胞凋亡和自噬,炎症,内质网应激,氧化应激和线粒体功能障碍等多种脑卒中后病理生理过程。这提示干预circRNAs的表达有可能成为脑缺血再灌注损伤的有效治疗手段。
前期研究证实,CIRI后,针刺大椎、百会和水沟穴对缺血再灌注损伤脑组织具有一定的保护作用[7-8],且针刺调控circRNA在其它疾病中的研究已有相关报道[9],因此本研究以circRNA为切入点,通过观察针刺大椎、百会和水沟穴对CIRI大鼠神经功能评分,脑梗死面积比、神经元发育标记物神经元核抗原(neuron nuclear antigen, NeuN)表达量、缺血侧海马组织神经元尼氏染色阳性细胞数、缺血侧海马组织circRNAs差异表达谱的影响,以及针刺干预后共同差异表达circRNAs功能的分析,探讨针刺对CIRI大鼠脑组织的保护作用与差异表达circRNAs功能之间存在的联系,为探究环状RNA介导针刺促CIRI修复机制、进一步寻找到治疗CIRI的靶点提供参考资料。
材料和方法
1 实验动物及分组
6~8周龄SPF级雄性SD大鼠54只,体重为220~250 g,由湖南中医药大学动物实验中心提供,动物生产合格证号为SCXK(湘)2019-0004,饲养于湖南中医药大学SPF级动物房。室温为:24~26 ℃,湿度为:40%~60%,光照12 h/d,自由摄食饮水。适应性饲养1周后,采用随机数字法,随机取18只大鼠作为假手术(sham)组,其余大鼠进行造模,造模后将模型成功的大鼠随机分为模型(model)组和针刺(AC)组,每组18只。本实验过程中对动物的处置符合《关于善待实验动物的指导性意见》,本实验研究通过湖南中医药大学实验动物福利和伦理委员会审查(批准编号:LL2021081203)。
2 主要试剂及器械
TTC染液(索莱宝生物科技有限公司);水合氯醛(中国上海阿拉丁生物试剂有限公司);注射用青霉素钠(中国华北医药股份有限公司);多聚甲醛(长沙维尔生物科技有限公司);PBS(索莱宝生物科技有限公司);抗体(艾方生物科技有限公司);二甲苯(国药集团化学试剂有限公司);甲苯胺蓝染液(武汉塞维尔生物科技有限公司)。
MCAO线栓(北京西浓科技有限公司);针灸针(北京中研太和医疗器械有限公司);HHS-2型恒温水浴锅(上海南阳仪器有限公司);circRNA芯片及芯片标记试剂盒(Arraystar);安捷伦G2505C扫描仪(Agilent);包埋机(武汉俊杰电子有限公司);匀浆仪(康涛科技有限公司);病理切片机(上海徕卡仪器有限公司)。
3 主要方法
3.1造模方法及脑缺血再灌注损伤模型的建立造模方法采用改良的从颈总动脉插线栓的Longa线栓法[10]制备大脑中动脉闭塞(middle cerebral artery occlusion, MCAO)模型,造模前大鼠禁食不禁水24 h,用10%的水合氯醛按3 mL/kg体重剂量进行腹腔麻醉。取仰卧位将大鼠固定在鼠板上,备皮消毒后,选择右侧距前正中线3 mm处,作长约10 mm纵行的切口,钝性剥离皮下筋膜等组织,暴露颈总动脉(common carotid artery,CCA)及分支颈外动脉(external carotid artery,ECA)、颈内动脉(internal carotid artery,ICA),钝性剥离CCA、ECA、ICA及迷走神经。分别在CCA、ECA、ICA处挂线,于近心端结扎CCA、ECA,然后用微动脉夹暂时夹闭ICA。在距CCA分支形成ICA、ECA分叉部3 mm处剪一小口,将线栓由CCA插入至ICA,当遇到动脉夹阻挡后松开动脉夹,再迅速向内插入(手法宜轻柔,避免戳破血管),进线长度约距颈总动脉分叉处19 mm时,线栓有轻微阻力,结扎ICA远心端的细线以防止线栓脱出和出血。缝合伤口并标记线栓外端,予以青霉素抗炎。2 h后,将拴线拔出10 mm左右[11]。假手术组只分离血管,不插入线栓。造模后待生命体征平稳,参照Longa[10]五分制评分法进行评分,造模组评分在1~3分的大鼠纳入后续实验。
为评估大鼠脑缺血再灌注损伤模型的有效性,在造模前、MCAO手术后30 min以及再灌注后30 min分别实时监测大鼠右侧半球脑血流量(cerebral blood flow, CBF)。在麻醉后将大鼠固定于脑立体定位仪上,备皮、消毒后剪开头皮,充分暴露颅骨,分离骨膜,用生理盐水保持表面湿润,用棉签清除颅骨表面杂质,用颅骨钻将右侧颅骨磨薄至清晰可见颅骨下血管,随后用激光散斑血流成像系统扫描大鼠右侧半球CBF。图像显示在MCAO术后,右侧半球脑血流量显著降低,再灌注后,脑血流量得到恢复但不及造模前(见图1),这提示脑缺血再灌注损伤模型的建立。
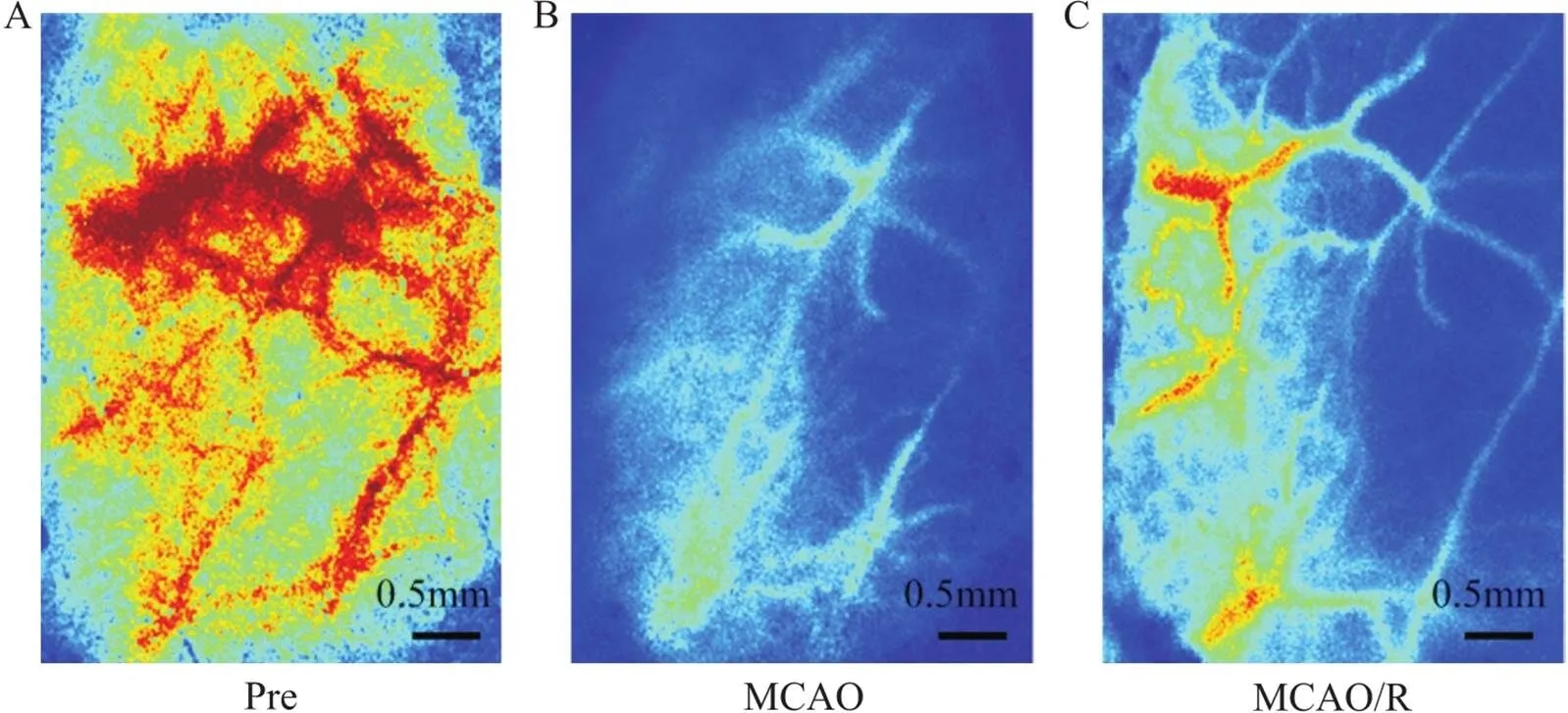
Figure 1. Establishment of middle cerebral artery occlusion/reperfusion (MCAO/R) rat model. The scale bar=0.5 mm. A:laser speckle imager showed the cerebral blood flow in the right cerebral hemisphere is full before modeling; B:after MCAO, the cerebral blood flow decreased significantly; C:after reperfusion, the cerebral blood flow recovered.
3.2干预方法实验动物造模完成,待大鼠呼吸、心跳等生命体征稳定,在造模后2 h进行干预,各组处理如下:假手术组和模型组:只捆绑30 min,不针刺,每12 h一次,共7次;针刺组:针刺大椎、水沟、百会穴(取穴参照2007年中国中医药出版社出版的《实验针灸学》[12]动物穴位图谱及拟人比照法定位。大椎:第7颈椎与第1胸椎间,直刺5 mm;百会:顶骨正中,平刺2 mm;水沟:唇裂鼻尖下1 mm正中处,向鼻中隔方向斜刺2 mm),留针30 min(期间行捻转手法),每12 h一次,共7次。
3.3取材方法干预期结束,所有大鼠完成改良Garcia评分[13]后,经10%的水合氯醛腹腔麻醉后迅速断头取脑。取全脑组织:将全脑组织至于一次性密封袋放入-20 ℃冰箱内冷冻10 min,于冰上去除嗅球、小脑和低位脑干,将脑组织平均切5个冠状脑切片,每片厚约2 mm,每组5只用于TTC染色;生理盐水冲洗后,用4%多聚甲醛固定全脑组织24 h以上,每组5只用于尼氏染色。取海马组织:于冰上迅速分离缺血侧海马组织,将组织置于冻存管内迅速投入液氮中速冻,后转入-80 ℃冰箱保存,每组3只用于基因芯片检测、每组5只用于Western blot检测。
3.4改良Garcia评分法评估神经功能损伤程度在CIRI后、干预72 h后两个时点进行评分;主要从以下6个方面进行判断:大鼠的自主运动,前肢伸展运动功能,体态对称性,攀爬运动,身体双侧的触觉,双侧胡须触碰反应。总分为18分,分数区间为3~18分,得分越低,表示神经功能损伤越严重,18分为无神经功能缺损。
3.5TTC染色法检测大鼠脑梗死面积将切片浸没在37 ℃、2% TTC染液中10 min,隔5 min翻一次面,使切片染色均匀;在染色结束后,用4%多聚甲醛固定24 h后用相机拍照,用ImageJ软件测量脑梗死面积,参考Swanson公式[14]算出脑梗死面积百分比。
3.6Western blot法检测神经元特异性核蛋白NeuN的表达每组取5只-80 ℃冰箱冻存的海马组织10 mg,步骤如下:(1)组织中加入蛋白抽提液,匀浆及冰浴30 s,组织裂解后12 000×离心15 min,上清转移到新管;(2)参照BCA蛋白定量步骤绘制标准曲线,计算目的蛋白浓度(mg/L);(3)制胶、灌胶,插下齿梳,分别凝胶聚合1 h;(4)拔去齿梳,倒入电泳液,上样,使用BioRad电泳装置,4 ℃恒压80 V电泳至染料靠近分离胶顶部,再改用120 V至溴酚蓝到胶底部;(5)将吸水纸、滤纸、PVDF膜装配于转移装置上,在200 mA恒流下4 ℃转膜1.5 h,后将膜浸入5% BSA溶液中封闭2 h;(6)加Ⅰ抗4 ℃过夜,Ⅱ抗孵育1 h,分别TBST洗膜6次×5 min;(7)发光混合液滴注在膜上,室温孵育2 min,CCD相机曝光,以ImageJ软件测量出各条带的灰度值,以目的蛋白与内参GAPDH的比值作为目的蛋白的相对表达量。
3.7尼氏染色法观察缺血侧海马组织神经元损伤程度取出4%多聚甲醛固定的全脑组织,每组5只,石蜡切片脱蜡至水后,组织切片入染液5 min,水洗,1%的冰醋酸稍分化,自来水洗终止反应,显微镜下控制分化程度,自来水洗后,将切片置于烤箱烤干,切片入干净的二甲苯透明5 min,中性树胶透明封片。使用CaseViewer成像系统扫描图像,在成像系统500 μm标尺下观察完整海马结构,50 μm标尺下观察海马CA1区神经元存活情况,每组每张切片随机采集3张50 μm标尺下海马CA1区图片,应用ImageJ软件对缺血侧海马CA1区尼氏染色阳性细胞进行计数。
3.8基因芯片技术筛选差异表达基因及基因本体(gene ontology,GO)功能富集分析每组随机抽取3只大鼠冻存海马组织,用NanoDrop nd-1000检测样品总RNA的纯度和浓度,用RNase R处理每个样本的总RNA去除线性RNA以富集circRNA,然后根据Arraystar超级RNA标记试剂盒,利用随机引物法扩增富集的circRNA,并将其转录为荧光cRNA。将标记的cRNA杂交到大鼠circRNA阵列(8×15k,Arraystar),在安捷伦杂交箱中65 ℃孵育17 h。洗涤后,用安捷伦G2505C扫描仪扫描切片,用Feature Extraction软件提取芯片数据,用GeneSpring软件进行数据分析,按照差异倍数(fold change, FC)>1.25、<0.05的筛选标准,寻找假手术组、模型组、针刺组间差异表达的circRNAs,并对差异表达circRNAs的来源基因进行GO功能富集分析。
4 统计学处理
本实验采用完全随机设计,所有数据为计量资料,用SPSS 25.0软件进行统计学分析,组内干预前后比较:差值符合正态分布使用配对检验;不符合则使用配对秩和检验。各组间比较:所有数据进行正态性检验,满足正态性分布使用单因素方差分析,方差齐者用LSD检验,方差不齐者用Tamhane's T2检验,数据用均数±标准差(mean±SD)表示;不符合正态性分布则使用非参数检验,以中位数与四分位数间距[median ()]表示。以0.05为差异有统计学意义。
结果
1 实验大鼠死亡率
本实验共纳入大鼠65只,未达到纳入标准大鼠2只,死亡大鼠9只,假手术组无大鼠死亡,死亡率为0;模型组死亡大鼠4只,死亡率为18.1%;针刺组死亡大鼠5只,死亡率为21.7%;最终各组以18只大鼠纳入统计。
2 改良Garcia评分观察各组大鼠神经功能状况
造模后,对所有大鼠进行神经功能评分,与假手术组比较,模型组和针刺组Garcia神经功能评分显著降低(<0.01);72 h干预后,与假手术组比较,模型组评分显著降低(<0.01);与模型组比较,针刺组评分显著升高(<0.01);与干预前比较,干预后针刺组评分显著升高(<0.01),见图2。
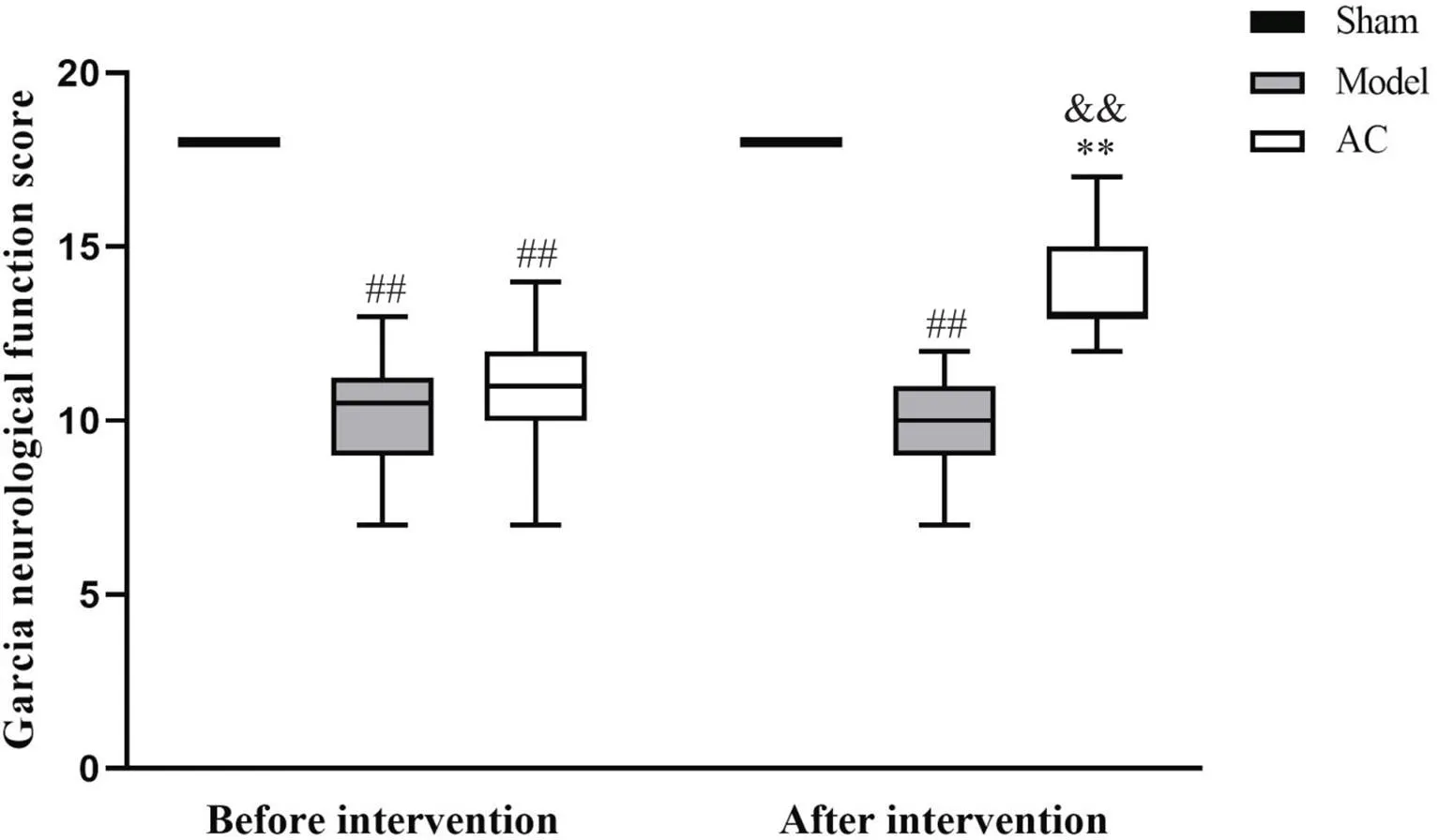
Figure 2. Garcia scoring was used to observe the neurological function of the rats in different groups. Median (Q). n=18. ##P<0.01 vs sham group; **P<0.01 vs model group; &&P<0.01 vs acupuncture (AC) groupbefore intervention.
3 TTC染色观察各组大鼠脑梗死面积比
假手术组大鼠脑组织未见显著梗死灶;与假手术组相比,模型组和针刺组脑梗死面积百分比均显著升高(<0.01);与模型组相比,针刺组梗死面积比显著降低(<0.01),见图3。
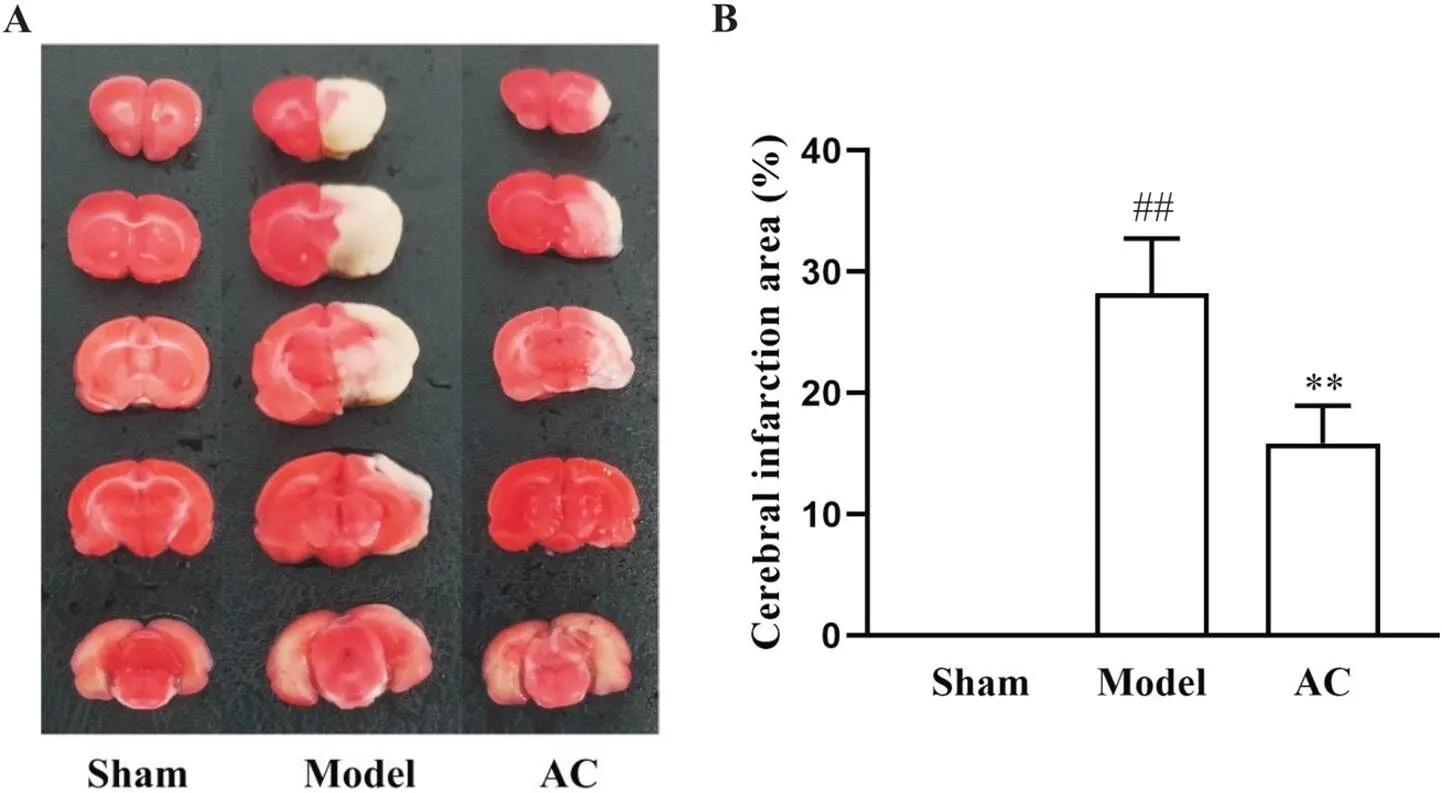
Figure 3. TTC staining was used to observe the cerebral infarction area changes of the rats in different groups. A: TTC staining picture; B: percentage of cerebral infarction area. Mean±SD. n=5. ##P<0.01 vs sham group; **P<0.01 vs model group.
4 Western blot观察各组大鼠缺血侧海马组织NeuN蛋白表达水平
与假手术组比较,模型组大鼠缺血侧海马组织NeuN表达量显著降低(<0.01);与模型组比较,针刺组NeuN表达量显著上调(<0.05),见图4。
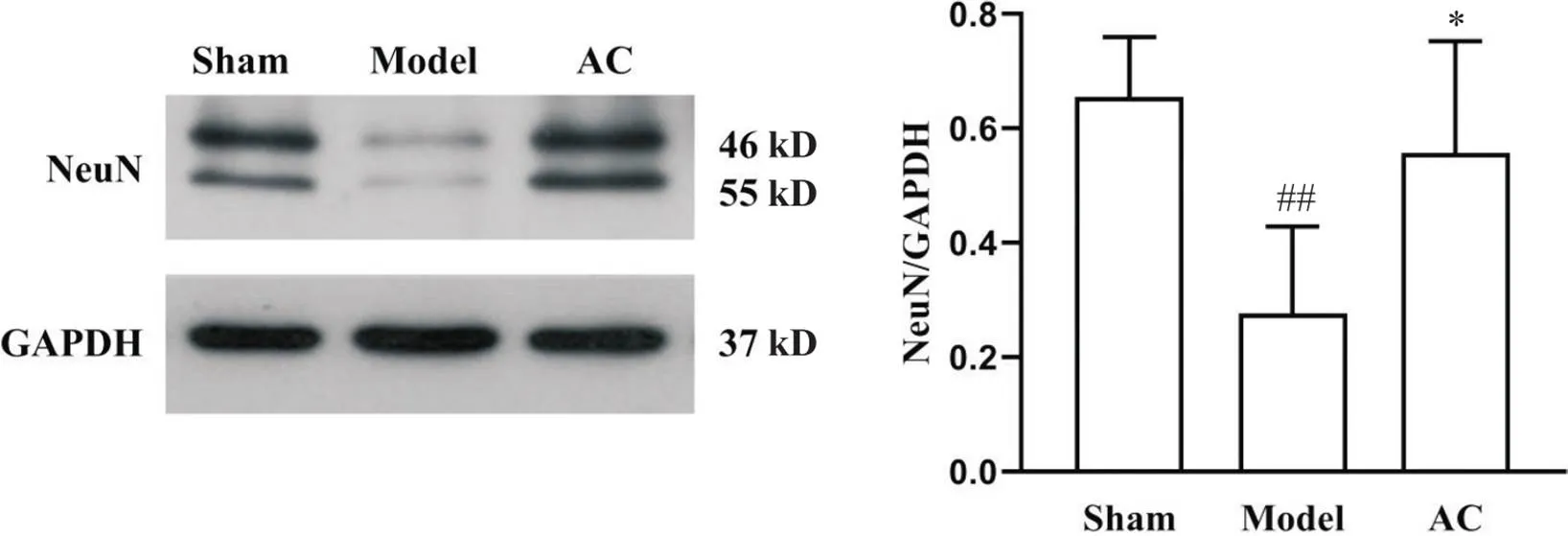
Figure 4. Western blot was used to observe the protein level of NeuN in hippocampal tissues on ischemic side of the rats in different groups. Mean±SD. n=5. ##P<0.01 vs sham group; *P<0.05 vs model group.
5 尼氏染色观察各组大鼠缺血侧海马CA1区神经元
大鼠海马神经元尼氏染色结果显示,假手术组细胞结构完整、密度大,排列整齐且紧密,胞体饱满,胞浆均匀着色,高倍镜下细胞核核仁显著,尼氏体染色较深,数量较多;模型组大部分细胞结构不完整,呈空泡状改变,尼氏体溶解甚至消失;针刺组细胞结构较为完整,偶有空泡状改变,神经元损伤较模型组降低,见图5A。与假手术组比较,模型组缺血侧海马CA1区神经元尼氏染色阳性细胞数显著降低(<0.05);与模型组比较,针刺组神经元尼氏染色阳性细胞数显著升高(<0.05),见图5B。
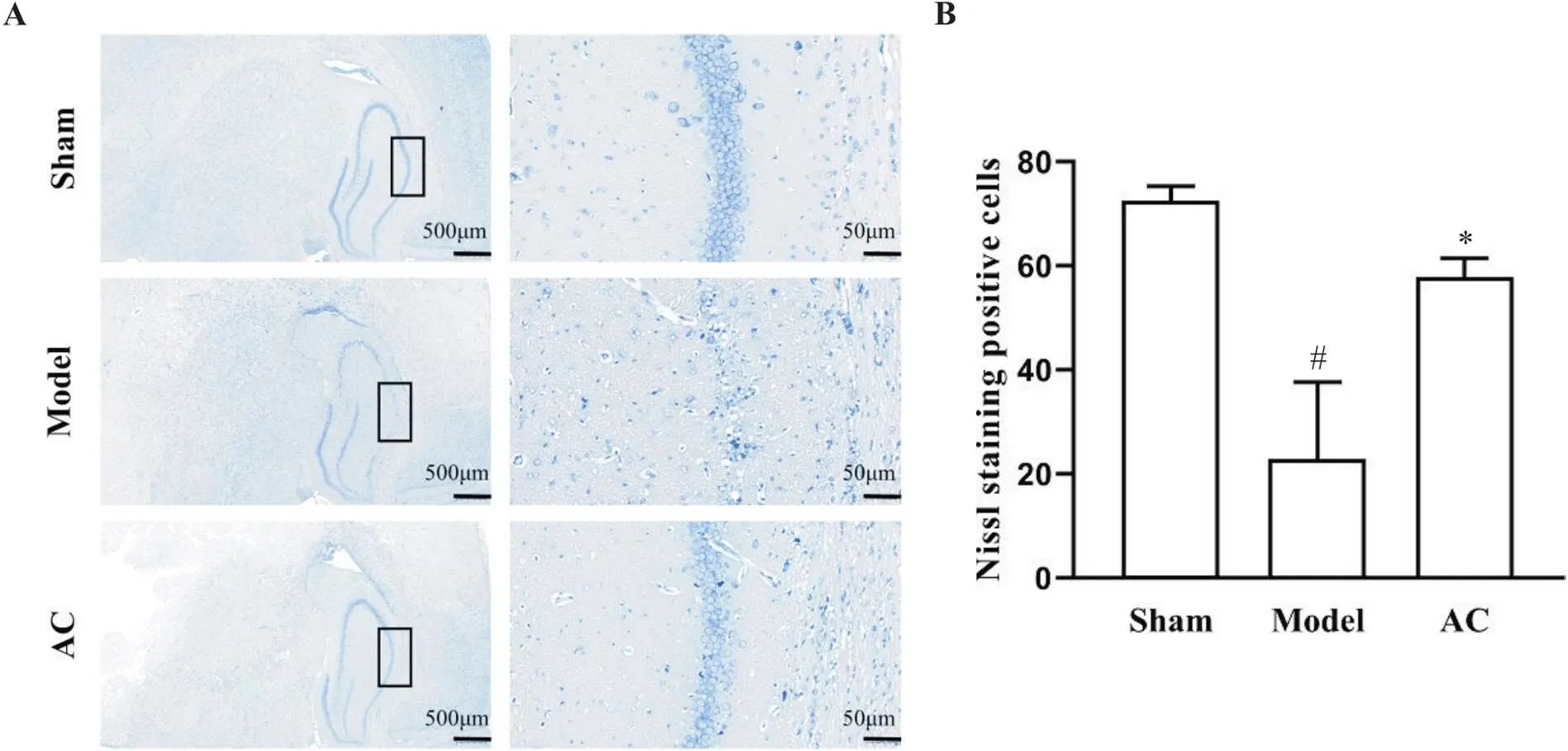
Figure 5. Nissl staining was used to observe neurons in hippocampal CA1 area on ischemic side of the rats in different groups. A: the left picture showed Nissl staining results of the whole hippocampus of the right hemisphere of the brain (scale bar=500 μm), while the right picture is the enlarged result of hippocampal CA1 area in the left picture (scale bar=50 μm); B: Nissl staining positive cells. Mean±SD. n=5. #P<0.05 vs sham group; *P<0.05 vs model group.
6 基因芯片检测
6.1大鼠缺血侧海马组织circRNAs差异表达谱差异circRNAs火山图见图6A、6B,垂直线分别对应上下差异倍数1.25倍,水平线代表0.05的值,因此,图中的红点代表具有统计意义的差异表达的circRNAs。聚类分析图见图6C、6D,横列为差异表达circRNAs,纵列为样本名称,红色荧光为高表达,绿色为低表达。
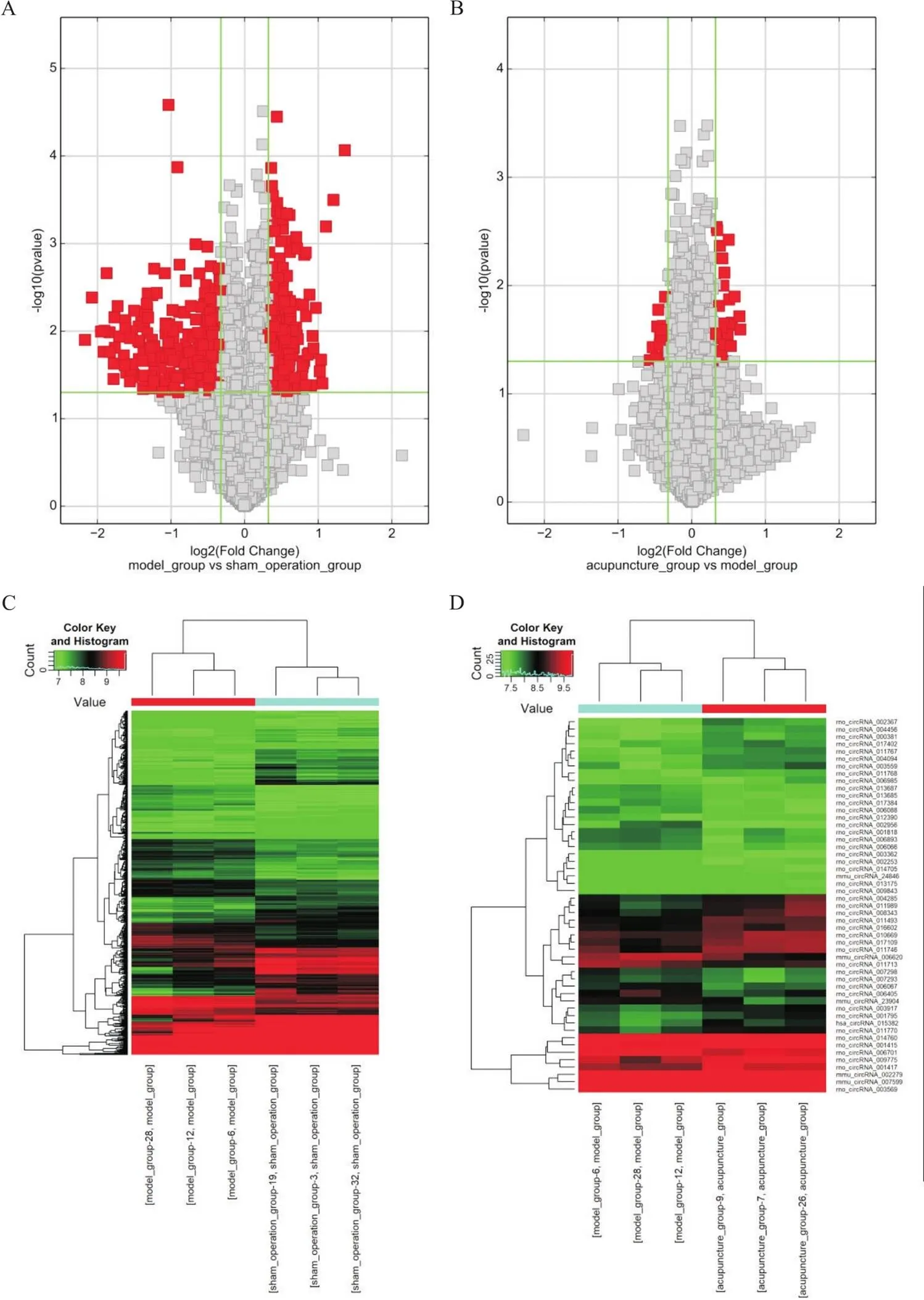
Figure 6. Differential expression profile of circRNAs. A and B: volcano plots. The vertical lines correspond to 1.25-fold up and down, and the horizontal line represents a P-value of 0.05. The red point in the plot represents the differentially expressed circRNAs with statistical significance. A: model group vs sham group; B: acupuncture group vs model group. C and D: heatmaps. The horizontal column is the differentially expressed circRNAs, the vertical column is the sample name, the red fluorescence is the high expression, and the green is the low expression. C: model group vs sham group; D: acupuncture group vs model group. n=3.
6.2大鼠缺血侧海马组织差异表达circRNAs数量与假手术组比较,模型组上调的差异表达circRNAs个数为288,下调个数为315;与模型组比较,针刺组上调的差异表达circRNAs个数为33,下调个数为18。其中造模后下调、针刺后上调的共同差异表达circRNAs为16个,分别是rno_circRNA_009775、rno_circRNA_011989、rno_circRNA_003569、mmu_circRNA_002279、rno_circRNA_008343、rno_circRNA_004285、rno_circRNA_011770、rno_circRNA_017109、rno_circRNA_001795、rno_circRNA_004094、rno_circRNA_011768、rno_circRNA_003559、rno_circRNA_006985、rno_circRNA_013175、rno_circRNA_003917、rno_circRNA_011767,见图7A,造模后上调、针刺后下调的共同差异表达circRNAs为7个,分别为mmu_circRNA_23904、mmu_circRNA_006620、rno_circRNA_006405、rno_circRNA_012390、rno_circRNA_006701、rno_circRNA_007293、rno_circRNA_006893,见图7B。
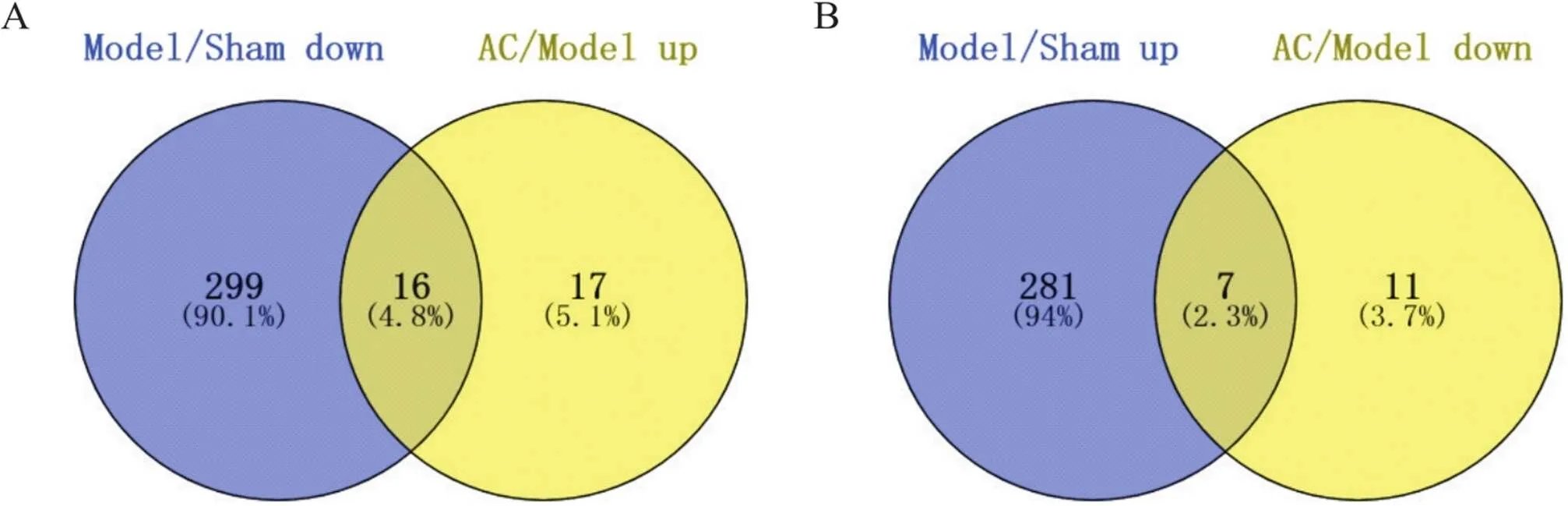
Figure 7. The numbers of common differentially expressed circRNAs. A: the blue part is the number of down-regulated circRNAs in model group compared with sham group, the yellow part is the number of up-regulated circRNAs in acupuncture (AC) group compared with model group, and the middle part is the numder of common differentially expressed circRNAs; B: the blue part is the number of up-regulated circRNAs in model group compared with sham group, the yellow part is the number of down-regulated circRNAs in AC group compared with model group, and the middle part is the number of common differentially expressed circRNAs. n=3.
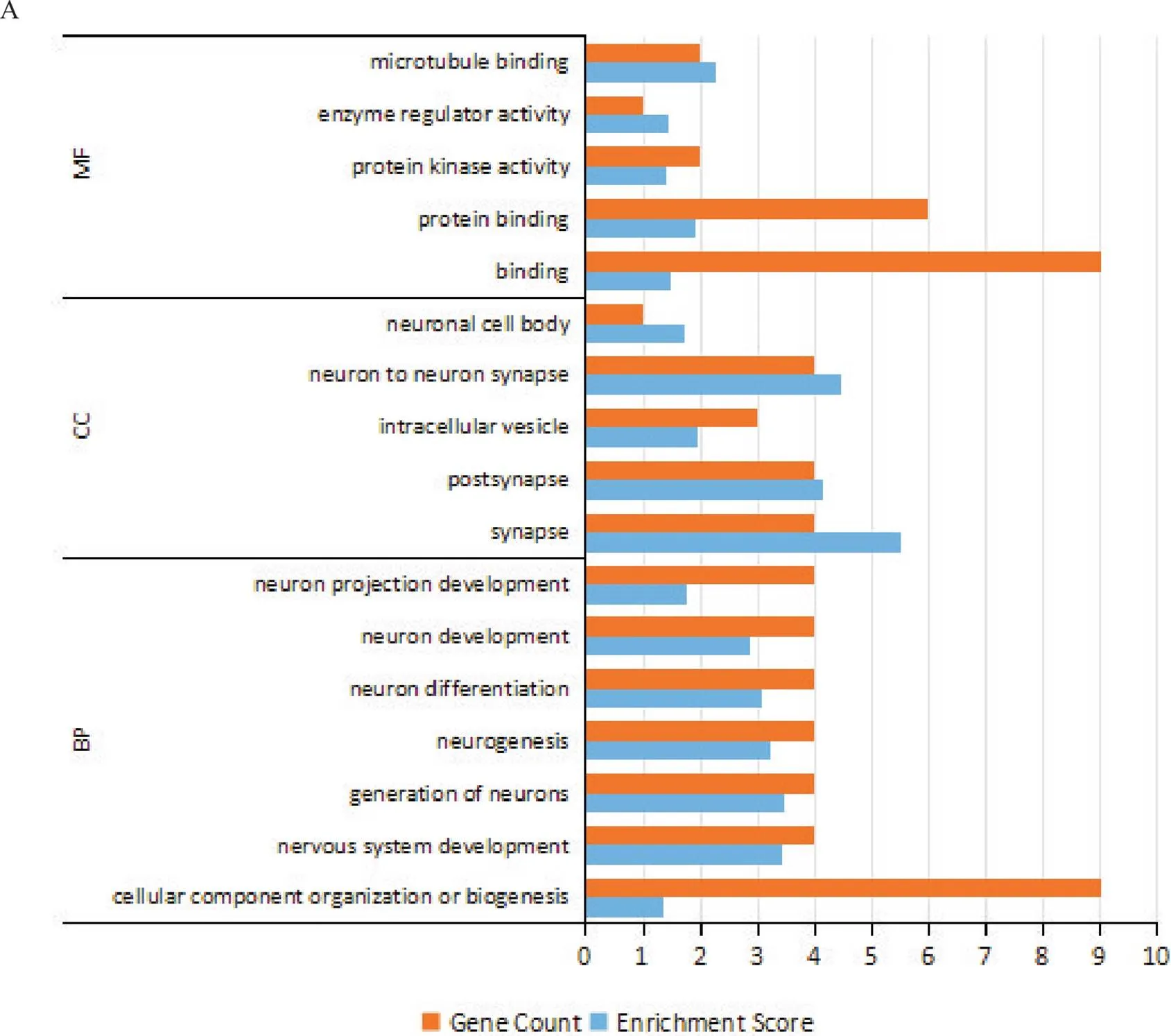
6.3针刺干预后共同差异表达circRNAs来源基因的GO功能富集分析通过对共同差异表达circRNAs来源基因进行的GO分析,可以初步探讨共同差异表达circRNAs在生物体中的潜在功能,GO分析包括生物进程(biological process, BP)、细胞成分(cellular component, CC)和分子功能(molecular function, MF)三个领域,根据来源基因的“基因计数”和GO条目的“富集分数”,针刺干预后上调的共同差异表达circRNAs其来源基因在BP方面,主要富集在神经系统发育、神经元的产生、神经元发育、神经元分化、神经元投射发育、神经发生和生物发生等进程;在CC方面,主要富集在突触、突触后膜、神经元间突触、细胞内囊泡和神经元细胞体等;在MF方面,主要富集在结合、蛋白质结合、蛋白激酶活性、酶调节活性和微管结合等,见图8A。下调的共同差异表达circRNAs其来源基因在BP方面,主要富集在大脑皮层发育、神经元投射发育、神经元分化、蛋白质代谢过程、生物过程的调节、基因表达的调控等进程;在CC方面,主要富集在细胞内膜结合细胞器、细胞质、细胞内囊泡、内质网、兴奋性突触等;在MF方面,主要富集在结合、转移酶活性、神经营养素结合、激酶结合、转运活性等,见图8B。
具体GO分析的GOID、GO条目、基因分布计数、富集分数等详见表1。
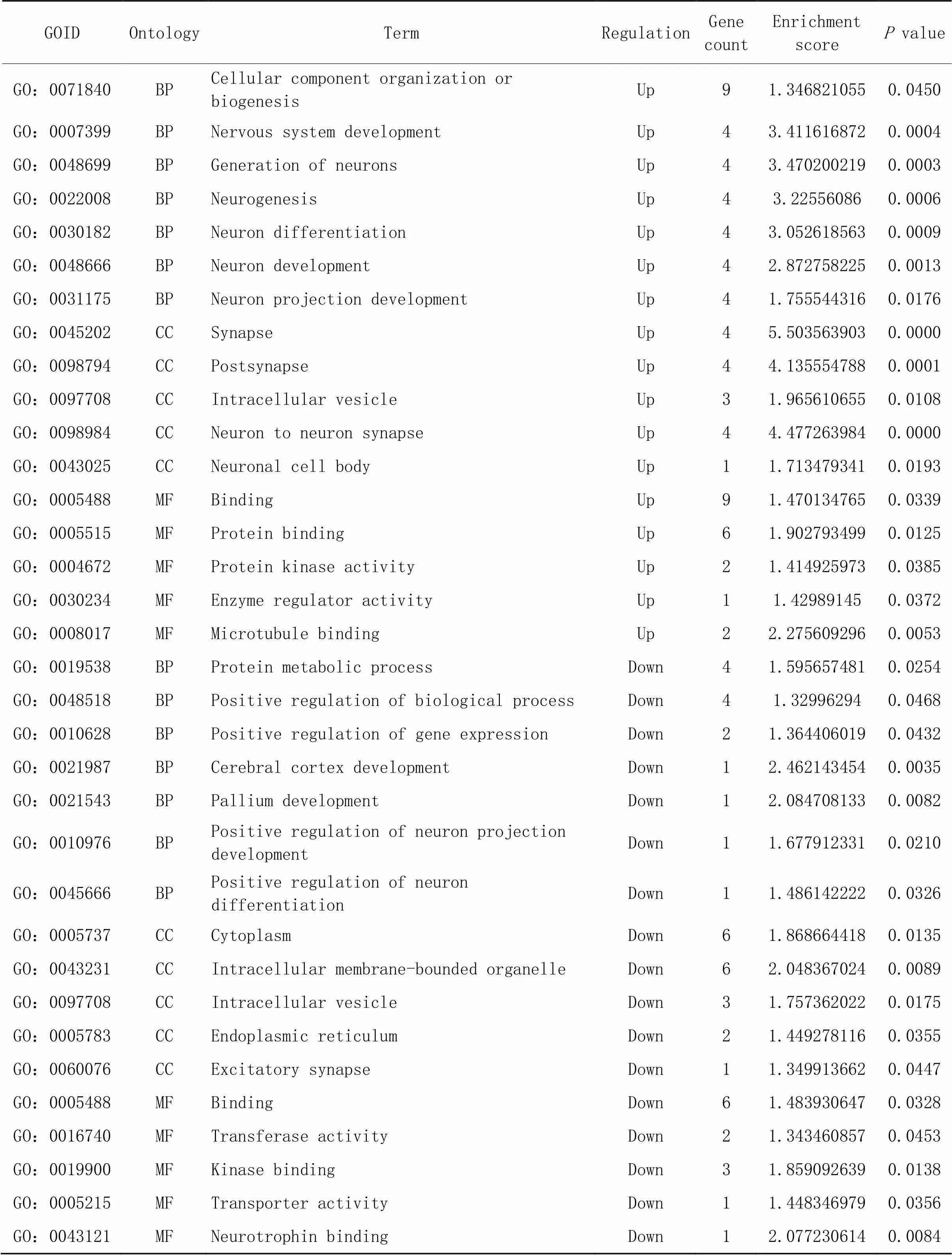
表1 针刺干预后共同差异表达circRNAs来源基因的GO分析
GOID代表GO条目的ID,对共同差异表达circRNAs来源基因富集的GOID数量统计见图9,其中富集GOID数量前三的基因分别为(118个)、(97个)和(84个),其对应的circRNA分别为:rno_circRNA_006893、rno_circRNA_009775和rno_circRNA_017109。
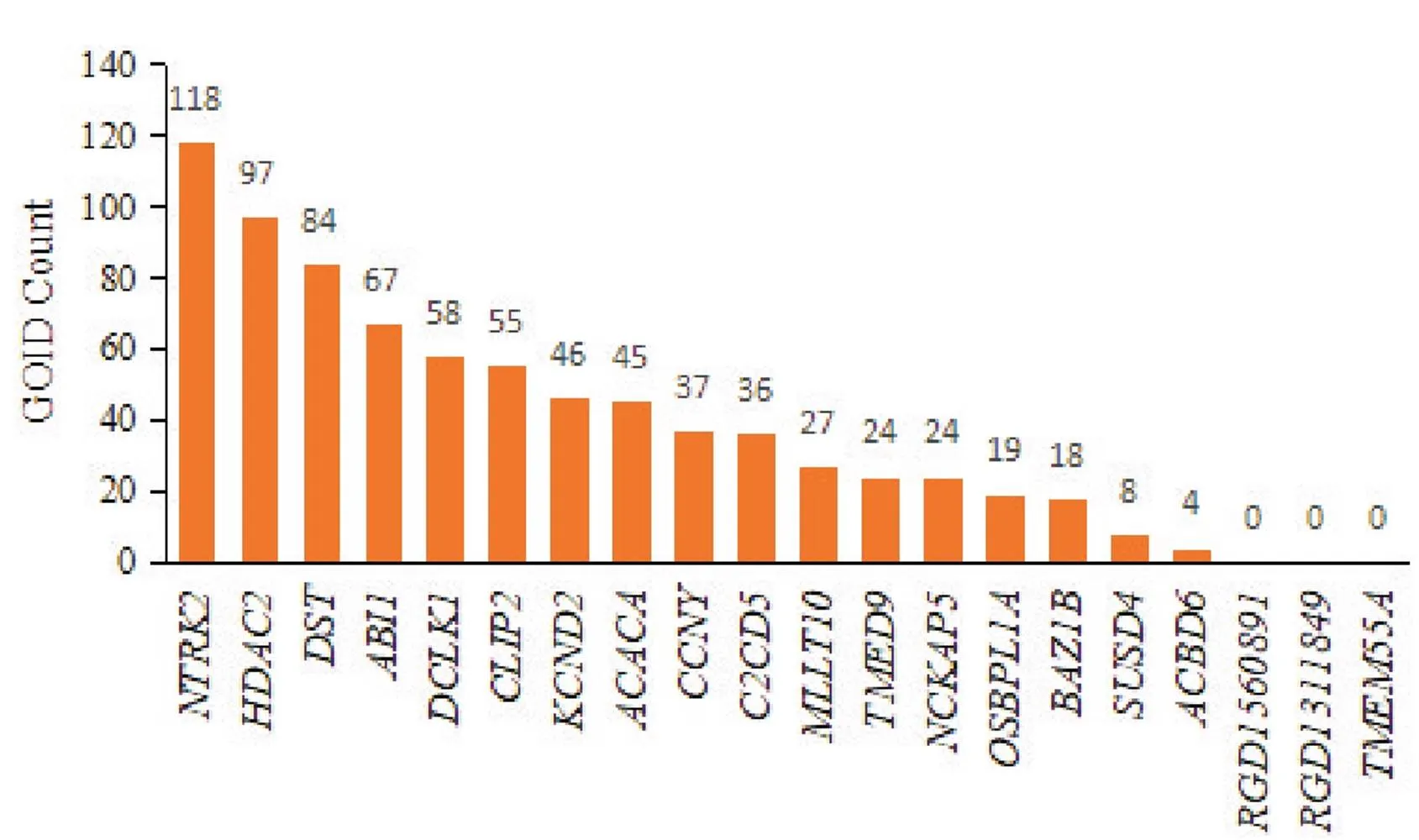
Figure 9. The number of GOID enriched by source genes of common differentially expressed circRNAs. n=3. P<0.05.
讨论
CIRI是缺血性脑卒中的主要并发症,因其发病症状与中医中风类似,故将其归属于中医中风范畴。中医学认为“脑髓损伤,神机失用”、“督脉瘀阻,阳气不振”是中风发病的病机关键[15],因此中风病的诊治常选取督脉穴,历代医家也认为“督脉痹阻”是中风病发病的经络学基础[16]。《灵枢·邪气脏腑病形》曰:“病变在脑,首取督脉”,本实验研究所选取的大椎、百会、水沟穴均为督脉要穴,其中大椎穴为督脉入脑的关键穴位,刺之可开通督脉,活血行气;百会穴位于巅顶,又称为三阳五会,是百脉聚会之处,是治疗内外风的关键穴位,刺之可使气血上荣,补益脑髓;水沟穴为督脉和手足阳明经的交会穴,刺之可回阳救逆,开窍醒神。课题组前期研究显示[17-18],针刺大椎、百会、水沟穴治疗CIRI疗效确切,可有效改善神经功能和神经元超微结构的病理改变,减轻神经损伤,促进机能恢复和血管新生,从而达到一定程度的脑保护作用。近年来课题组致力于从基因学视角研究针刺促CIRI修复机制,其机制可能是针刺激活多种类微小RNA(microRNA,miRNA)的表达,靶向多条信号转导通路,从多途径和多网络调控CIRI[19-20],可能与其调控miR-34c-5p表达,进而调控细胞自噬抗凋亡有关[21]。本实验研究结果显示,在造模后,大鼠Garcia神经功能评分显著降低,脑血流量显著减少,脑梗死面积显著增加,针刺干预后都能在一定程度上改善CIRI大鼠神经功能,减少脑梗死面积,减轻神经损伤,发挥脑保护效应。
大量circRNAs在哺乳动物脑中的表达丰富度要高于其他的检测组织,且circRNA在神经发育过程中,在大脑各个部位均有较高的表达[22-24]。circRNA在神经形成和发育及突触可塑性中的高表达,提示circRNA在中枢神经系统中的重要作用[25]。circRNA可作为海绵吸附miRNA和RNA结合蛋白参与转录后调控、调控亲本基因的表达等途径在神经系统疾病中发挥作用[26]。Han等[27]研究发现短暂性MCAO模型缺血脑组织中circHECTD1水平显著增加,在敲减表达之后,可缓解脑血管闭塞和神经元的亏损从而发挥神经保护作用。Chen等[28]研究表明局灶性脑缺血再灌注小鼠模型的脑组织中circUCK2的水平显著降低,升高的circUCK2水平显著降低了梗死体积,减轻了神经元损伤,并改善了神经功能缺损,其机制是circUCK2可以作为内源性miR-125b-5p海绵来抑制miR-125b-5p活性,从而导致生长分化因子11的表达增加,并随后改善神经元损伤。以上研究均表明调控差异表达的circRNAs可以实现对脑缺血后神经损伤的修复作用,改善神经功能缺损,减轻神经元损伤,从而发挥神经保护作用,因此circRNA在CIRI的诊疗中具有重要的研究意义。目前已有circRNA在大鼠脑缺血后海马组织中表达谱的研究[29];但针刺调控circRNA在CIRI大鼠海马组织中的差异表达谱仍未见报道,本研究主要以circRNA为切入点、针刺为干预手段,探索针刺调控circRNAs的差异表达抗脑缺血再灌注损伤。
本实验研究结果显示,造模后及针刺后大鼠缺血侧海马组织circRNAs表达谱均发生改变,且造模后与针刺后存在共同差异表达的circRNAs,这23个共表达的基因可能成为针刺调控circRNA抗CIRI的关键靶点。此外,共同差异表达circRNAs的来源基因GO分析结果显示,其广泛参与神经系统发育,神经元的产生、发育、分化及投射,头部、大脑及海马的发育,突触的形成、发育、延伸及运输等,且富集GOID数量前三的核心基因、和功能多富集在与神经系统发育与神经元发育分化有关。因此,我们可以推断出共同差异表达circRNAs在神经系统中具有促进神经元发育分化、减轻神经元损伤等功能;本实验采用尼氏染色法观察缺血侧海马组织神经元的损伤程度、WB检测神经元特异性核蛋白NeuN的表达以探究针刺对缺血侧海马组织神经元的作用。因海马CA1区神经元最为敏感[30],且CIRI后海马CA1区神经元是损伤率最高的区域[31],故本实验采用海马CA1区域的染色进行计数,模型组缺血侧海马CA1区神经元尼氏染色阳性细胞数显著降低、NeuN表达量显著降低,针刺干预后均显著升高,这表明针刺可以减轻缺血侧海马组织神经元的损伤。以上GO分析的结果与本实验针刺所表现出的效应相一致。
综上所述,针刺能显著改善CIRI大鼠的神经功能评分和脑梗死面积比,减轻海马组织神经元损伤,其机制可能与针刺调控缺血侧海马组织circRNAs差异表达以及激发共同差异表达circRNAs的促神经元发育分化、抗神经损伤等功能有关。本实验为探究针刺调控circRNA表达抗CIRI提供了关键基因,为进一步治疗CIRI提供了关键靶点。但本实验未对核心circRNA进行验证,且未在基因层面对核心circRNA进行沉默或者过表达相关研究。另外,circRNA的差异表达是通过何种途径起到的减轻神经元损伤的作用,有待进一步研究。
[1] Liu Q, Zhou S, Wang Y, et al. A feasible strategy for focal cerebral ischemia-reperfusion injury: remote ischemic postconditioning[J]. Neural Regener Res, 2014, 9(15):1460.
[2] Wang Y, Ren Q, Zhang X, et al. Neuroprotective mechanisms of calycosin against focal cerebralischemia and reperfusion injury in rats[J]. Cell Physiol Biochem, 2018, 45(2):537-546.
[3]臧瑞, 郭涛, 李旭华, 等. 五味子醇甲通过调控自噬流减轻大鼠脑缺血再灌注损伤[J]. 中国病理生理杂志, 2021, 37(2):269-276.
Zang Y, Guo T, Li XH, et al. Schisandrin A alleviates cerebral ischemia-reperfusion injury in rats by regulating autophagic flux[J]. Chin J Pathophysiol, 2021, 37(2):269-276.
[4] Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs[J]. Nat Biotechnol, 2014, 32(5):453-461.
[5] Ebbesen KK, Kjems J, Hansen TB. Circular RNAs: identification, biogenesis and function[J]. Biochim Biophys Acta, 2016, 1859(1):163-168.
[6] Mehta SL, Pandi G, Vemuganti R. Circular RNA expression profiles alter significantly in mouse brain after transient focal ischemia[J]. Stroke, 2017, 48(9):2541-2548.
[7]肖姮, 阳仁达, 田浩梅, 等. 针刺联合亚低温对脑缺血/再灌注损伤大鼠Bcl-2、Bax、caspase-3蛋白表达的影响[J]. 湖南中医药大学学报, 2016, 36(2):58-61.
Xiao H, Yang RD, Tian HM, et al. Effect of acupuncture combined hypothermia on Bcl-2, Bax and caspase-3 expressions of cerebral ischemia reperfusion injury rats[J]. J Hunan Univ Chin Med, 2016, 36(2):58-61.
[8]刘琴, 林亚平, 陈文, 等. 针刺联合亚低温对脑缺血再灌注损伤大鼠脑组织p-Raf1、p-ERK1/2的影响[J]. 湖南中医药大学学报, 2016, 36(1):58-62.
Liu Q, Lin YP, Chen W, et al. Effect of acupuncture combined with mild hypothermia on p-Raf1 and p-ERK1/2 in brain tissue of rats with cerebral ischemia reperfusion injury[J]. J Hunan Univ Chin Med, 2016, 36(1):58-62.
[9]侯瑜超, 刘璐慜, 陈晓桐, 等. 基于环状RNA研究针灸对慢性萎缩性胃炎细胞凋亡机制的思考[J]. 针刺研究, 2020, 45(8):676-681.
Hou YC, Liu LM, Chen XT, et al. Research strategy thoughts acpuncture-moxibustion treatment of chronic atrophic gastritis by suppressing apoptosis via circular RNA[J]. Acupunct Res, 2020, 45(8):676-681.
[10] Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats[J]. Stroke, 1989, 20(1):84-91.
[11] Wang HL, Liu FL, Li RQ, et al. Electroacupuncture improves learning and memory functions in a rat cerebral ischemia/reperfusion injury model through PI3K/Akt signaling pathway activation[J]. Neural Regener Res, 2021, 16(6):1011.
[12] 李忠仁. 实验针灸学[M]. 北京: 中国中医药出版社, 2007:314.
Li ZR. Experimental acupuncture science[M]. Beijing: Chinese Medicine Press, 2007:314.
[13] Garcia JH, Wagner S, Liu KF, et al. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats: statistical validation[J]. Stroke, 1995, 26(4):627-635.
[14] Swanson RA, Morton MT, Tsao-Wu G, et al. A semiautomated method for measuring brain infarct volume[J]. J Cereb Blood Flow Metab, 1990, 10(2):290-293.
[15] 林志诚, 薛偕华, 江一静, 等. 中医康复临床实践指南·脑卒中[J]. 康复学报, 2019, 29(6):6-9.
Lin ZC, Xue XH, Jiang YJ, et al. Clinical practice guide of traditional chinese medicine rehabilitation: stroke[J]. Rehabilit Med, 2019, 29(6):6-9.
[16] 何兴伟. 中风病从督脉论治探讨[J]. 中国中医基础医学杂志, 2006, 12(8):561-561.
He XW. Discussion on treatment of apoplexy from the governor vessel[J]. J Basic Chin Med, 2006, 12(8):561-561.
[17] 武姿含, 蒋素容, 陈芯仪, 等. 针刺大椎、百会、人中穴对大鼠脑缺血再灌注损伤后ES蛋白表达的影响[J]. 湖南中医药大学学报, 2019, 39(4):507-510.
Wu ZH, Jiang SR, Chen XY, et al. Effects of acupuncture,,on ES protein expression after cerebral ischemia reperfusion injury in rats[J]. J Hunan Univ Chin Med, 2019, 39(4):507-510.
[18] 贺平, 颜虹, 蒋素容, 等. 针刺大椎、人中、百会穴对脑缺血再灌注损伤大鼠脑线粒体超微结构的影响[J]. 湖南中医药大学学报, 2018, 38(1):55-58.
He P, Yan H, Jiang SR, et al. Effects of Acupuncture,,Points on ultrastructure of cerebral mitochondria in rats with cerebral ischemia reperfusio[J]. J Hunan Univ Chin Med, 2018, 38(1):55-58.
[19] 郑慧娥, 何灏龙, 陈芯仪, 等. 针刺对CIRI大鼠缺血侧海马组织miRNA表达及miR-20a-5p和miR-22-5p的影响[J]. 湖南中医药大学学报, 2020, 40(10):1226-1231.
Zheng HE, He HL, Chen XY, et al. Effects of acupuncture on miRNA expression and miR-20a-5p and miR-22-5p in ischemic hippocampal tissues in CIRI rats[J]. J Hunan Univ Chin Med, 2020, 40(10):1226-1231.
[20] 何灏龙, 郑慧娥, 高音来, 等. 针刺调控脑缺血再灌注损伤大鼠缺血侧海马差异miRNA信号归属通路及mir-34c-3p表达的研究[J]. 中国康复医学杂志, 2020, 35(11):1290-1295.
He HL, Zheng HE, Gao YL, et al. Research on acupuncture regulation of differential miRNA signal attribution pathway and mir-34c-3p expression in ischemic hippocampus of rats with cerebral ischemia reperfusion injury[J]. Chin J Rehabil Med, 2020, 35(11):1290-1295.
[21] 卢小叶, 何灏龙, 吕倩忆, 等. 针刺介导miR-34c-5p调控脑缺血再灌注损伤大鼠海马神经细胞自噬的研究[J]. 针刺研究, 2022, 47(5):415-421.
Lu XY, He HL, Lyu QY, et al. Research on acupuncture mediated miR-34c-5p regulating autophagy of hippocampal neurons in rats with cerebral ischemia-reperfusion injury[J]. Acupunct Res, 2022, 47(5):415-421.
[22] You X, Vlatkovic I, Babic A, et al. Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity[J]. Nat Neurosci, 2015, 18(4):603-610.
[23] Rybak-Wolf A, Stottmeister C, Glažar P, et al. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed[J]. Mol Cell, 2015, 58(5):870-885.
[24] You X, Vlatkovic I, Babic A, et al. Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity[J]. Nat Neurosci, 2015, 18(4):603-610.
[25] Constantin L. Circular RNAs and neuronal development[J]. Circular RNAs, 2018:205-213.
[26] 刘燕芳, 逯丹, 徐安定. 脑血管病环状RNA的转录后调控及展望[J]. 中国病理生理杂志, 2018, 34(4):760-763.
Liu YF, Lu D, Xu AD. Post-transcriptional regulation and prospect of circular RNA in cerebrovascular disease[J]. Chin J Pathophysiol, 2018, 34(4):760-763.
[27] Han B, Zhang Y, Zhang Y, et al. Novel insight into circular RNA HECTD1 in astrocyte activation via autophagy by targeting MIR142-TIPARP: implications for cerebral ischemic stroke[J]. Autophagy, 2018, 14(7):1164-1184.
[28] Chen W, Wang H, Feng J, et al. Overexpression of circRNA circUCK2 attenuates cell apoptosis in cerebral ischemia-reperfusion injury via miR-125b-5p/GDF11 signaling[J]. Mol Ther Nucleic Acids, 2020, 22:673-683.
[29] 陈博威, 唐荣梅, 徐雅倩, 等. 大脑中动脉栓塞模型大鼠海马组织circRNA-miRNA-mRNA三元转录网络分析[J]. 中国病理生理杂志, 2022, 38(3):479-486.
Chen BW, Tang RM, Xu YQ, et al. Analysis of circRNA-miRNA-mRNA ternary transcriptional network in hippocampus of a rat model of middle cerebral artery occlusion[J]. Chin J Pathophysiol, 2022, 38(3):479-486.
[30] Petito CK, Morgello S, Felix JC, et al. The two patterns of reactive astrocytosis in postischemic rat brain[J]. J Cereb Blood Flow Metab, 1990, 10(6):850-859.
[31] Newrzella D, Pahlavan PS, Krüger C, et al. The functional genome of CA1 and CA3 neurons under native conditions and in response to ischemia[J]. BMC genomics, 2007, 8:370.
Research on function of acupuncture regulating differential expression of circRNAs in hippocampus of CIRI rats
JIANG Shanshan1, TANG Hong1, WANG Hongjuan1, LÜ Qianyi2, XIE Canming1, WANG Yao1, CHEN Chutao1, TIAN Haomei1△
(1,,,410208,;2,610021,)
To explore the protective effect of acupuncture on brain tissue of rats with cerebral ischemia reperfusion injury (CIRI), observe the effect of acupuncture on the differential expression of circular RNA (circRNA) in the hippocampus of the ischemic side of CIRI rats, and carry out gene ontology (GO) analysis on it.Fifty four 6~8 week old SD rats were randomly divided into modeling group and sham operation group (sham group). CIRI rats were randomly divided into model group(model group) and acupuncture group(AC group) with 18 rats in each group. Establishment of middle cerebral artery occlusion reperfusion (MCAO/R) model by using Longa monofilament method. The cerebral blood flow was monitored by laser speckle imager. In the sham operation group, only the blood vessels were stripped without inserting thread plugs. The acupuncture group was bound+acupuncture every 12 h, 30 minutes for 7 times, during which twirling manipulation was performed. The nerve function was evaluated by the modified Garcia scoring method. Cerebral infarction area was measured by TTC staining. Detection of the expression of neuronal nuclear antigen (NeuN) by Western blot. Observation of neuronal damage in ischemic hippocampus by Nissl staining. Gene chip microarray analysis was used to screen out the circRNA differentially expressed in the hippocampus of ischemic side, and GO analysis was performed on the source genes of common differentially expressed circRNA in model groupsham group and AC groupmodel group.Before intervention, compared with the sham group, the Garcia neural function score in the modeling group was significantly lower (<0.01). After intervention, compared with the sham group, the score of the Model group decreased significantly (<0.01) . Compared with the model group, the score of AC group increased significantly (<0.01). Compared with before intervention, the score of AC group increased significantly after intervention (<0.01). Compared with the sham group, the area ratio of cerebral infarction in the model group was significantly increased (<0.01), the expression of NeuN was significantly decreased (<0.01), and the number of Nielsen staining positive cells in hippocampal CA1 neurons in the ischemic side was significantly decreased (<0.05). Compared with the model group, the area ratio of cerebral infarction in the AC group was significantly reduced (<0.01), the expression of NeuN was significantly increased (<0.05), and the number of neurons with positive Nissl staining was significantly increased (<0.05). The results of circRNA chip screening showed that, compared with the sham group, the number of up regulated circRNA in the model group was 288, and the number of down regulated circRNA was 315. Compared with the model group, the number of differential expression circRNA up-regulated and down-regulated in the AC group was 33 and 18 respectively (FC>1.25,<0.05). The number of common differential expression circRNA in model groupsham group and AC groupmodel group is 23. GO analysis showed that the source genes with common differential expression of circRNA may regulate cerebral ischemia-reperfusion injury by participating in the development of nervous system, the generation, development, differentiation and projection of neurons, the development of head, brain and hippocampus, and the formation, development, extension and transportation of synapses.Acupuncture improves the neurological function score and cerebral infarction area ratio of CIRI rats, and reduce neuronal damage in the hippocampus. The mechanism may be related to acupuncture regulating the differential expression of multiple circRNA in the ischemic hippocampus.
acupuncture; cerebral ischemia reperfusion injury; neuronal injury; circular RNA; differential expression
R743; R363.2
A
10.3969/j.issn.1000-4718.2023.02.004
1000-4718(2023)02-0220-13
2022-08-22
2022-12-23
[基金项目]国家自然科学基金资助项目(No. 81874508; No. 82274662);湖南省自然科学基金资助项目(No. 2020JJ4065; No. 2021JJ30490);长沙市科技局自然科学基金项目(No. kq2014094);湖南省研究生科研创新项目(No. CX20220799);湖南中医药大学研究生创新课题(No. 2021CX39; No. 2022CX97)
Tel: 13548574270; E-mail: 451358104@qq.com
(责任编辑:宋延君,李淑媛)
