焙烧氧化PdCo非预期的乙二醇电催化氧化性能的研究
2021-07-29胡天军贾文玉贾建峰
胡天军 ,赵 瑾 ,贾文玉 ,王 瀛 ,贾建峰
(山西师范大学 教育部磁性分子与磁信息材料重点实验室和山西省分子磁体重点实验室, 山西 临汾, 041004)
Proton exchange membrane fuel cells (PEMFCs)have been proved to be the ideal choice for energy conversion devices that provide clean and sustainable energy. The electrocatalytic materials for the electrooxidation of hydrocarbons (methanol, ethanol,ethylene glycol and formic acid) are the key to the next generation of electrocatalytic devices[1-4]. Among these liquid fuels, ethanol was considered to be the most suitable liquid fuel due to its following advantages: (i)It was a low toxic liquid. (ii) It was easily produced from biomass, and therefore carbon neutral which mitigates the increasing amount of carbon dioxide in the atmosphere. (iii) If completely oxidized to CO2,ethanol has a higher energy density than methanol[5,6].However, when ethanol was used as fuel in PEMFCs,even if the state of the art electrocatalysts was used, it was difficult to break the C-C bond at the temperature below 100 °C. Therefore, the electron transfer number of ethanol electrocatalytic oxidation process was only 4, far lower than the theoretical number of complete oxidation 12[6]. Due to the high electron transfer number in electrocatalytic oxidation process, ethylene glycol,with high boiling point (198 °C) and low volatility that can be synthesized by electrochemical method using renewable energy and thus act as an alternative liquid fuel for ethanol, has been widely concerned by researchers[7-10]. However, the low activity and low stability seriously restrict its commercial application in alkaline direct oxidation fuel cells.
In view of the above problems, the catalytic materials for alkaline direct oxidation fuel cells have been extensively investigated[11-13]. At present, the reported catalytic materials for alkaline direct oxidation fuel cells mainly include Pt, Pd, Au and their alloys[14,15].The crystal structure and lattice parameters of Pd are similar to those of Pt. So Pd-based catalysts are deemed to be one of the most promising materials to replace Pt catalysts. In particular, Pd-based catalysts have been widely studied for their high electrocatalytic activity in alkaline media. The efforts in the rational catalyst design strategies include: (i) controlling the structure and morphology of Pd-based catalysts which includes porous, nanosheets, nanowires and multidimensional structures with preferential facets[15-19], (ii) doping of other elements to form composite materials[20-23], (iii)loading on metal oxides to modify the electronic structure of Pd and improve the electrocatalytic performance of the catalysts[24-27]. Among them,supported electrocatalysts have been widely investigated because of their low price, high catalytic activity and good stability.
On the one hand, metal oxides can significantly improve the adsorption of reactants[28]. For example,Muneeb et al[29]found that the decisive step of alcohol oxidation was the adsorption of alcohol and OH on the surface of catalyst, while OH can hardly be adsorbed on the surface of Pd metal. On the other hand, the interaction between metal and support was known as“strong metal support interaction”, which can change the electronic structure of Pd, thus improving the catalytic activity and stability. For example, Schalow et al[30]prepared Pd/Fe3O4catalysts and found that a thin film of PdOxoxide layer was formed at the interface of metal and support. The interaction between metal Pd and support changed the electronic structure of Pd and improved the activity and stability of the electrocatalytic alcohol oxidation. Krittayavathananon et al[25]also found that the surface oxide layer significantly improved the electrocatalytic activity of Pd for ethanol oxidation in alkaline solution. The electrocatalytic oxidation of alcohols was a structure sensitive reaction, and the interaction between the surface active center and oxygen species directly affects the catalytic activity. Therefore, PdOxcatalysts are expected to be used in alkaline direct oxidation fuel cells.
Here, the PdCo nanomaterials were prepared by chemical reduction method. The PdO-Co3O4were obtained by thermal treatment of PdCo. Then, the change of electrocatalytic performance of PdCo and PdO-Co3O4were investigated. The experimental results showed that the formed PdO-Co3O4multicomponent hybrid nanocrystals comprised of PdO and Co3O4not only promoted the formation of OH species on the surface of the catalyst, but also improved the redox properties of the catalyst and the electrocatalytic oxidation activity toward electrooxidation of ethylene glycol. Furthermore, the formed PdO-Co3O4with special structure, comprised of the amorphous and crystalline domains joined together through PdO and Co3O4interfaces, could facilitate synergistic interaction and the charge and energy flow between the amorphous and crystalline domains, leading to the improved catalytic activity and stability of the nano-particles.DFT calculation confirmed that the adsorption energies of O2and OH on the Co doped PdO (101) surface decreased, which was beneficial to stabilize the intermediate C2H4OHO*. As a result, the energy barrier for the O-H dissociation on the Co-doped surface decreased. The strong binding of Co doped PdO (101)with ethylene glycol and its intermediate species led to different electrochemical kinetics and reaction path that produce excellent electric catalytic activity.
1 Experimental
1.1 Preparation of the PdCo and PdO-Co3O4
The PdCo were prepared following the reported procedures[8]. In a typical synthesis, 575 mg of EDTA-2Na and 379 mg of Co(NO3)2·6H2O were added into 30 mL of deionized water with stirring. Then, 3 mL of H2PdCl4(0.06 mol/L) solution was added. After stirring for 5 min, the mixed solution was quickly injected into the newly prepared NaBH4solution (NaBH4∶292 mg,H2O 77 mL). The PdCo could be formed within 5 min.The resulting black deposits were collected by magnetic field sedimentation separation and washed with water for several times. The PdO-Co3O4was synthesized by thermal treatment of PdCo at 300 °C for 120 min with a ramping rate of 5 °C/min.
1.2 Electrochemical test
The electrochemical tests were carried out according to our previous reports without any change[8].The electrochemically active surface area (ECSA), the specific activity (SA), and mass activities (MA) were calculated according to our previous reports without any change[8,14].
2 Results and discussion
2.1 XRD analysis
The XRD patterns of PdO-Co3O4and PdCo were shown in Figure 1. For the sample of PdCo, the diffraction peaks at about 40.1°,46.7°,68.1° and 82.2°were corresponded to the (111),(200),(220) and (311)planes of Pd (PDF# 46-1043), respectively. No characteristic diffraction peak of metal Co was observed for PdCo, which might due to the Co are in amorphous or poorly crystalline structure. After calcination, two weak diffraction peaks appeared at 36.9° and 65.4°, corresponding to (311) and (440)crystal faces of Co3O4(PDF# 74-1657), respectively,indicating the formation of Co3O4in poor crystallinity.In addition, there were five diffraction peaks at 33.8°,41.9°,54.7°,60.2° and 71.5°, corresponding to (101),(110), (112), (103) and (211) crystal planes of PdO(PDF # 41-1107), respectively. The composition of the prepared PdCo and PdO-Co3O4was measured by ICP-MS. The Pd and Co mass ratio were determined to be 20.5∶79.5 and 22.8∶77.2 for the samples of PdCo and PdO-Co3O4, respectively. Combined with the results of XRD and ICP, we believed that element Co does exist in the sample whose crystallinity was poor.In addition, after oxidative calcination treatment of PdCo, 38.18 mg of PdO-Co3O4was obtained, which was close to the theoretical yield of 39.56 mg. These results further confirmed that the precursor PdCo was transformed into PdO and Co3O4after calcination.
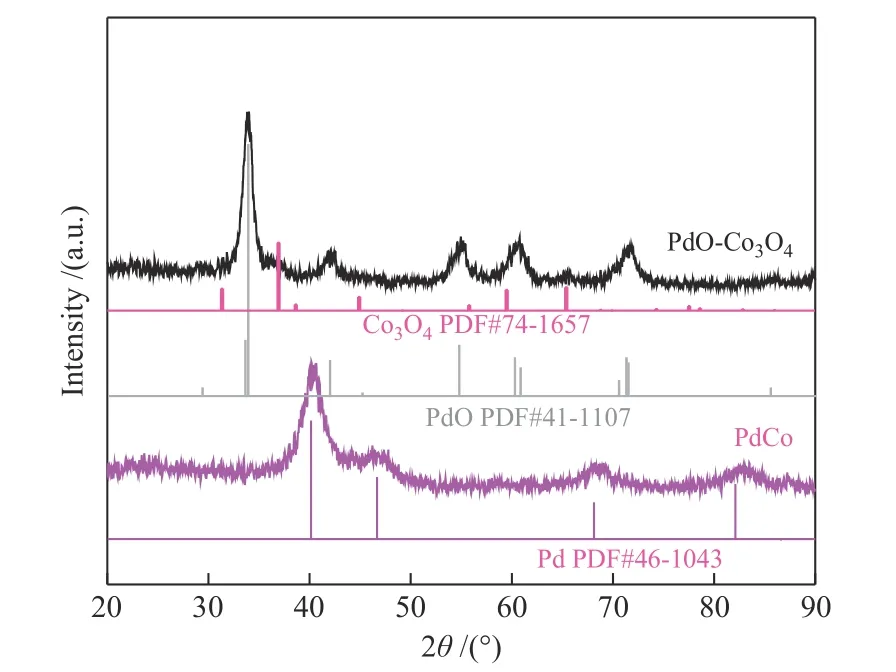
Figure 1 XRD patterns of PdCo and PdO-Co3O4
2.2 TEM analysis
The morphology of the samples was characterized by TEM. The metal PdCo (Figure 2) showed an interconnected irregular nanoparticle structure with clear edges. After calcination, the boundaries of PdOCo3O4, (Figure 3, Figure 4) nanoparticles become more blurred. There was no obvious boundary between the nanoparticles,whichfurtherindicatesthep oor crystallinity of Co3O4forthe samplesofPdO-Co3O4[31, 32].The result was consistent with the XRD analysis results. Compared with high crystallinity materials, low crystallinity and amorphous materials usually have long-range disordered structure and rich internal defects, which lead to not only diversity in composition and structure, but also rich active sites and good charge transfer ability. As the high-resolution transmission electron microscopy (HRTEM) image shown in Figure 5, the fringe lattice parameter of the PdO-Co3O4was determined to be 2.47 and 2.64 nm, which could be assigned to the spacing distance of the (311) plane of Co3O4and the (101) plane of PdO, respectively. It was worth noting that the lattice spacing of PdO increased to 2.78 nm at the interface between Co3O4and PdO (The red area in the Figure 5), which indicated the formation of heteromixture by Co doping with PdO[33]. The lattice spacing of Co doped PdO increased from 2.64 to 2.78 nm, which could alter the surface electronic structure by modifying the distances between surface atoms and in turn the catalytic activity[34]. As can be seen from the high-angle annular dark field(HADDF) image and element mapping image in Figure 6, O, Pd and Co were evenly distributed in PdOCo3O4, further confirming the existence of cobalt element in the sample. All these results indicated that PdCo transformed into PdO-Co3O4after calcination in which Co3O4mainly existed in the form of amorphous state. A multicomponent nanocomposite crystal was formed at the interface of PdO and Co3O4by joining different inorganic materials through PdO and poorly crystalline Co3O4interfaces, which have unique physical and chemical properties. This kind of material could improve the stability of energy transfer and catalytic activity of metal oxides.
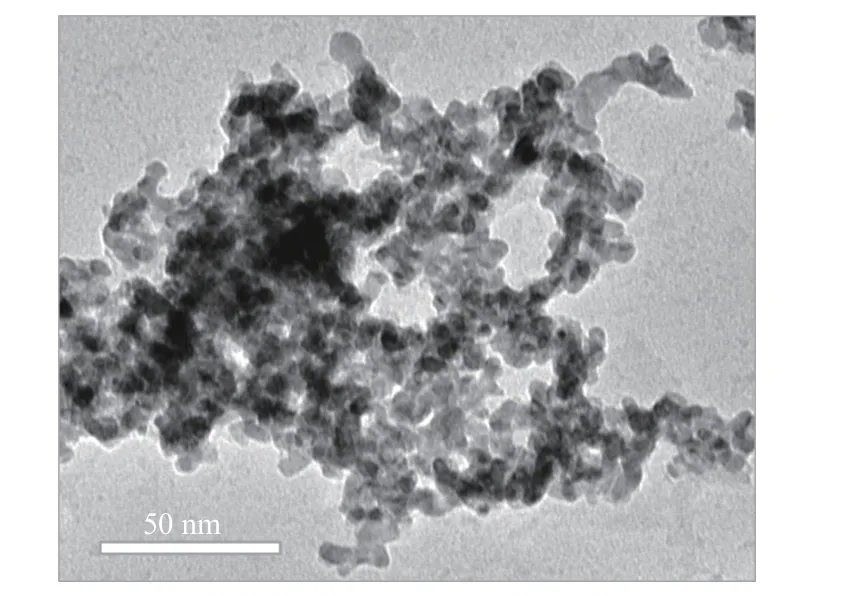
Figure 2 TEM image of PdCo

Figure 3 TEM image of PdO-Co3O4
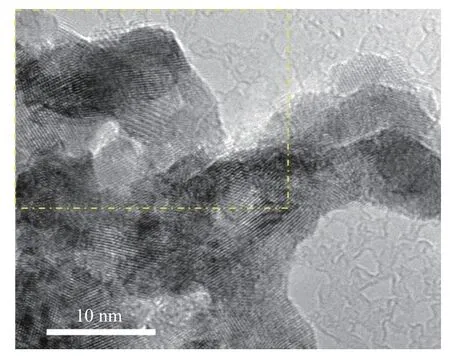
Figure 4 TEM image of PdO-Co3O4 at different magnification

Figure 5 HRTEM image of PdO-Co3O4

Figure 6 HADDF image of PdO-Co3O4 and elemental mapping of Co, Pd, and O
2.3 XPS analysis
2.3.1 Pd 3d XPS analysis
The surface composition and valence state of the catalysts were characterized by XPS (Figure 7). The peaks at 335.5 and 340.8 eV belong to the Pd03d5/2and 3d3/2spin-orbit, and those at 337.2 and 342.6 eV can be ascribed to the characteristic peaks of Pd2+3d5/2and 3d3/2spin-orbit. For PdO-Co3O4, the characteristic peaks of Pd03d5/2and 3d3/2spin-orbit can be observed even after calcination in air atmosphere. Compared with PdCo, the Pd 3dbinding energy of PdO-Co3O4was obviously shifted to high energy. The positive shift could lead to the decrease of thed-band center of Pd and the coverage of the generated OH on the catalyst surface, which can release more active sites on the catalyst surface for oxygen adsorption and thus enhance the electrocatalytic activity[35].

Figure 7 XPS spectra of Pd 3d for PdCo and PdO-Co3O4
2.3.2 Co 2p XPS analysis
The Co 2pXPS spectra of PdCo and PdO-Co3O4were shown in Figure 8. Two main peaks could be observed at about 780.4 and 796.3 eV, corresponding to the Co 2p3/2and Co 2p1/2spin-orbit of Co3O4,respectively[36]. The two peaks at 785.9 and 801.8 eV could be assigned to Co 2p3/2and Co 2p1/2oscillation satellite peaks of Co3O4, respectively[37]. More importantly, for PdO-Co3O4, the binding energies of Co 2p3/2and Co 2p1/2shifted to high energy by 0.4 and 0.8 eV, respectively, and the intensity of the satellite peak increased, indicating more Co2+components in PdOCo3O4[38]. Co on the surface of PdCo was oxidized to form Co3O4in air, and part of Co3+transformed into Co2+during heat treatment[36].

Figure 8 XPS spectra of Co 2p for PdCo and PdO-Co3O4
2.4 Evaluation of electrocatalytic performance of catalyst for ethylene glycol oxidation
2.4.1 Cyclic voltammograms analysis
The cyclic voltammograms of PdO-Co3O4, PdCo and commercial Pt/C in nitrogen saturated 1 mol/L KOH solution were shown in Figure 9. It could be seen that for the positive scanning process, all samples had a wide current peak between -0.65 and -0.35 V (vs.Hg/HgO) attributed to the oxidation peak of absorbed and adsorbed H[39]. While the current peak at -0.2 V belonged to the OH adsorption[40]. It was shown that OH group was an important active substance in alkaline medium whose adsorption was the rate determining step of alcohol oxidation. When the hydroxyl group was adsorbed at a lower potential, the overpotential of alcohol oxidation could be effectively reduced and the higher electrocatalytic activity could be achieved[41,42]. The current peak at about 0.1 V could be ascribed to the oxidation of Pd on the catalyst surface[43]. An obvious oxidation peak of Pd in PdOCo3O4were found because the synergistic effect of PdO and Co3O4could enhance the redox performance of the catalyst[44]. Previous studies had shown that the surface oxidation was closely related to the formation of oxygen species on the surface of Pd particles which were necessary intermediates for electrocatalytic oxidation[43,45]. In the reverse scanning, the current peak at -0.32 V could be attributed to the reduction peak of PdO. In the range of 0.15 to 0.3 V, two redox peaks were observed for PdO-Co3O4due to the redox of high valence cobalt oxides or cobalt hydroxides.
It was found that PdO-Co3O4exhibit excellent electrocatalytic oxidation of ethylene glycol. Figure 10 showed the CV curves of PdO-Co3O4in 1 mol/L KOH +0.5 mol/L ethylene glycol solution. For comparison, the PdCo and commercial Pt/C were also tested at the same condition. The onset potential of ethylene glycol oxidation for PdO-Co3O4appeared at about -0.26 V(vs Hg/HgO), while that for commercial Pt/C and PdCo was about -0.21 and -0.19 V, respectively. The peak current of PdO-Co3O4was about 21.3 mA, while that of commercial Pt/C and PdCo were about 18.7 and 13.4 mA, respectively. The electrochemically active surface area (ECSA) was calculated according to the area of Pd-oxides reduction peak (Figure 9), and the current density of different catalysts was normalized by ECSA to show the specific activity (SA) and mass activities (MA). As shown in Figure 11, it was clear that PdO-Co3O4showed a SA of 6.703 mA/cm2, which was 1.2 times larger than that of PdCo (5.74 mA/cm2).The MA was 6187 mA/mgPd, which was 1.6 times higher that of PdCo (3897 mA/mgPd). The SA and MA of PdO-Co3O4were 3.8 and 2.4 times of the commercial Pt/C, respectively. The results revealed that the calcination and oxidation treatment significantly affects the electrocatalytic activity of PdCo towards the ethylene glycol oxidation in alkaline solution.
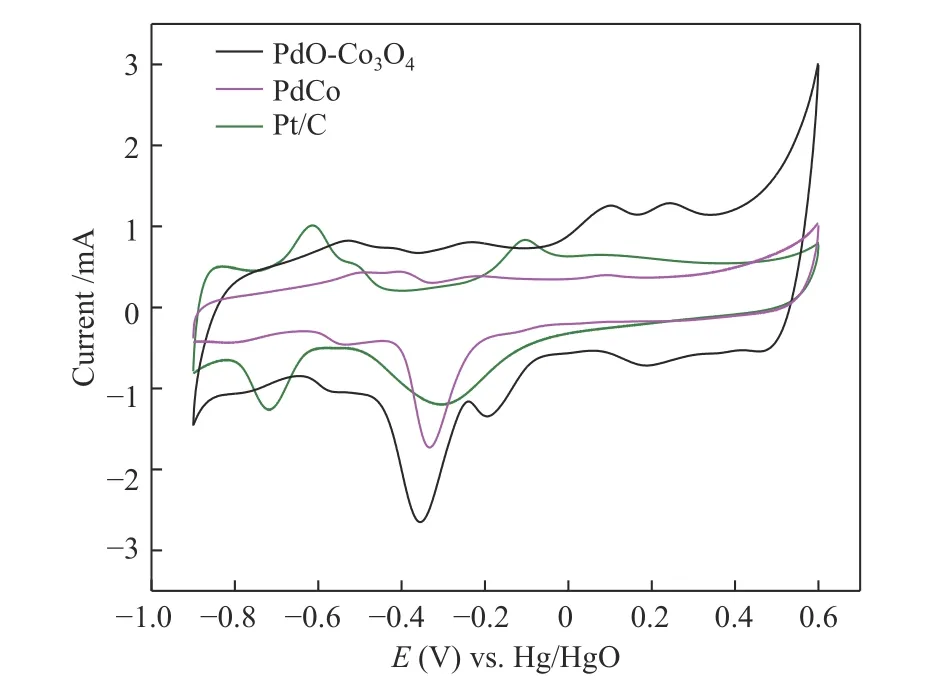
Figure 9 CV curves of PdO-Co3O4, PdCo and commercial Pd/C in N2-saturated 1.0 mol/L KOH with the scan rate of 100 mV/s
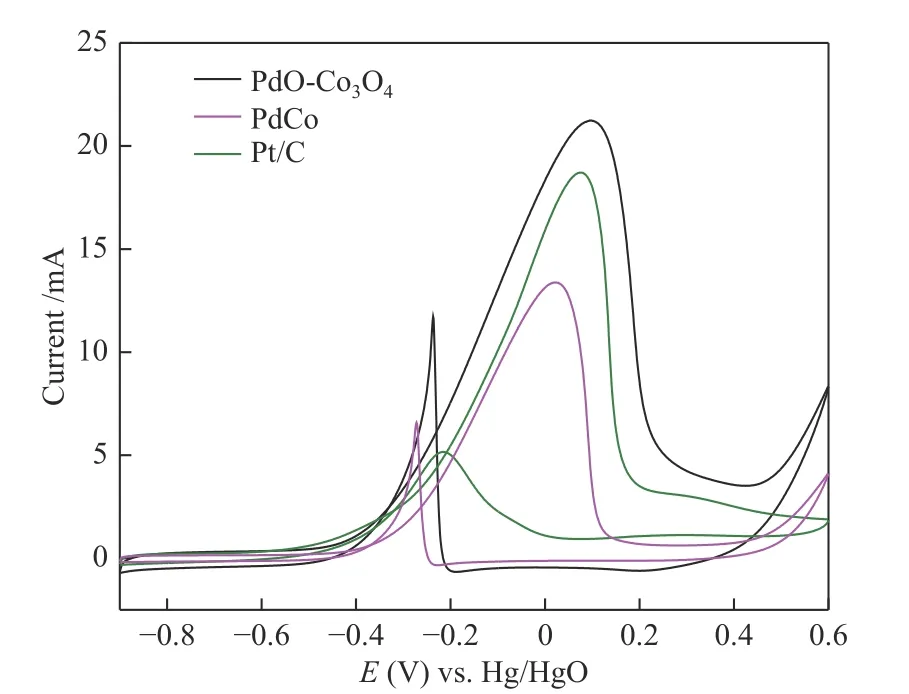
Figure 10 CV curves of PdO-Co3O4, PdCo and commercial Pd/C in 1.0 mol/L KOH + 0.5 mol/L EG with the scan rate of 100 mV/s for EG electrooxidation

Figure 11 SA and MA of PdO-Co3O4, PdCo and commercial Pd/C for EGOR
A long term current-time method was used to measure the stability of PdO-Co3O4for ethylene glycol electrooxidation. As shown in Figure 12, the current density on PdO-Co3O4catalyst (7.7 mA) after 10000 s was higher than that on Pt/C (3.9 mA) which clearly demonstrated the superior stability of PdO-Co3O4nanoparticles during ethylene glycol electrocatalytic oxidation reaction.
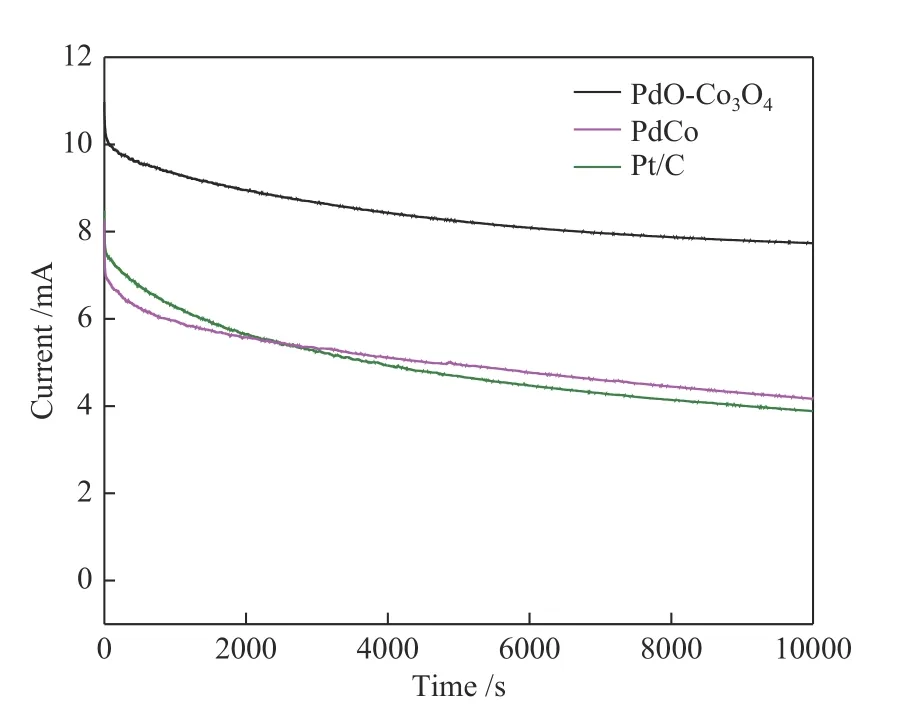
Figure 12 Current-time chronoamperometric response of PdO-Co3O4, PdCo and commercial Pd/C at -0.15 V in 1.0 mol/L KOH + 0.5 mol/L EG solution
2.5 Origin of the superior electrocatalytic activity of PdO-Co3O4
It was clear that the electrocatalytic activity of SA and MA for ethylene glycol electrocatalytic oxidation was in the order of PdO-Co3O4> PdCo > commercial Pt/C. According to the results of XRD and TEM, the main exposed surface was the PdO (101) for PdO.Furthermore, there was mutually connected interfaces between PdO and Co3O4on which Co doped PdO to formed heteromixture. To elucidate the effect of the coexisted Pd and Co ions in the interface of PdO-Co3O4,the interaction of O2, OH with pure Pd (101) and Co doped PdO (101) were compared by DFT calculations.The Co-doped PdO (101) were used to mimic the interface between PdO and Co3O4. As shown in Figure 13(a) and 13(b), the calculated adsorption energies of O2and OH on the Co-doped Pd (101)surface were -1.69 and -2.80 eV, respectively. While on pure Pd (101) surface, the calculated adsorption energies of O2and OH were -1.46 and -2.60 eV,respectively. It was shown in Figure 13(a) that the O-O bond length (1.371 Å) on Co-doped Pd (101) was longer than that (1.334 Å) on pure PdO (101) surface,indicating that the O2on Co-doped Pd (101) was more active i.e. the synergy between Pd and Co enhanced the interaction between active oxygen species and the catalyst surface. For the electrocatalytic oxidation of alcohol, the initial O-H breaking might be the ratedetermining step[46]. Based on the DFT calculations, the adsorption of ethylene glycol and the process of the first O-H dissociation on both on pure PdO (101) and Co-doped Pd (101) surface were investigated. Although the adsorption energy of ethylene glycol on pure PdO(101) surface was higher than that on Co-doped surface, the Co-doped surface was more favorable to stabilize the intermediate C2H4OHO*. As a result, the energy barrier for the O-H dissociation on the Codoped surface was only 0.04 eV. While on the pure PdO (101), it was 0.17 eV (Figure 13(c)) i.e. the synergy between Pd and Co facilitates the electrocatalytic oxidation of ethylene glycol.
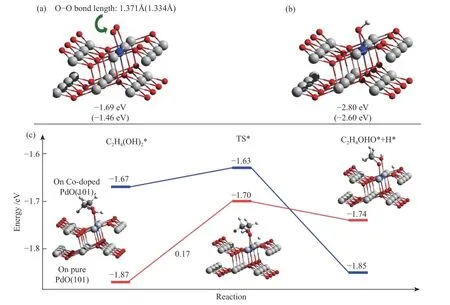
Figure 13 (a): The bond length and adsorption energy of O2 on Co-doped PdO (101) surface; (b): The bond length and adsorption energy of OH on Co-doped PdO (101) surface; (c): The adsorption and O-H dissociation of EG on Co-doped PdO (101)
The enhanced electrocatalytic activities was mainly attributed to the following factors: (i) The structure of PdO-Co3O4, in which multicomponent hybrid nanocrystals comprised of distinct inorganic domains joined together through PdO and Co3O4interfaces and enhanced the electrocatalytic activity.Specifically, formation of PdO-doped Co3O4crystals resulted in a tight and interconnected interface, which led to surface reconstruction around the interface,lattice mismatch and electron interaction/transfer on the interface, thus providing new active sites for electrocatalytic reactions. (ii) The synergistic effect between PdO and Co3O4provides more active sites for O2adsorption. At the same time, surface defects formed on the surface of PdO-Co3O4nanoparticles could produce high activity electron oxygen species and enhance the electrocatalytic activity.
3 Conclusions
In this work, metal PdCo was prepared by a simple and convenient wet chemical reduction method,and the effect of calcination and oxidation treatment on the electrocatalytic oxidation of ethylene glycol was studied. The results of XRD, XPS and TEM showed that the metal PdCo had a tightly connected heterostructure due to the simultaneous reduction of Pd and Co. After calcination and oxidation, the morphology,composition, and electronic structure of the active component of PdO-Co3O4changed obviously. The mass specific activity and area specific activity of PdO-Co3O4nanocomposites increased by 1.6 and 1.2 times, respectively in alkaline solution. The experimental and computational results suggested that the synergistic effect of PdO and Co3O4could enhance the interaction between the active oxygen species and the catalyst surface, which was not only beneficial to the formation of superoxide species on the catalyst surface, but also promoted the electrocatalytic activity for oxidation of ethylene glycol. Therefore, controlling the surface chemical environment of metal catalysts was the key to improve the electrocatalytic performance.
