多壁碳纳米管-二氧化硅吸附剂对甲苯的吸附性能
2021-07-29张绍鹏建伟伟马丹竹
刘 微 ,李 壮 ,张绍鹏 ,建伟伟 ,马丹竹
(辽宁石油化工大学,辽宁 抚顺 113001,中国)
Volatile organic compounds (VOCs) are ubiquitous air pollutants, which are precursors of fine particulate matter and ground level ozone[1,2].Photochemical smog, which is a mixture of pollutants(ozone, nitric acid, aldehydes, peroxyacyl nitrates(PANs), and other secondary pollutants)[3]formed when nitrogen oxides interact with VOCs, creating a brownish-gray haze above urban areas. VOCs may lead to eye, nose, and throat irritation and respiratory diseases, and some of them are highly toxic[4,5]. Because of their adverse effects on the human health and the environment[6], control and reduction of VOC concentrations have become a popular research topic in the field of environmental science and engineering.Volatile organic compounds are emitted from a variety of sources including automobile coating; 1 kg of coating releases 0.02-0.09 kg VOCs, and more household products coated with paint also generate large amounts of VOCs[7]. Volatile organic compounds include nonmethane hydrocarbons such as alkanes, olefins, and aromatic hydrocarbons, and oxygen-containing organic compounds such as aldehydes, ketones, alcohols, and ethers[8,9], halogenated hydrocarbons, nitrogenous compounds, and sulfur-containing compounds. Toluene is a widely used solvent in various applications, and thus was used as the research object in this study[5,10].
Widely used VOC emission reduction technologies include incineration, catalytic combustion,biodegradation, plasma, photocatalysis[8], adsorption,condensation, and membrane separation[11-13]. Parmar et al[14]showed that the incineration method and the catalytic combustion method consume high energy and the products can cause secondary pollution,and the biodegradation method is time-consuming. Ulrich et al[15]use a nanoporous, hydrophobic membrane to separate VOCs from a stream of water-saturated air.Their experiments have proved that although membrane separation method is safe and pollution-free,its mass transfer rate drops very fast and requires regular cleaning, which is not suitable for the treatment of high concentration VOCs. The adsorption method is popular because of its low cost and large benefits[16-19];traditional activated carbon is widely used as an adsorbent because of its high recovery efficiency and low cost[20-23]. Han et al[24]doped silicon on activated carbon to prepare an adsorbent. The newly prepared AC-Si composite had a mesoporous structure with a specific surface area of 729 m2/g. Silicon doping improved the adsorption performance; the maximum adsorption capacity ofp-xylene was 292 mg/g, and the adsorption material was renewable. Dou et al[25]showed that the adsorption capacity of a carbon silicon composite at 25, 35, and 45 °C to remove benzene was increased by 7.5%, 9.7%, and 15.7% compared to activated carbon (AC). Mohammadi et al[26]also showed that the adsorption capacity of carbon silicon composite to remove ethylbenzene and benzene was 1.3 and 1.8 times that of AC under the same conditions,respectively.
Compared with activated carbon, multi-walled carbon nanotubes (MWCNTs) have a highly porous and hollow structure, larger specific surface area,lighter mass density, and higher thermal and chemical stability[27]. Owing to their unique properties and potential application prospects, they have attracted widespread attention[28-33]and can be modified to improve the adsorption performance[29,31]. Su et al[34]oxidized MWCNTs in a NaOCl solution to increase their carbon purity, surface carboxyl groups, and negative charge and demonstrated that MWCNTs showed a good adsorption capacity for benzene,toluene, ethylbenzene, andp-xylene (BTEX) in aqueous solutions[30,35,36]. Ganguponu et al[37]studied the treatment of single-walled CNTs with acid oxidation to enhance the adsorption potential of their surfactants and generate more internal adsorption sites[38], and their specific surface area increased from 1136 to 1347 m2/g.The ability to absorb toluene was 1.6 times that of granular activated carbon (GAC).
To date, only a few studies have focused on modifying MWCNTs with silicon as an effective adsorbent for VOCs. The aim of this study is to investigate the adsorption properties of silicon-doped MWCNTs for toluene. The sol-gel method was used to modify MWCNTs by loading SiO2to synthesize adsorbents CS2, CS4, and CS6 with different MWCNT molar contents. A temperature-variable fixed-bed device was established, and a dynamic VOC adsorption technique was used to comprehensively evaluate the adsorption performance of CS2, CS4, and CS6 for toluene. Based on this system, the influence of various factors (MWCNT molar content, temperature, water vapor concentration, and cycle regeneration times) on the adsorption capacity was explored, and the operating parameters were obtained when the adsorption capacity was optimal. Then, the kinetic models were fitted with experimental data, and the best adsorption kinetic model was selected.
1 Experiments
1.1 Material synthesis
l g MWCNTs was placed in a mixed acid solution of concentrated sulfuric acid and concentrated nitric acid for 2 h, then the MWCNTs were separated with a centrifuge, rinsed repeatedly with deionized water to make them neutral, and finally placed in a drying oven(80 °C) for 16 h .
MWCNTs with 2%, 4%, and 6% ethanol mass fraction were added to an adequate amount of ethanol(10 mL) and stirred for 30 min. Then, 20% of tetraethyl silicate/water solution was added followed by concentrated hydrochloric acid until the pH dropped to 2.0-3.0, and reacted for 30 min. The temperature of the solution was then raised to 40 °C, and a 10% ammonia/ethanol solution was added dropwise to form a gel.
The surface of the gel was covered with ethanol for 24 h to prevent the reverse reaction of surface modification[39]. Then, an appropriate amount of nhexane (10 mL) was added to replace water and ethanol in the gel to reduce the surface tension of the gel. Later, a 10% trimethylchlorosilane/n-hexane solution was added dropwise to complete the surface modification. The modified MWCNTs-SiO2was dried at 50 °C, 80 °C for 2 h and at 120 °C, 150 °C for 1 h.Finally, adsorbents MWCNTs-SiO2-2, MWCNTs-SiO2-4, MWCNTs-SiO2-6 (CS2, CS4, and CS6) were obtained by reduction in nitrogen at 500 °C for 2 h.
1.2 Parameter setting measurements
A gas chromatograph equipped with a flame ionization detector was used to detect toluene. The carrier gas was high-purity nitrogen, and the chromatographic column was an ATOV-225 capillary column (50 m × 0.32 mm × 1 μm). The parameters were set as follows: column box temperature 80 °C,detector 230 °C, and capillary column temperature 80 °C. A standard gas with 300 mg/L toluene was passed into the pipeline and operated at a flow rate of 100 mL/min for 10 min, and 1 mL of gas was extracted with a gas injector from the injection port 15 cm away from the exhaust port. The peak of toluene was determined via gas chromatography. This operation was repeated until the peak time and peak area of toluene were stable and set as the standard curve of toluene. Similarly, inject 1 mL of the gas to be detected into the gas chromatograph, and the peak curve was compared with the toluene standard curve to determine the toluene concentration.
1.3 Characterization methods
The main adsorbent characterization methods used in the experiment were JEM-2100F transmission electron microscopy (TEM)[0.20 nm (accelerating voltage 200 kV)] and SU8010 scanning electron microscope (SEM)[1.0 nm (accelerating voltage 15 kV,WD = 4 mm), 1.3 nm (accelerating voltage 1 kV, WD =1.5 mm)]. The automatic physical static analysis[Brunauer-Emmett-Teller (BET) adsorption] using Autosorb-IQ2-MP calculates the total volume of pores using the data under maximum pressure[24]. Fourier transform infrared (FTIR) spectrometer of Nicolet iS50 has a spectral range of 7000-350 cm-1(host type of KBr) and 5100-600 cm-1(host type of ZnSe), resolution >2 cm-1, wavenumber accuracy of 0.05 cm-1, and wavenumber reproducibility of 0.005 cm-1.
1.4 Dynamic adsorption process
The experimental system (Figure 1) consists of a gas supply device, a fixed-bed equipment, and an analysis system[40]. In the experiment, N2was used as the gas source and divided through two pipes. One channel of N2produced toluene through a gas bubbler containing liquid toluene, and the other produced steam through a constant temperature water bath. The water vapor content is controlled by the N2flow rate and the temperature of the water. Quartz wool with a certain thickness is placed in the slot inside the adsorption tube to support the adsorbent. The quartz tube can be placed in a heating furnace with a programmed temperature control function to achieve accurate temperature control. The experiment was carried out on an adsorption reactor. Gas chromatograph GC-9600 (China Inesa Analytical Instrument Co., Ltd.) was used for measurement at the end of the gas supply device, and then the adsorption results were fed back through the N2000 GC data workstation (Zhejiang University,China). The experimental conditions for adsorption were as follows: VOC concentration of 700 mg/L, flow rate of 40 mL/min, and 1 g adsorbent.

Figure 1 Experimental system
2 Results and discussion
2.1 Characterization
2.1.1 TEM
Figure 2(a) shows that the MWCNTs without any treatment are long and easy to aggregate, the surface of the MWCNTs is relatively smooth. A small amount of impurities and amorphous carbon can be observed on the surface. Figure 2(b) shows the MWCNTs after oxidation treatment in the mixed acid solution, the length of a single MWCNT became shorter than before, and the external surface of the oxidized MWCNTs was no longer smooth and the surface area was increased compared with that before oxidation treatment. In addition, a large number of oxidation openings appeared on MWCNTs, which increased the number of adsorption sites and was conducive to the adsorption of VOCs.
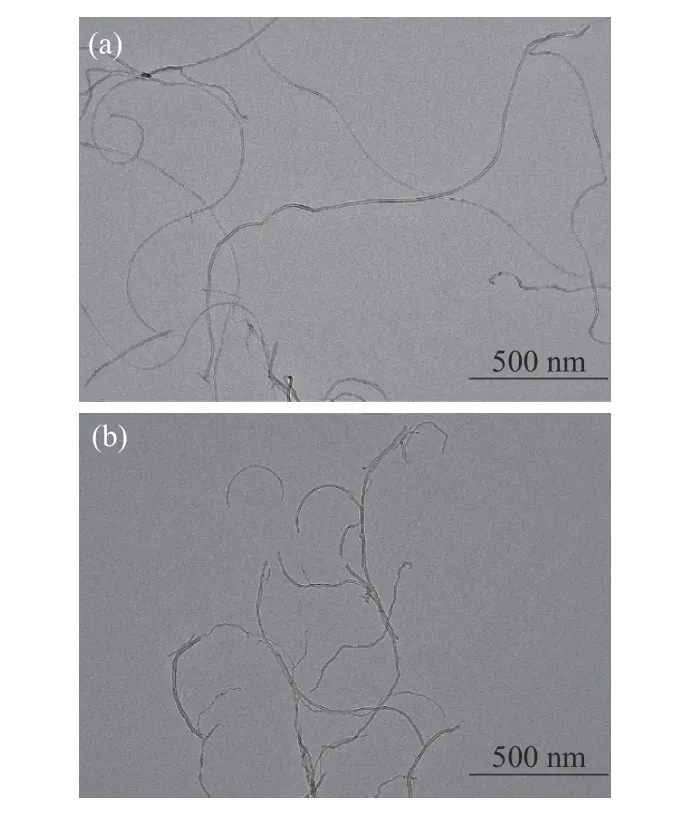
Figure 2 TEM images: (a) MWCNTs, (b) purified MWCNTs
2.1.2 SEM
Figure 3 shows the SEM morphology of the MWCNTs-SiO2-6 adsorbent. The resolutions are 30 μm,2 μm, 1 μm, and 500 nm, respectively. Figure 3(a)displays the presence of SiO2particles, which indicates that the surface modification reaction in the MWCNTs-SiO2adsorbent has been fully completed, as also observed in the FT-IR analysis. Figure 3(b) demonstrates that the structure of the MWCNTs-SiO2adsorbent contains spherical solid clusters and shows a porous network structure. The MWCNTs loaded with SiO2were wrapped and exposed to the outside of SiO2, and hundreds of nano-sized holes were observed. Figure 3(c)depicts that SiO2and MWCNTs are combined by van der Waals forces. Figure 3(d) reveals the image of the sampling part of the Energy Dispersive Spectrometer(EDS) analysis. The EDS analysis results are: the content of Cu element accounts for about 60%, and the element C and Si each account for about 20%.
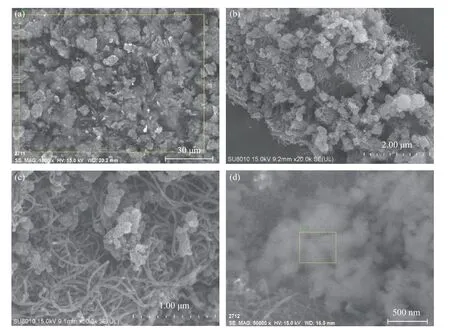
Figure 3 SEM: (a) 30 μm, (b) 2 μm, (c) 1 μm and (d) 500 nm
2.1.3 FT-IR
The FT-IR characterization results of CS2, CS4,and CS6 are shown in Figure 4. FT-IR analysis indicated the completion of the surface modification of the MWCNTs. The band around at 568 cm-1belongs to the curved O-Si-O vibration. The peak vibrations around at 800 cm-1are attributed to the three modes of asymmetry, symmetry, and bending in SiO2[41]. The peaks near 845 cm-1is caused by the bending vibration of the Si-O-H hydroxyl group. The vibration band around 1095 cm-1is due to the tensile vibration of the C-O bond or the Si-O bond. The absorption peak corresponding to the CH3functional group at 1400 cm-1is very strong. Vibration of the peak near 1400 cm-1was also observed in the FT-IR spectrum, which was attributed to the C-H bond.

Figure 4 FT-IR spectra of pattern for CS2, CS4, CS6
The peak at 1600 cm-1in the FT-IR spectrum can be attributed to the OH group. The intensity of the OH peak in CS6 (1600 cm-1) was less than that in CS2(1600 cm-1), which indicates that CS6 is more hydrophobic. Subsequent adsorption experiments also verified this result, which is caused by the surface modification effect of trimethylchlorosilane on the aerogel of SiO2. The vibrational peaks corresponding to the Si-H bond at 2120 cm-1and 2190 cm-1were not observed in FT-IR characterization. Therefore, according to reaction formula (1), the Si-H bond of siloxane may react with acidic groups on the CNT surface. The discovery of the C-O bond or Si-O bond peak confirms this result.
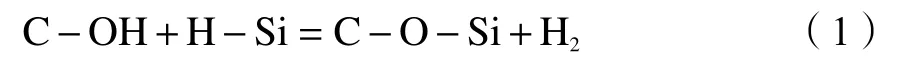
The analysis showed that the surface modification reaction in the samples containing SiO2was fully completed, and the hydrophobicity of the MWCNTs-SiO2adsorbent increased with the increasing MWCNT content. The OH peak intensity was small and hydrophobicity was strong.
2.1.4 BET
The pore size, specific surface area, and pore volume of CS2, CS4, CS6, and AC were compared by using the Barrett-Joyner-Halenda (BJH) model. As shown in Table 1, microporosity of the MWCNTs-SiO2adsorbent increased as the MWCNT content increased,while the total pore volume decreased. The FTIR analysis results also confirmed that the amount of supported Si(CH3)3increased with the increase in the molar content of the MWCNT, which increased the average pore diameter[26]. In general, the adsorption process relies on microporous adsorption, and microporous adsorption is controlled by the micropore surface area, which is consistent with the BET detection results (Figure 5). Therefore, the increase in hydrophobicity and the decrease in adsorption performance can be attributed to the decrease in BET surface area and micropore structure[42].

Table 1 Textural properties of MWCNTs-SiO2 composites
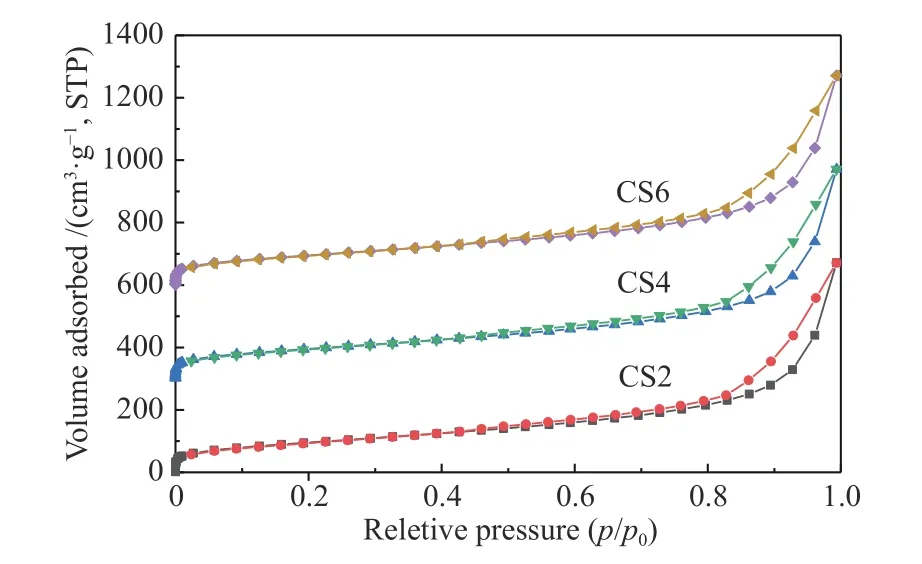
Figure 5 Nitrogen adsorption and desorption isotherms
2.2 Adsorption performance of MWCNTs-SiO2 adsorbent
2.2.1 Influence of temperature
Temperature has a significant impact on the removal of VOCs by the MWCNTs-SiO2adsorption.Breakthrough measurements are typically used to analyze the adsorption performance of MWCNTs-SiO2adsorbents for VOCs. Figure 6 shows the relationship between the outlet concentration and adsorption time of toluene when AC, CS2, CS4, and CS6 are adsorbed at 30, 40, 50, and 60 °C. Figure 7 shows the relationship between adsorption time, equilibrium adsorption capacity, and adsorption rate of CS2, CS4, and CS6 at 30, 40, 50, and 60 °C. Other experimental variables were kept constant during the experiment.
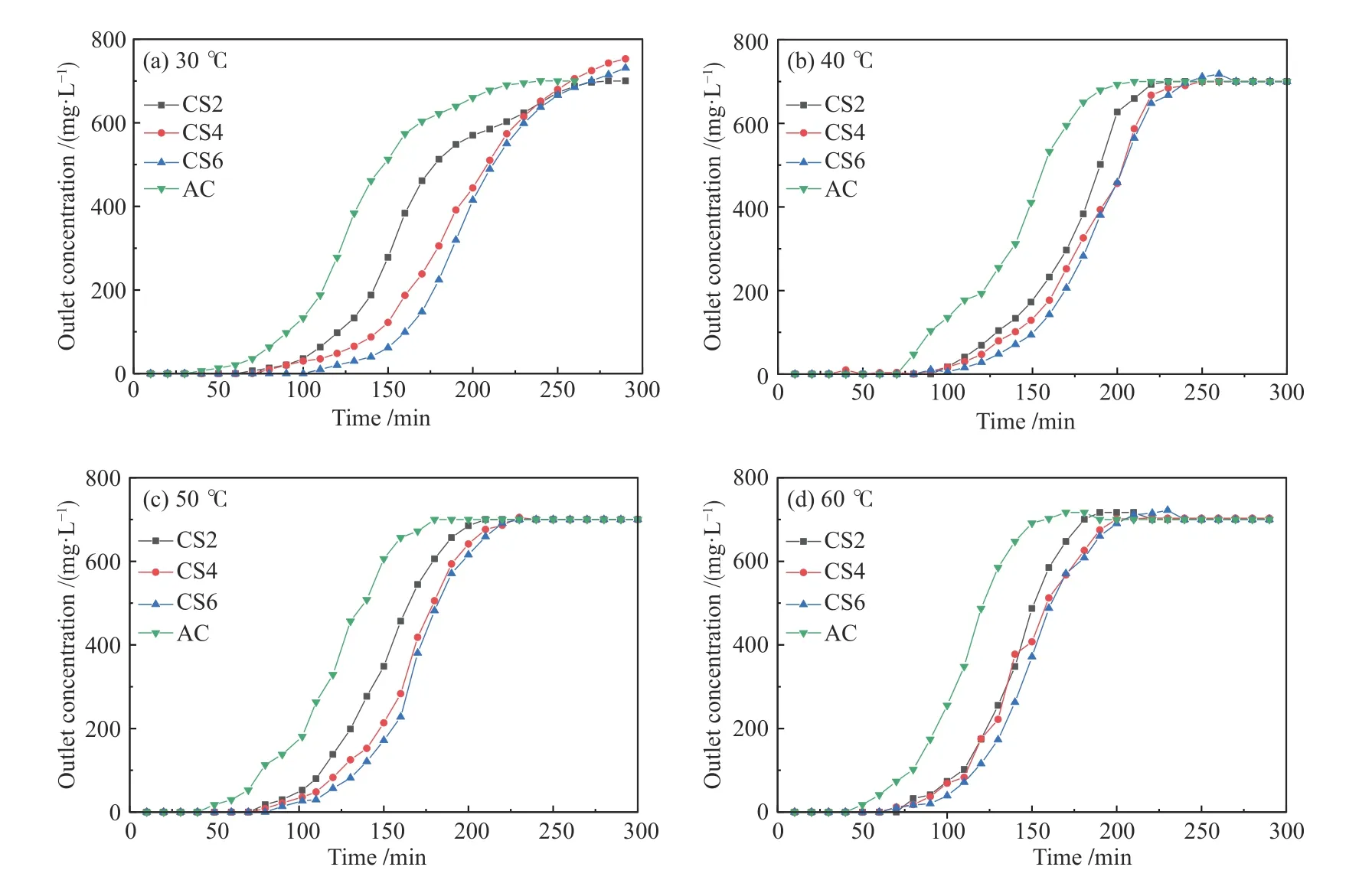
Figure 6 Influence of temperature on outlet concentration
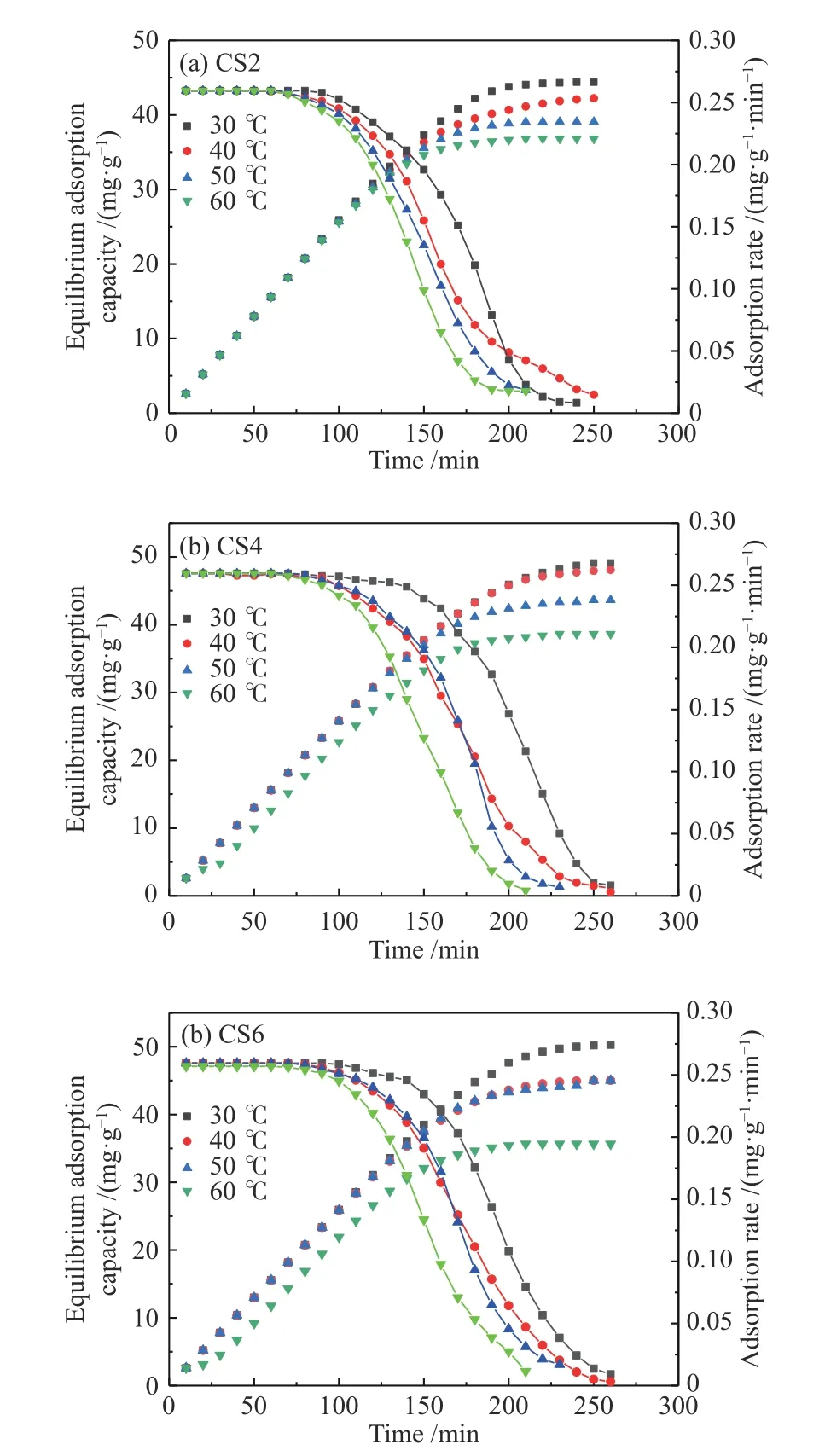
Figure 7 Adsorption performance of MWCNTs-SiO2 adsorbent at different temperatures
During the entire adsorption process at the same temperature, the amount of toluene adsorbed on the MWCNTs-SiO2adsorbent was directly proportional to the MWCNT content (CS6 > CS4 > CS2 > AC), which is consistent with the micropore size of the MWCNTs-SiO2adsorbent[43]. The adsorption of MWCNTs-SiO2for toluene mainly depends on micropores, and the adsorption by micropores is controlled by the surface area of the micropores, which is in accordance with the BET detection results. As the adsorption temperature increased from 30 to 60 °C, the mass transfer rate of toluene in the MWCNTs-SiO2adsorbent decreased. For every 10 °C increase in temperature, the adsorption time of CS2 and CS6 decreased by 30 and 20 min,respectively. The adsorption capacity decreased by approximately 16 and 4 mg/g, respectively, because the structure of the MWCNTs-SiO2adsorbent is mainly microporous, and the microporous structure is conducive to the physical adsorption of toluene by MWCNTs-SiO2.
The adsorption capacities of CS2, CS4, and CS6 for toluene are proportional to the breakthrough time.For adsorption temperatures at 30, 40, and 50 °C, the capacities of CS2, CS4, and CS6 increased by 8.08,2.87, and 2.00 mg/g, respectively, for every 10 min increase in the breakthrough time. When the adsorption temperature increases, the impact of breakthrough time on the adsorption capacity also decreases because the increase in temperature reduces the time for fully saturated adsorption[44]. Therefore, the adsorption temperature is in the range of 30-60 °C. The relatively low adsorption temperature can increase the adsorption capacity of the MWCNTs-SiO2adsorbent and prolong the breakthrough time, and the influence of temperature on adsorption capacity decreases with the increase of temperature.
2.2.2 Influence of water vapor concentration
In this experiment, the range of water vapor volume concentration was 1%-5%. As shown in Figure 8, AC is a hydrophilic material, and the adsorption capacity of AC decreased to 34% of the original adsorption amount when the volume concentration of water vapor was 5%. When the volume concentration of water vapor was 1%, the amount of toluene adsorbed on MWCNTs-SiO2was almost unchanged. The adsorption capacity of CS2, CS4, and CS6 for toluene decreased with increasing water vapor concentration. When the water vapor concentration was 3%, the adsorption capacities of CS2, CS4 and CS6 were 38.8, 46.28, and 47.1 mg/g, which were 92%, 93.1%, and 93.8% of those under dry conditions. When the water vapor concentration was 5%, the adsorption capacities of CS2, CS4, and CS6 were 35.8, 42.6, and 43.6 mg/g,which were 84.9%, 85.7%, and 86.9% of those under dry conditions. When the water vapor volume concentration increased by 1%, the adsorption capacity of MWCNTs-SiO2for toluene decreased by 3.5%, and the reduction of CS6 adsorption was less than that of CS2. The FTIR spectrum shows the content of OH groups; the OH peak intensity in CS6 (1600 cm-1) is less than that in CS2, indicating that the hydrophobicity of CS6 is stronger, which is consistent with the conclusion drawn from the experimental data.

Figure 8 Influence of water vapor content on outlet concentration
2.2.3 Analysis of adsorption kinetics
Experimental data of toluene on the CS2, CS4,and CS6 adsorbents were fitted using the quasi first-order kinetic model[45-48], quasi second-order kinetic model[47-49], intraparticle diffusion model[50,51], and Elovich kinetic model[47,48]. The relevant parameters of model fitting are shown in Table 2.
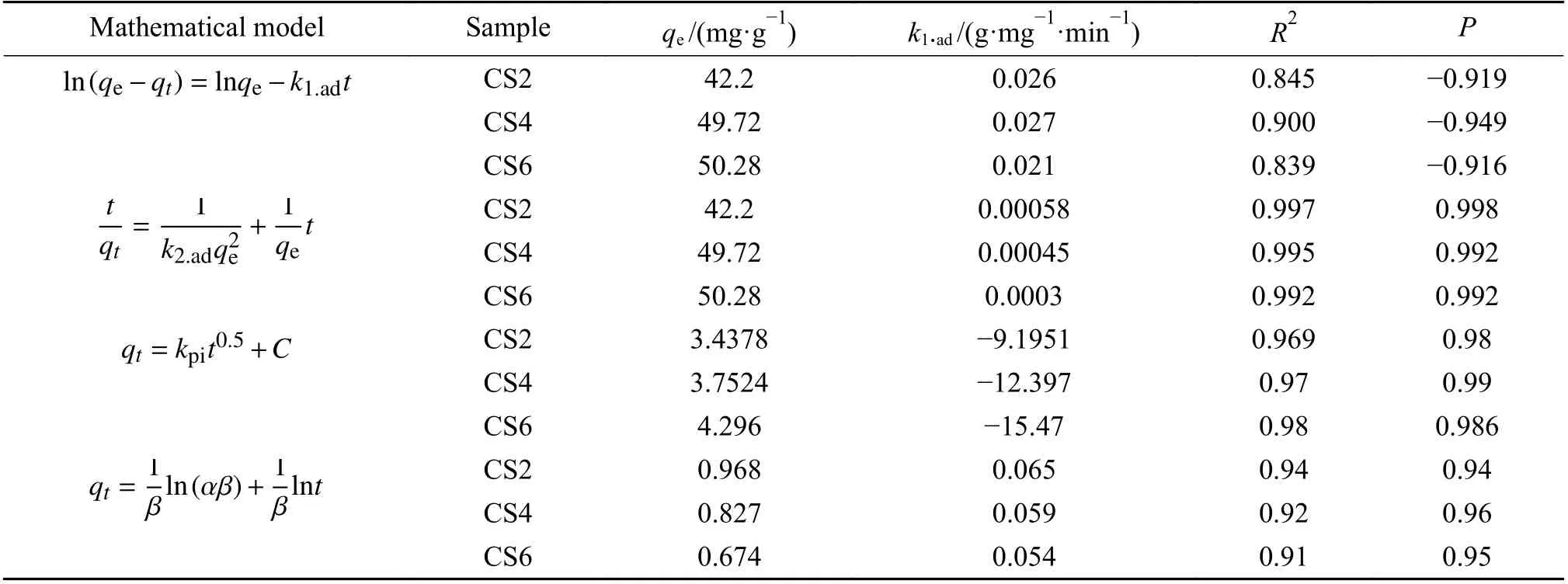
Table 2 Fitting related parameters of each sample model
The results of the pseudo-first-order adsorption kinetic equation for toluene adsorption on CS2, CS4,and CS6 were fitted (Figure 9(a)). The quasi-first-order kinetic equation fitting curve of CS6 did not show a linear correlation (R2= 0.839), whileR2of CS4 was 0.900, which was higher thanR2of CS2 and CS6. The fitting of the quasi-first-order equation in the entire process has a strong linear correlation only at 50-75 min, indicating that the absorption rate in the entire process of toluene removal is not completely controlled by the absorption performance of the outer surface layer.
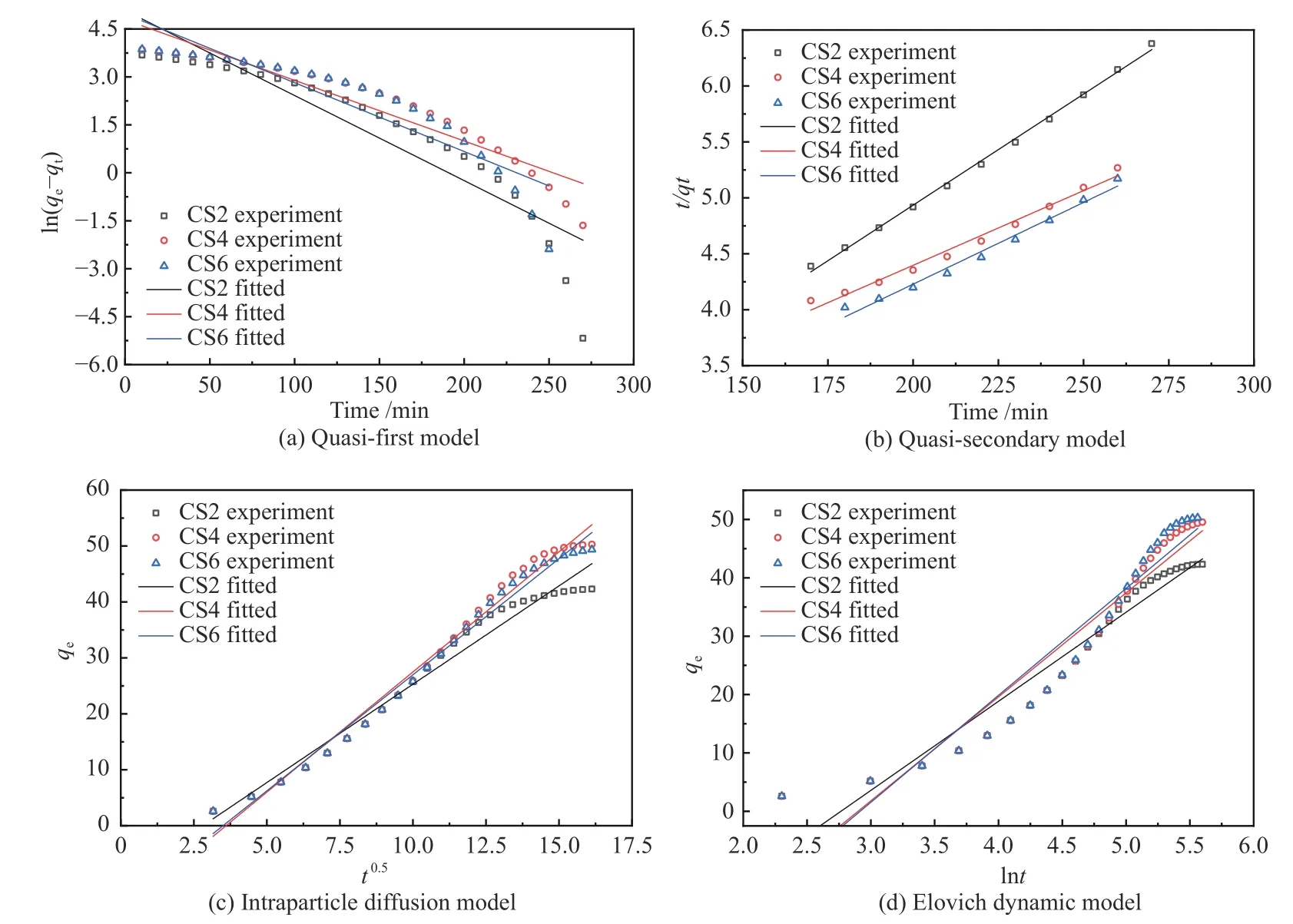
Figure 9 Adsorption kinetic model fitting
The dynamic adsorption process of toluene on CS2, CS4, and CS6 was fitted by using a quasi-secondorder equation (Figure 9(b)). Multiple fittings of the pseudo-second-order equation of CS2, CS4, and CS6 showed that CS2, CS4, and CS6 had strong linear relationships at 170-270 min, 180-260 min, and 180-260 min, withR2values of 0.997, 0.995, and 0.992, respectively. However, the deviation from the straight line in other time periods was larger, which indicates that the removal rate of toluene during the entire adsorption process is not completely dominated by the surface absorption performance. In the adsorption process, 170-270 min and 180-260 min were the stages where toluene had the fastest mass transfer rate in the sample. The fitting line of the Quasi-secondary kinetic model passed almost all data points, indicating that the dynamic process of toluene adsorption on CS2, CS4, and CS6 could be described accurately by the pseudo second-order kinetic model.
Figure 9(c) shows the relationship betweenqtandt0.5of samples CS2, CS4, and CS6. The linear correlation between CS2, CS4, and CS6 is strong in the initial stage of absorption during the entire process, but it becomes weaker as the adsorption progresses to the later stage of the experiment. During the adsorption of toluene on CS2, CS4, and CS6, toluene diffused into the adsorbent after the active sites on the surface were completely covered, and the intraparticle diffusion played a major role in this stage. Therefore, adsorption was more relevant in the initial stage of the experiment.When the adsorption experiment progressed to the later stage, the intraparticle diffusion no longer played a major role, and the linear correlation became weaker.Table 3 shows thatR2of each sample is close to 1,indicating that the adsorption of toluene by CS2, CS4,and CS6 conforms to the intraparticle diffusion equation. Furthermore, the intraparticle diffusion rate constantkpiincreased with an increase in the MWCNT content of the adsorbent. This result can be explained by the increase in the MWCNT content of the adsorbent, which is beneficial to the adsorption of toluene.
The fitting diagram of the Elovich kinetic equation for the dynamic adsorption of toluene on CS2,CS4, and CS6 is shown in Figure 9(d). As the molar content of MWCNTs increased,R2gradually decreased;R2of CS2, CS4, and CS6 were 0.94, 0.92,and 0.91, respectively. The desorption rate constantsβof toluene on the surface of CS2, CS4, and CS6 were 0.065, 0.059, and 0.054, respectively; the lower the MWCNT content of the MWCNTs-SiO2adsorbent, the easier the desorption. The adsorption rate constants α of toluene on the surface of CS2, CS4, and CS6 were 0.968, 0.827, and 1.003, respectively; the higher the MWCNT content, the easier the adsorption of toluene on the surface.
2.2.4 Recycling performance
The inability of an adsorbent to be recycled and reused has always been an important factor that restricts its industrialization. In this study, the solvent extraction-heat treatment method was used to regenerate the MWCNTs-SiO2adsorbent. The specific operation scheme is as follows: first, the saturated MWCNTs-SiO2adsorbent was immersed in ethanol for 1 h. Then, the liquid on the surface of the MWCNTs-SiO2adsorbent was evacuated with a syringe, and the MWCNTs-SiO2adsorbent soaked in ethanol was placed in a drying oven at 80 °C until the absolute ethanol and toluene in the micropores of the adsorbent were completely removed.
The adsorption-regeneration cycle was repeated seven times (Figure 10), and the adsorption experiment of toluene was carried out using CS6 at 30 °C. The regenerated CS6 was analyzed using FT-IR. Figure 11 shows the change in the adsorption capacity of CS6 after regeneration. The adsorption capacity of CS6 for toluene was 50.28 mg/g, which was reduced to 44 mg/g after the first regeneration and no less than 87% of that before regeneration. After seven regenerations, the adsorption capacity of CS6 for toluene was not less than 73% of the original capacity. Therefore, the pore structure of CS6 showed no obvious changes after regeneration, which suggests that MWCNTs-SiO2adsorbs toluene mainly by physical adsorption. Figure 12 shows the FT-IR detection results of CS6 after solvent extraction and heat treatment. The FT-IR analysis of the regenerated MWCNTs-SiO2adsorbent did not change significantly. The structure of MWCNTs-SiO2was not changed by this method, the width of the C-O or Si-O bond widened, and the strength of the O-Si-O and CH3functional groups decreased significantly.Hence, the decrease in the number of O-Si-O and CH3functional groups is the likely reason for the decrease in the adsorption capacity. In addition to micropores,the MWCNTs-SiO2adsorbent also uses O-Si-O and CH3functional groups on the surface to adsorb toluene.The OH group peak at 1600 cm-1confirms that the MWCNTs-SiO2adsorbent remains hydrophobic after regeneration.
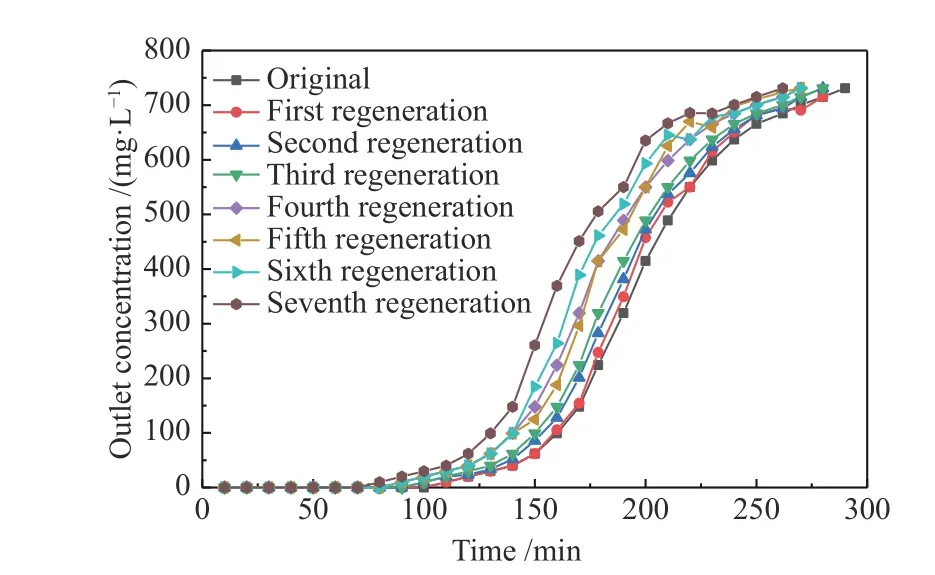
Figure 10 Regeneration performance of CS6
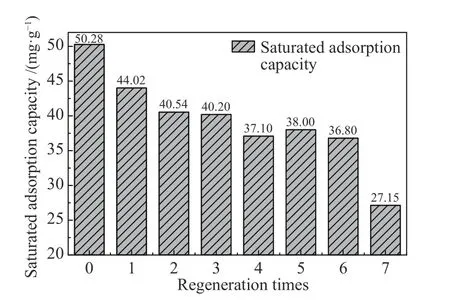
Figure 11 Adsorption capacity of CS6 after regeneration
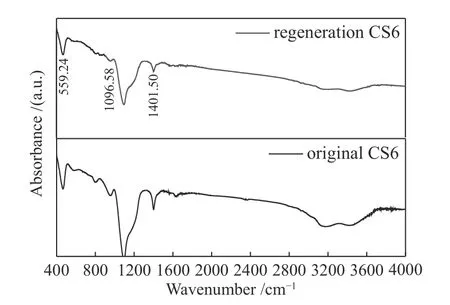
Figure 12 FT-IR spectra of pattern for CS6
3 Conclusions
Three types of MWCNTs-SiO2adsorbents were prepared by using the sol-gel method and analyzed by TEM, SEM, BET, and FT-IR. The adsorption performance of adsorbents AC, CS2, CS4, and CS6 for toluene were studied in a fixed-bed device. The effects of temperature and water vapor concentration on the adsorption process were explored, and the cyclic regeneration performance was investigated. The results can be summarized as follows:
High temperature and water vapor inhibit the adsorption of toluene onto the MWCNTs-SiO2adsorbent. Within 30-60 °C, the toluene adsorption capacity of the adsorbent was AC < CS2 < CS4 < CS6.Under optimal conditions (7 × 10-4toluene, 0 H2O, 30°C, and 100 mL/min), the adsorption capacity of CS6 was 50.28 mg/g. The breakthrough time decreased by 10-20 min when the temperature increased by 10 °C,and the adsorption content decreased by 3.5% when the water vapor concentration increased by 1%.
The rate of adsorption of toluene on the MWCNTs-SiO2adsorbent was not completely controlled by the adsorption reaction of the outer surface layer. When toluene diffused into the adsorbent,intraparticle diffusion played a major role in the adsorption process.
The MWCNTs-SiO2adsorbent showed satisfactory recycling performance. FTIR characterization indicated that the surface chemical functional groups of the regenerated MWCNTs-SiO2adsorbent remained unchanged. After seven cycles of regeneration, the adsorption capacity was still no less than 73% of the original adsorption capacity.
The results of this study suggest that the MWCNTs-SiO2adsorbent has a great potential to control VOC emissions.
