樟脑基喹喔啉类化合物的合成及其生物活性
2020-07-22卜俊文段文贵林桂汕
卜俊文,段文贵,林桂汕,何 云,姚 池,岑 波
(广西大学 化学化工学院,广西 南宁 530004)
樟脑是一种具有双环单萜结构的非木质林产品,天然存在于樟科植物中,是我国的可再生天然优势生物质资源,也可由α-蒎烯经多步反应合成之。樟脑及其衍生物具有广泛的生物活性,如强心[1]、止痛[2-3]、止咳[4-5]、抗菌[6-8]、除草[9]、抗病毒[10]、驱虫杀虫[11-14]等,在医药和农药等领域有着广泛的应用。
此外,含有喹喔啉骨架的化合物显示多种生物活性,如杀菌[15-16]、杀虫[17]、抗病毒[18-19]、抗肿瘤[20-21]、除草[22-24]、降血糖[25]、抑制胆固醇酯转移蛋白酶[26]等。喹喔啉骨架是重要的有机合成单元并广泛存在于天然产物、医药及农药分子中。
基于近年来本课题组对松香松节油基生物活性化合物的研究成果[27-32],利用活性基团拼接原理,通过氧化、缩合等反应将喹喔啉骨架与D-(+)-樟脑骨架相结合,合成得到一系列樟脑基喹喔啉类化合物(3a~3s,Scheme 1)。利用UV-Vis,1H NMR,13C NMR,FT-IR及HR-MS(ESI)等进行表征,并测试了目标化合物的抑菌活性以及除草活性。
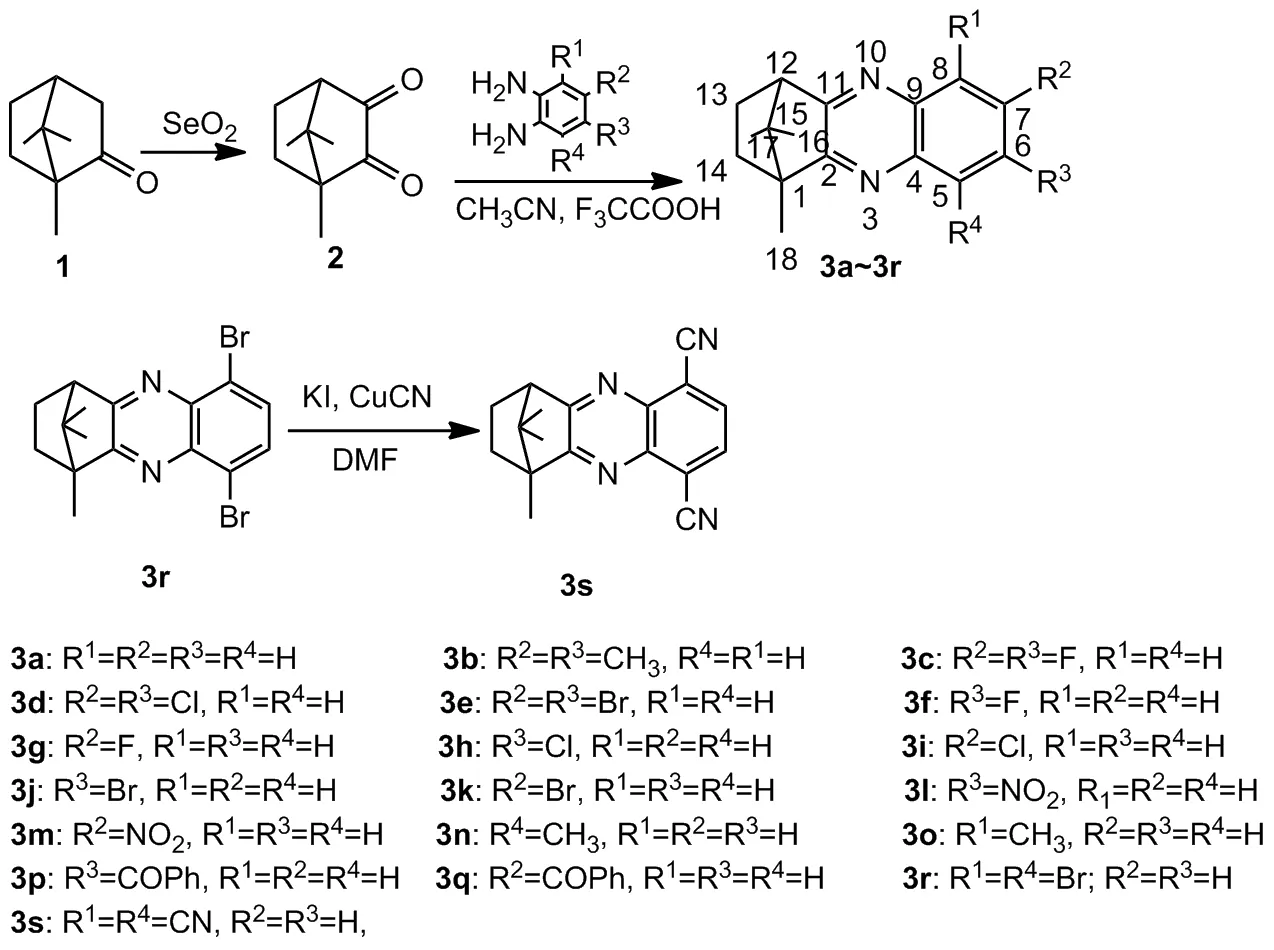
Scheme 1
1 实验部分
1.1 仪器与试剂
MP420型全自动熔点仪;SHIMADZU UV-1800型紫外光谱仪;Nicolet iS 50 FT-IR型红外光谱仪;AVANCE Ⅲ HD型600 MHz超导核磁共振仪(CDCl3为溶剂,TMS为内标);TSQ Quantum Access MAX型液相色谱-质谱联用仪;Waters 1525型高效液相色谱仪。
D-(+)-樟脑(AR,GC纯度96.0%),上海晶纯试剂有限公司;3,6-二溴-1,2-苯二胺参照文献[33]自制;D-(+)-樟脑醌(2)参照文献[34]合成。其余所用试剂均为分析纯。
1.2 合成
(1)3a~3r的合成(以3a为例)
将0.50 g(3 mmol)D-(+)-樟脑醌、10 mL乙腈、5滴三氟乙酸和0.34(3 mmol)邻苯二胺混合,80 ℃搅拌1~4 h(TLC监测)。除去溶剂,残渣溶于10 mL乙酸乙酯中,分别用饱和碳酸钠溶液(3×5 mL)和去离子水(3×5 mL)洗涤,无水硫酸钠干燥后,经硅胶柱层析[洗脱剂:V(石油醚)/V(乙酸乙酯)=5/1]纯化得白色固体3a。
用类似方法合成3b~3r。
樟脑基喹喔啉(3a):白色固体,收率83%,m.p.75.6~76.3 ℃;UV-Vis(EtOH)λmax:314.50,241.00 nm;1H NMR(600 MHz,CDCl3)δ:8.08~8.01(m,1H,5-H or 8-H),8.01~7.96,(m,1H,5-H or 8-H),7.67~7.61(m,2H,6,7-H),3.06(d,J=4.5 Hz,1H,12-H),2.36~2.25(m,1H,13-Hexo),2.13~2.00(m,1H,14-Hendo),1.46~1.39(m,5H,13-Hendo,14-Hexo,18-H),1.12(s,3H,16-H),0.63(s,3H,17-H);13C NMR(151 MHz,CDCl3)δ:165.52,163.75,141.51,141.31,128.78,128.65,128.07,128.05,54.21,53.79,53.29,31.84,24.63,20.29,18.52,10.04;IR(KBr)ν:3059,2957,2926,2869,1510,1471,1444 cm-1;MS(ESI)m/z:238.12{[M+H]+}。
樟脑基6,7-二甲基喹喔啉(3b):淡黄色固体,收率82%,m.p.77.1~79.0 ℃;UV-Vis(EtOH)λmax:336.96,322.00,247.00 nm;1H NMR(600 MHz,CDCl3)δ:7.80(s,1H,5-H or 8-H),7.72(s,1H,5-H or 8-H),3.02(d,J=4.4 Hz,1H,12-H),2.45(s,6H,Ar-CH3),2.31~2.22(m,1H,13-Hexo),2.08~1.99(m,1H,14-Hendo),1.43~1.36(m,5H,13-Hendo,14-Hexo,18-H),1.10(s,3H,16-H),0.61(s,3H,17-H);13C NMR(151 MHz,CDCl3)δ:164.56,162.82,140.07,139.91,137.91,137.90,128.30,128.13,54.18,53.64,53.26,31.91,24.70,20.23,20.08,20.04,18.55,10.06;IR(KBr)ν:3044,2959,2922,2873,1606,1504,1451 cm-1;MS(ESI)m/z:267.11 {[M+H]+}。
樟脑基6,7-二氟喹喔啉(3c):白色固体,收率86%,m.p.158.2~159.1 ℃;UV-Vis(EtOH)λmax:312.50,240.50 nm;1H NMR(600 MHz,CDCl3)δ:7.82~7.76(m,1H,5-H or 8-H),7.76~7.70(m,1H,5-H or 8-H),3.05(d,J=4.5 Hz,1H,12-H),2.35~2.27(m,1H,13-Hexo),2.12~2.02(m,1H,14-Hendo),1.44~1.36(m,5H,13-Hendo,14-Hexo,18-H),1.12(s,3H,16-H),0.61(s,3H,17-H);13C NMR(151 MHz,CDCl3)δ:165.87,164.09,151.63,149.95,138.39,138.18,115.03,114.80,54.41,53.85,53.18,31.71,24.52,20.23,18.46,9.92;IR(KBr)ν:3089,3028,2983,2961,2924,2879,1612,1587,1528,1471 cm-1;MS(ESI)m/z:274.06{[M+H]+}。
樟脑基6,7-二氯喹喔啉(3d):淡红色固体,收率88%,m.p.144.0~147.0 ℃;UV-Vis(EtOH)λmax:322.50,247.00 nm;1H NMR(600 MHz,CDCl3)δ:8.33(s,1H,5-H or 8-H),8.26(s,1H,5-H or 8-H),3.05(d,J=4.5 Hz,1H,12-H),2.35~2.26(m,1H,13-Hexo),2.12~2.02(m,1H,14-Hendo),1.42~1.37(m,5H,13-Hendo,14-Hexo,18-H),1.12(s,3H,16-H),0.61(s,3H,17-H);13C NMR(151 MHz,CDCl3)δ:166.83,165.03,140.93,140.77,132.98,132.80,124.04,124.03,54.15,53.96,53.24,31.64,24.45,20.27,18.41,9.88;IR(KBr)ν:3091,3055,2961,2928,2869,1585,1573,1475,1461 cm-1;MS(ESI)m/z:306.00 {[M+H]+}。
樟脑基6,7-二溴喹喔啉(3e):淡红色固体,收率90%,m.p.138.5~140.2 ℃;UV-Vis(EtOH)λmax:336.25,324.00,250.00 nm;1H NMR(600 MHz,CDCl3)δ:8.15(s,1H,5-H or 8-H),8.08(s,1H,5-H or 8-H),3.06(d,J=4.5 Hz,1H,12-H),2.35~2.30(m,1H,13-Hexo),2.13~2.04(m,1H,14-Hendo),1.44~1.39(m,5H,13-Hendo,14-Hexo,18-H),1.13(s,3H,16-H),0.62(s,3H,17-H);13C NMR(151 MHz,CDCl3)δ:166.74,164.95,140.36,140.19,132.17,132.16,129.61,129.42,54.18,53.94,53.23,31.67,24.48,20.27,18.4,9.89;IR(KBr)ν:3077,2959,2930,2873,1579,1469,1457 cm-1;MS(ESI)m/z:395.87{[M+H]+}。
樟脑基6-氟喹喔啉(3f):白色固体,收率38%,m.p.153.8~155.6 ℃;UV-Vis(EtOH)λmax:315.50,241.00 nm;1H NMR(600 MHz,CDCl3)δ:7.97(dd,J=9.1 Hz,5.8 Hz,1H,8-H),7.69(dd,J=9.5 Hz,2.8 Hz,1H,5-H),7.41(td,J=8.6 Hz,2.8 Hz,1H,7-H),3.06(d,J=4.4 Hz,1H,12-H),2.35~2.26(m,1H,13-Hexo),2.12~2.02(m,1H,14-Hendo),1.46~1.38(m,5H,13-Hendo,14-Hexo,18-H),1.13(s,3H,16-H),0.63(s,3H,17-H);13C NMR(151 MHz,CDCl3)δ:166.40,163.19,161.73,142.30,138.21,130.21,117.41,113.07,54.29,53.89,53.14,31.78,24.58,20.27,18.49,9.96;IR(KBr)ν:3053,2957,2930,2875,1632,1597,1514,1457 cm-1;MS(ESI)m/z:256.08{[M+H]+}。
樟脑基7-氟喹喔啉(3g):白色固体,收率40%,m.p.153.2~154.4 ℃;UV-Vis(EtOH)λmax:314.50,241.00 nm;1H NMR(600 MHz,CDCl3)δ:8.01(dd,J=9.1 Hz,5.8 Hz,1H,5-H),7.62(dd,J=9.4 Hz,2.8 Hz,1H,8-H),7.40(td,J=8.6 Hz,2.8 Hz,1H,6-H),3.06(d,J=4.4 Hz,1H,12-H),2.36~2.26(m,1H,13-Hexo),2.12~2.01(m,1H,14-Hendo),1.45~1.37(m,5H,13-Hendo,14-Hexo,18-H),1.12(s,3H,16-H),0.62(s,3H,17-H);13C NMR (151 MHz,CDCl3)δ:164.98,164.65,161.73,142.10,138.44,130.38,117.40,112.92,54.30,53.71,53.31,31.78,24.59,20.28,18.49,9.99;IR(KBr)ν:2987,2961,2932,2869,1618,1561,1479,1440 cm-1;MS(ESI)m/z:256.09{[M+H]+}。
樟脑基6-氯喹喔啉(3h):白色固体,收率37%,m.p.140.6~143.8 ℃;UV-Vis(EtOH)λmax:319.50,244.00 nm;1H NMR(600 MHz,CDCl3)δ:8.04(d,J=2.3 Hz,1H,5-H),7.91(d,J=8.8 Hz,1H,8-H),7.59(dd,J=8.8 Hz,2.3 Hz,1H,7-H),3.06(d,J=4.4 Hz,1H,12-H),2.36~2.26(m,1H,13-Hexo),2.14~2.02(m,1H,14-Hendo),1.44~1.38(m,5H,13-Hendo,14-Hexo,18-H),1.13(s,3H,16-H),0.63(s,3H,17-H);13C NMR(151 MHz,CDCl3)δ:166.47,164.01,141.96,139.83,133.59,129.74,128.77,128.0,54.25,53.90,53.22,31.75,24.54,20.28,18.47,9.94;IR(KBr)ν:3067,3046,2995,2955,2924,2867,1585,1473,1448 cm-1;MS(ESI)m/z:272.05{[M+H]+}。
樟脑基7-氯喹喔啉(3i):白色固体,收率41%,m.p.139.8~142.8 ℃;UV-Vis(EtOH)λmax:315.50,241.00 nm;1H NMR(600 MHz,CDCl3)δ:7.99~7.94(m,2H,5,8-H),7.60(dd,J=8.8 Hz,2.3 Hz,1H,6-H),3.07(d,J=4.4 Hz,1H,12-H),2.35~2.3(m,1H,13-Hexo),2.1~2.01(m,1H,14-Hendo),1.49~1.36(m,5H,13-Hendo,14-Hexo,18-H),1.13(s,3H,16-H),0.63(s,3H,17-H);13C NMR(151 MHz,CDCl3)δ:165.80,164.70,141.79,140.04,133.59,129.90,128.76,127.85,54.24,53.82,53.28,31.74,24.55,20.28,18.47,9.97;IR(KBr)ν:3051,2961,2926,2875,1593,1508,1473,1448 cm-1;MS(ESI)m/z:272.05{[M+H]+}。
樟脑基6-溴喹喔啉(3j):白色固体,收率39%,m.p.134.3~136.8 ℃;UV-Vis(EtOH)λmax:319.50,245.00 nm;1H NMR(600 MHz,CDCl3)δ:8.22(d,J=2.2 Hz,1H,5-H),7.85(d,J=8.8 Hz,1H,8-H),7.72(dd,J=8.8 Hz,2.2 Hz,1H,7-H),3.06(d,J=4.5 Hz,1H,12-H),2.34~2.27(m,1H,13-Hexo),2.11~2.04(m,1H,14-Hendo),1.44~1.39(m,5H,13-Hendo,14-Hexo,18-H),1.13(s,3H,16-H),0.62(s,3H,17-H);13C NMR(151 MHz,CDCl3)δ:166.43,164.13,142.28,140.14,131.41,131.31,129.93,121.58,54.24,53.91,53.27,31.76,24.51,20.28,18.47,9.93;IR(KBr)ν:3061,3038,2951,2920,2883,2869,1585,1504,1473,1453 cm-1;MS(ESI)m/z:316.98{[M+H]+}。
樟脑基7-溴喹喔啉(3k):白色固体,收率40%,m.p.133.4~135.2 ℃;UV-Vis(EtOH)λmax:319.50,245.00 nm;1H NMR(600 MHz,CDCl3)δ:8.15(d,J=2.2 Hz,1H,8-H),7.90(d,J=8.8 Hz,1H,5-H),7.73(dd,J=8.8 Hz,2.2 Hz,1H,6-H),3.07(d,J=4.5 Hz,1H,12-H),2.36~2.28(m,1H,13-Hexo),2.12~2.03(m,1H,14-Hendo),1.44~1.38(m,5H,13-Hendo,14-Hexo,18-H),1.13(s,3H,16-H),0.63(s,3H,17-H);13C NMR(151 MHz,CDCl3)δ:165.91,164.65,142.10,140.34,131.40,131.12,130.08,121.57,54.2,53.86,53.26,31.70,24.55,20.27,18.46,9.96;IR(KBr)ν:3063,2959,2930,2875,1587,1500,1469,1451 cm-1;MS(ESI)m/z:316.98{[M+H]+}。
樟脑基6-硝基喹喔啉(3l):白色固体,收率38%;m.p.154.8~156.7 ℃;UV-Vis(EtOH) λmax:297.00,257.00 nm;1H NMR(600 MHz,CDCl3)δ:8.88(d,J=2.5 Hz,1H,8-H),8.44(dd,J=9.0 Hz,2.5 Hz,1H,7-H),8.15(d,J=9.0 Hz,1H,5-H),3.14(d,J=4.5 Hz,1H,12-H),2.41~2.33(m,1H,13-Hexo),2.17~2.09(m,1H,14-Hendo),1.48~1.42(m,5H,13-Hendo,14-Hexo,18-H),1.17(s,3H,16-H),0.65(s,3H,17-H);13C NMR(151 MHz,CDCl3)δ:169.01,166.12,146.66,144.75,140.50,130.08,124.96,121.83,54.31,54.30,53.20,31.63,24.4,20.35,18.40,9.87;IR(KBr)ν:3089,2965,2926,2876,1606,1540,1504,1477,1448 cm-1;MS(ESI)m/z:283.07{[M+H]+}。
樟脑基7-硝基喹喔啉(3m):白色固体,收率42%;m.p.154.6~156.3 ℃;UV-Vis(EtOH)λmax:297.00,256.50 nm;1H NMR(600 MHz,CDCl3)δ:8.95(d,J=2.5 Hz,1H,5-H),8.43(dd,J=9.0 Hz,2.5 Hz,1H,6-H),8.10(d,J=9.0 Hz,1H,8-H),3.13(d,J=4.5 Hz,1H,12-H),2.40~2.34(m,1H,13-Hexo),2.17~2.09(m,1H,14-Hendo),1.47~1.42(m,5H,13-Hendo,14-Hexo,18-H),1.17(s,3H,16-H),0.65(s,3H,17-H);13C NMR(151 MHz,CDCl3)δ:167.93,167.18,146.67,144.57,140.66,129.94,125.11,121.86,54.33,54.08,53.47,31.59,24.42,20.35,18.40,9.87;IR(KBr)ν:3110,3063,2965,2928,2873,1600,1540,1504,1448 cm-1;MS(ESI)m/z:283.07{[M+H]+}。
樟脑基5-甲基喹喔啉(3n):淡黄色固体,收率42%,m.p.133.6~135.3 ℃;UV-Vis(EtOH)λmax:317.50,245.50 nm;1H NMR(600 MHz,CDCl3)δ:7.90(dd,J=8.1 Hz,0.6 Hz,1 H,8-H),7.53(t,J=7.6 Hz,1 H,7-H),7.51~7.47(m,1 H,6-H),3.12(d,J=4.4 Hz,1H,12-H),2.80(s,3H,Ar-CH3),2.34~2.26(m,1H,13-Hexo),2.09~2.04(m,1H,14-Hendo),1.46~1.39(m,5H,13-Hendo,14-Hexo,18-H),1.13(s,3H,16-H),0.64(s,3H,17-H);13C NMR(151 MHz,CDCl3)δ:164.94,162.61,141.53,140.38,136.72,128.56,127.52,126.76,54.19,53.65,53.35,31.80,24.85,20.32,18.59,17.70,10.06;IR(KBr)ν:3065,3028,2965,2926,2877,1591,1512,1479,1451 cm-1;MS(ESI)m/z:252.11{[M+H]+}。
樟脑基8-甲基喹喔啉(3o):淡黄色固体,收率46%,m.p.132.3~134.2 ℃;UV-Vis(EtOH)λmax:317.00,245.50 nm;1H NMR(600 MHz,CDCl3)δ:7.83(d,J=7.3 Hz,1H,5-H),7.55~7.51(m,1H,6-H),7.50(d,J=7.1 Hz,1H,7-H),3.06(d,J=4.5 Hz,1H,12-H),2.81(s,3H,Ar-CH3),2.34~2.27(m,1H,13-Hexo),2.09~2.03(m,1H,14-Hendo),1.45~1.39(m,5H,13-Hendo,14-Hexo,18-H),1.13(s,3H,16-H),0.63(s,3H,17-H);13C NMR(151 MHz,CDCl3)δ:164.11,163.13,141.20,140.54,137.17,128.40,127.50,126.44,54.26,53.83,53.25,31.96,24.72,20.31,18.5,17.25,9.97;IR(KBr)ν:3063,3024,2967,2928,2879,1587,1516,1479 cm-1;MS(ESI)m/z:252.11{[M+H]+}。
樟脑基6-苯甲酰基喹喔啉(3p):淡黄色固体,收率36%,m.p.105.1~106.9 ℃;UV-Vis(EtOH)λmax:322.50,258.00 nm;1H NMR(600 MHz,CDCl3)δ:8.37(d,J=0.9 Hz,1H,8-H),8.17~8.12(m,2H,5-H and 7-H),7.87(d,J=7.1 Hz,2H,COPh-H),7.62(t,J=7.5 Hz,1H,COPh-H),7.51(t,J=7.7 Hz,2H,COPh-H),3.08(d,J=4.4 Hz,1H,12-H),2.38~2.29(m,1H,13-Hexo),2.12~2.07(m,1H,14-Hendo),1.47~1.42(m,5H,13-Hendo,14-Hexo,18-H),1.14(s,3H,16-H),0.65(s,3H,17-H);13C NMR(151 MHz,CDCl3)δ:195.93,167.62,164.92,143.79,140.43,137.44,136.75,132.58,131.88,130.10,129.21,128.42,128.38,54.29,54.12,53.27,31.77,24.54,20.34,18.47,9.97;IR(KBr)ν:3059,2959,2926,2871,1661,1628,1600,1577,1451 cm-1;MS(ESI)m/z:342.08{[M+H]+}。
樟脑基7-苯甲酰基喹喔啉(3q):淡黄色固体,收率40%,m.p.104.6~106.5 ℃;UV-Vis(EtOH)λmax:322.50,258.00 nm;1H NMR(600 MHz,CDCl3)δ:8.43(d,J=1.8 Hz,1H,8-H),8.15(dd,J=8.5 Hz,1.9 Hz,1H,6-H),8.10(d,J=8.5 Hz,1H,5-H),7.88(d,J=7.0 Hz,2H,COPh-H),7.63(t,J=7.5 Hz,1H,COPh-H),7.52(t,J=7.7 Hz,2H,COPh-H),3.12(d,J=4.5 Hz,1H,12-H),2.40~2.3(m,1H,13-Hexo),2.15~2.06(m,1H,14-Hendo),1.51~1.41(m,5H,13-Hendo,14-Hexo,8-H),1.15(s,3H,16-H),0.66(s,3H,17-H);13C NMR(151 MHz,CDCl3)δ:196.07,166.71,165.81,143.59,140.63,137.55,136.85,132.55,131.90,130.06,129.08,128.45,128.40,54.28,53.92,53.45,31.74,24.55,20.33,18.46,9.96;IR(KBr)ν:3059,2965,2930, 2873,1663,1632,1602,1579,1446 cm-1;MS(ESI)m/z:342.08{[M+H]+}。
樟脑基5,8-二溴喹喔啉(3r):白色固体,收率88%,m.p.203.5~206.1 ℃;UV-Vis(EtOH)λmax:319.50,256.00 nm;1H NMR(600 MHz,CDCl3)δ:7.84~7.81(m,2H,6,7-H),3.27(d,J=4.5 Hz,1H,12-H),2.39~2.31(m,1H,13-Hexo),2.12~2.09(m,1H,14-Hendo),1.49(s,3H,18-H),1.48~1.43(m,2H,13-Hendo,14-Hexo),1.16(s,3H,16-H),0.63(s,3H,17-H);13C NMR(151 MHz,CDCl3)δ:167.13,165.39,140.43,140.26,131.79,131.77,123.76,123.06,54.52,54.07,53.08,31.64,24.63,20.50,18.47,9.94;IR(KBr)ν:2955,2928,2871,1573,1477,1451 cm-1;MS(ESI)m/z:395.87{[M+H]+}。
(2)3s的合成
将0.50 g(1.7 mmol)3r、0.38 g(4.25 mmol) CuCN、5 mL DMF和0.3 mg KI混合,150 ℃搅拌3 h(TLC监测)。反应结束后倒入100 mL三氯化铁水溶液中搅拌1 h,用乙酸乙酯萃取(3×5 mL),有机相分别用饱和氯化钠溶液(3×5 mL)和去离子水(3×5 mL)洗涤,无水硫酸钠干燥后,经硅胶柱层[洗脱剂:V(石油醚)/V(乙酸乙酯)=5/1]纯化得淡绿色固体3s。
樟脑基5,8-二氰基喹喔啉(3s):淡绿色固体,收率56%,m.p.236.9~238.5 ℃;UV-Vis(EtOH)λmax:322.50,247.00 nm;1H NMR(600 MHz,CDCl3)δ:8.07(s,2H,6,7-H),3.28(d,J=4.5 Hz,1H,12-H),2.42~2.38(m,1H,13-Hexo),2.21~2.11(m,1H,14-Hendo),1.48(s,3H,18-H),1.47~1.41(m,2H,13-Hendo,14-Hexo),1.17(s,3H,16-H),0.63(s,3H,17-H);13C NMR (151 MHz,CDCl3)δ:169.77,167.92,141.77,141.73,132.40,132.34,117.11,116.80,115.57,115.39,54.73,54.57,53.25,31.44,24.34,20.49,18.37,9.72;IR(KBr)ν:3073,2967,2934,2881,2236,1591,1477,1393 cm-1;MS(ESI)m/z:288.06{[M+H]+}。
(3) 目标产物的生物活性测试
参考文献[35],本文采用离体法(也称琼脂稀释法)测试目标化合物的抑菌活性,结果列于表1;采用油菜平皿法和稗草小杯法测试目标化合物的除草活性,结果列于表2。
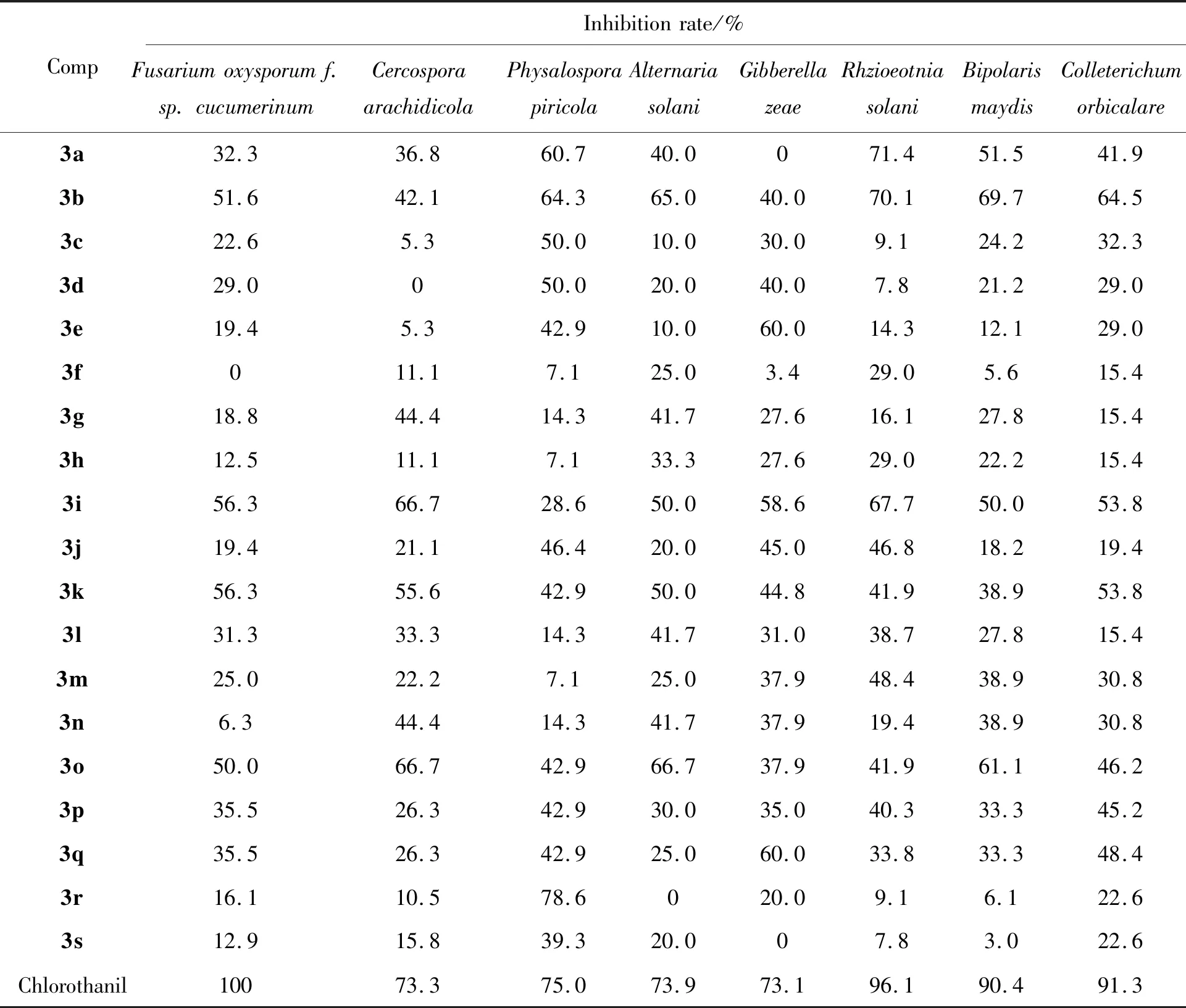
表1 化合物3a~3s的抑菌活性*Table 1 Antifungal activity of compounds 3a~3s
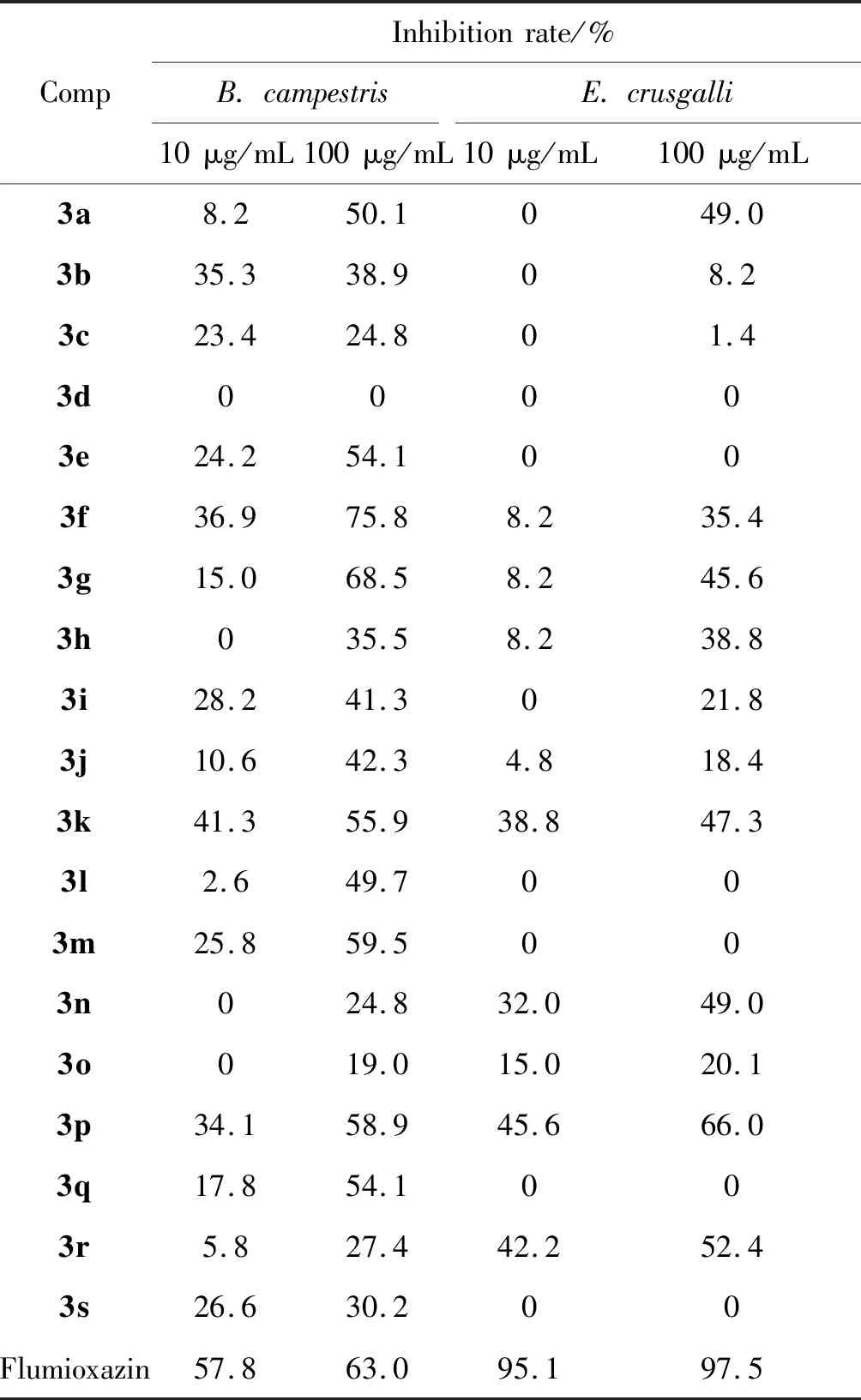
表2 化合物3a~3s的除草活性Table 2 Herbicidal activity of compounds 3a~3s
2 结果与讨论
2.1 合成
目标化合物3a~3s的合成过程中,樟脑醌与邻苯二胺的聚合会降低产率。采用较多量的溶剂可以减少聚合,提高目标化合物的产率。
2.2 表征
D-(+)-樟脑醌2与单取代邻苯二胺缩合将会产生两个异构体。以化合物3h和3i为例,通过2D-NMR中的HMBC对异构体进行了结构分析鉴别。
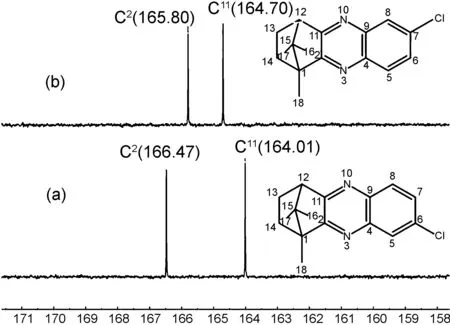
δ图1 化合物3h和3i的13C NMR谱图Figure 1 13C NMR spectra of compounds 3h and 3i
结合HMBC(谱图略)中的(a)与(b)均观察到的18-H与C2相关;图2(a)的HMBC中7-H与C11相关,不与C2相关,表明(a)为化合物3h;而图2(b)的HMBC中6-H与C2相关,不与C11相关,表明(b)为化合物3i。此外,从图1的13C NMR谱图可知(a)的C2(166.47)化学位移大于(b)的C2(165.80)化学位移,(a)的C11(164.01)化学位移小于(b)的C11(164.70)化学位移。总之,5-位与6-位取代的樟脑基喹喔啉的C2化学位移大于7-位与8-位取代的樟脑基喹喔啉的C2化学位移,5-位与6-位取代的樟脑基喹喔啉的C11化学位移小于7-位与8-位取代的樟脑基喹喔啉的C11化学位移。上述结论与GLII报道的4个单取代甲基樟脑基喹喔啉的13C NMR数据相符[36]。从目标产物的FT-IR数据可知,在3100~3000 cm-1出现的弱吸收峰为Ar—H的伸缩振动吸收峰;芳环骨架伸缩振动吸收峰出现在1600~1450 cm-1。这些HR-MS(ESI)数据均与目标化合物3的分子量相符。
2.3 生物活性测试结果
(1) 抑菌活性
如表1的活性数据所示,在50 μg·mL-1的浓度下,绝大部分樟脑基喹喔啉化合物对花生褐斑病菌、小麦赤霉病菌、黄瓜枯萎病菌、苹果轮纹病菌、番茄早疫病菌、玉米小斑病菌、西瓜炭疽病菌以及水稻纹枯病菌等8种病原菌有一定的抑制活性。从总体上看,目标化合物对苹果轮纹病菌、水稻纹枯病菌的抑制活性较好,其中化合物3a与3b对水稻纹枯病菌的抑制率分别为71.4%和70.1%(均达B级活性水平);而化合物3r对苹果轮纹病菌的抑制率达78.6%(B级活性水平),优于阳性对照百菌清。
(2) 除草活性
从表2可知,在100 μg·mL-1浓度下,目标化合物对油菜胚根生长有一定的抑制作用。化合物3f和3g对油菜胚根生长的抑制率分别为75.8%和68.5%(均达B级活性水平),均优阳性对照于丙炔氟草胺。
以D-(+)-樟脑为原料,合成得到19个樟脑基喹喔啉化合物3a~3s。初步生物活性测试显示,目标化合物对8种植物病原菌具有一定的抑制活性。化合物3a与3b对水稻纹枯病菌的抑制率分别为71.4%和70.1%;化合物3r对苹果轮纹病菌的抑制率达78.6%,优于阳性对照百菌清。目标化合物对油菜胚根生长具有一定的抑制作用,化合物3f与3g对油菜胚根生长的抑制率分别为75.8%和68.5%,均优于阳性对照丙炔氟草胺。
致谢:抑菌和除草活性测试由南开大学元素有机化学国家重点实验室生物活性测试室完成,谨表谢意!
