DEC1基因shRNA慢病毒表达载体的构建及其稳转胶质瘤细胞系的建立
2017-11-27魏学辉李晓明张耀茹懿王秦豪李霞林伟
魏学辉 李晓明 张耀 茹懿 王秦豪 李霞* 林伟*
(第四军医大学: 1西京医院神经外科; 2基础部生物化学与分子生物学教研室,陕西 西安 710032)
·脑胶质细胞瘤研究·
DEC1基因shRNA慢病毒表达载体的构建及其稳转胶质瘤细胞系的建立
魏学辉1李晓明1张耀1茹懿2王秦豪2李霞2*林伟1*
(第四军医大学:1西京医院神经外科;2基础部生物化学与分子生物学教研室,陕西 西安 710032)
目的构建人分化型胚胎软骨发育基因1 (DEC1)的短发卡RNA (shRNA)慢病毒表达载体并建立稳定敲减DEC1表达的人脑胶质瘤细胞株T98G。方法根据GenBank中DEC1基因cDNA序列设计合成两条特异性shRNA序列,同时设计1条非特异性序列作为阴性对照,分别克隆到Psi-LVRU6MP质粒载体内,经酶切电泳、DNA测序鉴定后包装成慢病毒颗粒。再以3组慢病毒感染胶质瘤细胞系T98G,用嘌呤霉素筛选后,荧光显微镜下观察mCherry的表达,Western Blot检测各组DEC1蛋白的表达水平。结果DEC1基因 shRNA慢病毒表达载体经酶切、测序鉴定证实克隆正确。荧光显微镜检测显示各组细胞均已被慢病毒高效感染。Western Blot结果显示,两干扰组细胞各自DEC1蛋白表达水平明显低于未处理组和阴性对照组,分别占未处理组的39.47%±0.69%和41.17%±0.89% (Plt;0.05),但两干扰组之间无显著性差异(Pgt;0.05);阴性对照组与未处理组相比亦无明显差异(Pgt;0.05)。结论成功构建DEC1基因shRNA慢病毒表达载体,感染胶质瘤T98G细胞系获得成功。两干扰组慢病毒均能明显敲减目的基因DEC1的表达,为进一步研究DEC1在胶质瘤细胞系T98G中的生物学功能和作用机制提供了实验基础。
分化型胚胎软骨发育基因1; 短发卡RNA; 慢病毒表达载体; 胶质瘤
人分化型胚胎软骨发育基因1 (differentiated embryo-chondrocyte expressed gene 1, DEC1) 是由Shen等[1]1997年首次从人类胚胎软骨组织分离得到,是碱性螺旋-环-螺旋 (basic helix-loop-helix, bHLH) 转录因子家族成员[2-3]。DEC1参与软骨形成[4]、免疫反应[5]、生物节律[6]、脂肪形成[7]等重要生理过程。且研究表明,DEC1作为癌基因,在结肠癌、肺癌、肾癌、乳腺癌等多种肿瘤组织中显著高表达,与部分肿瘤的恶性级别呈正相关[8-9]。特定肿瘤组织中DEC1的表达可被多种有害刺激所诱导,还与肿瘤的缺氧状态密切相关[10]。这些发现提示DEC1与肿瘤恶性表型密切相关,且在肿瘤发生、发展中发挥着重要作用。DEC1在胶质瘤中的作用尚未报道。
RNA干扰(RNA interference, RNAi)是一种由小干扰RNA (small interfeing RNA, siRNA)诱发的特定基因沉默,是细胞固有的对抗外源性核酸的一种自我保护现象,在生物体内普遍存在。RNAi主要发生在基因转录后mRNA的修饰或翻译水平上,具有特异性、高效性和长久性的特点[11]。基于此的RNAi技术因在基因功能研究以及肿瘤等疾病的基因治疗中具有潜在应用价值而受到人们的关注。本研究设计构建了针对人DEC1基因的特异性短发卡RNA (short hairpin RNA, shRNA)真核慢病毒表达载体,用此载体质粒先转染293T细胞获得病毒上清后,再感染人胶质瘤细胞T98G,鉴定并筛选稳定的细胞株,为下一步研究敲减DEC1表达后对胶质瘤细胞生物学行为及对化疗药替莫唑胺敏感性的影响等研究提供实验基础。
材料与方法
一、材料
1.细胞系、菌种、质粒:人脑胶质母细胞瘤T98G细胞源自美国模式培养物收集中心(American type culture collection, ATCC),并由第四军医大学西京医院神经外科研究所保存。大肠杆菌DH5α感受态细胞由第四军医大学基础部生物化学与分子生物学教研室提供。慢病毒包装质粒psPAX2、pMD2.G,psi-LVRU6MP质粒 (含嘌呤霉素抗性基因、氨苄霉素抗性基因) shRNA 载体(图1)购自GeneCopoeia公司。
2.主要试剂:限制性核酸内切酶BamHⅠ和EcoRⅠ、NheⅠ、T4 DNA连接酶(T4 DNA ligase)及PCR试剂盒、聚凝胺(polybrene)为日本TaKaRa公司产品;质粒提取试剂盒及凝胶回收纯化试剂盒为U-gene 公司产品;Lipofectamine 2000、puromycin及Trizol均为美国Invitrogen公司产品;兔抗人DEC1多克隆抗体为Abcam公司产品。小鼠抗人甘油醛-3-磷酸脱氢酶 (glyceraldehyde-3-phosphate dehydrogenase, GAPDH)单抗及 Western blot试剂盒为美国Sigma公司产品。
二、方法
1.人DEC1基因特异性shRNA片段的设计及合成:根据 GenBank中DEC1基因的 cDNA序列(NM_003670.1)和干扰载体的设计要求,利用生物信息学及茎环结构的特点,选择2段靶序列,并在其上游引物引入BamHⅠ酶切位点,下游引物引入EcoRⅠ酶切位点,设计序列为DEC1-shRNA-1和DEC1-shRNA-2,同时设计1条经Blast检索与现有基因文库中所有人源基因均无同源性的非特异性序列为 阴性对照序列Negative-shRNA,最终合成3对含茎环结构的正义与反义寡核苷酸链ssDNA (单链DNA)。DEC1-shRNA-1的正义链:5'-GATCCG-GGCGCAATTAAGCAAGAGTCC-TCAAGAG-GGACTCTTGCTTAATTGCGCC-TTTTTTGGAATT-3';反义链:5'-AATTCCAAAAAA-GGCGCAATTAAGCAA GAGTCC-CTCTTGA-GGACTCTTGCTTAATTGCGCC-CGGATC-3'。DEC1-shRNA-2的正义链:5'-GATCCG-GCAAGATTGTTGCATTGTGTA-TCAAGAG-TACACAATGCAACAATCTTGC-TTTTTTGGAATT-3';反义链:5'-AATTCCAAAAAA-GCAAGATTGTTGCATTGTGTA-CTCTTGA-TACACAATGCAACAATCTTGC-CGGATC-3'。阴性对照序列Negative-shRNA的正义链:5'-GATCCG-GCTTCGCGCCGTAGTCTTA-TCAAGAG-TAAGACTACGGCGCGAAGC-TTTTTTGGAATT-3';反义链:5'-AATTCCAAAAAA-GCTTCGCGCCGTAGTCTTA-CTCTTGA-TAAGACTACGGCGCGAAGC-CGGATC-3',序列由上海生工生物工程公司合成。
2.psi-LVRU6MP-DEC1-shRNA重组质粒载体构建:将上述3对单链寡核苷酸片段退火形成双链DNA。用BamHⅠ和EcoRⅠ双酶切线性化psi-LVRU6MP质粒载体。利用T4 DNA连接酶连接线性psi-LVRU6MP质粒载体和退火形成的双链DNA序列。连接产物经大肠杆菌感受态细胞DH5α转化提取质粒后,以限制性核酸内切酶EcoRⅠ和NheⅠ行双酶切,产物经1%琼脂糖凝胶电泳鉴定。将鉴定正确的质粒命名为:psi-LVRU6MP-DEC1-shRNA-1和psi-LVRU6MP-DEC1-shRNA-2,psi-LVRU6MP-Negative。将鉴定证实后的阳性克隆送上海生工生物工程公司测序鉴定。
3.慢病毒颗粒的包装:分别将包装质粒psPAX2(7.5 μg)、pMD2.G(2.5 μg)以及上述重组质粒载体中的一种(10 μg)混合于1200 μL无血清DMEM(Dulbecco's modified Eagle's medium, DMEM)液中,室温下温育5 min。同时将Lipofectamine 2000(40 μL)混合于1000 μL无血清DMEM液中,室温下温育5 min。将上述室温下稀释的质粒混合液分别与Lipofectamine 2000稀释液混合,室温下温育20 min后加入已接种293T细胞的培养皿中,调整体积至10 mL,于37 ℃、5%CO2饱和湿度培养箱中培养。6 h后换含10%胎牛血清的新DMEM培养基继续培养。转染48 h后,收集细胞上清液,-80 ℃保存。分别命名为DEC1-shRNA-1病毒,DEC1-shRNA-2病毒和阴性对照Negative-shRNA 病毒。

图1 psi-LVRU6MP载体的结构
Fig 1 Map of the psi-LVRU6MP vector
图2 重组质粒载体的酶切电泳鉴定
Fig 2 The identification of recombinant plasmid vector by enzyme digestion and electrophoresis
A: psi-LVRU6MP-Negative plasmid vector; B: psi-LVRU6MP-DEC1-shRNA-1 plasmid vector; C: psi-LVRU6MP-DEC1-shRNA-2 plasmid vector.
M: DL10000 Marker.
图3 重组质粒载体psi-LVRU6MP-DEC1-shRNA测序鉴定(下划线为DEC1基因特异性shRNA序列)
Fig 3 The identification of the recombinant plasmid vector psi-LVRU6MP-DEC1-shRNA by DNA sequencing (shRNA sequence targeting DEC1 gene were underlined)
A: psi-LVRU6MP-DEC1-shRNA-1 plasmid vector; B: psi-LVRU6MP-DEC1-shRNA-2 plasmid vector.
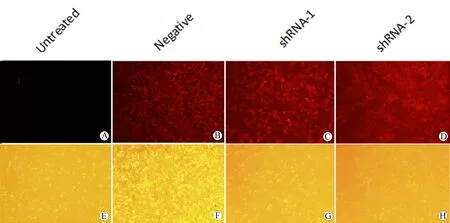
图4 荧光显微镜观察各组慢病毒感染的T98G细胞mcherry的表达(×40; 上:荧光视野;下:明场视野)
Fig 4 Fluorescence microscopy was used to observe the expression of mCherry in T98G cells infected with shRNA lentivirus (×40; Up: Fluorescence field; Down: Bright field of vision)
A: Fluorescence field of vision of untreated group; B: Flourescence field of vision of negative group; C: Flourescence field of vision of shRNA-1 group; D: Flourescence field of vision of shRNA-2 group; E: Bright field of vision of untreated group; F: Bright field of vision of negative group; G: Bright field of vision of shRNA-1 group; H: Bright field of vision of shRNA-2 group.
4.稳定转染T98G胶质瘤细胞系的建立:将上述三种病毒液各1 mL分别加入含有T98G细胞的6孔板中,再各加入1 mL含10%胎牛血清的DMEM培养液,另每孔加入聚凝胺(polybrene),使其终浓度为8 μg/mL,以促进感染。同时设立T98G的未处理对照组。感染72 h后,荧光显微镜下观察红色荧光(mCherry)的表达情况,初步评估各病毒液的感染效率。用含puromycin(0.8 μg/mL)的DMEM完全培养基持续筛选4 w,待不再有细胞死亡后,维持0.8 μg/mL的puromycin压力筛选,等待干扰效果鉴定。
5.Western Blot检测T98G稳转胶质瘤细胞DEC1蛋白的表达:收集6 cm培养皿中T98G未处理组、DEC1-shRNA-1组、DEC1-shRNA-2组、阴性对照组Negative-shRNA细胞,用RIPA裂解细胞,提取蛋白后行蛋白定量。蛋白提取液中加入1/4体积的5×十二烷基硫酸钠(sodium dodecyl sulfate, SDS)凝胶上样缓冲液,沸水中煮5 min,按照蛋白定量结果加样,10%SDS聚丙烯酰胺凝胶电泳90 min后,转膜2 h,将蛋白转移至硝酸纤维素膜(nitrocellulose membrane, NC)上。5%脱脂奶室温封闭NC膜1 h,加入一抗DEC1多克隆抗体(1 ∶5000)或GAPDH单克隆抗体(1 ∶3000)4 ℃封闭过夜。TBST缓冲液(tris buffered saline tween, TBST)洗涤3次,每次10 min,分别加入AP标记的山羊抗兔、山羊抗小鼠二抗(均为1 ∶5000)室温孵育1 h,TBST洗涤后AP显色。

结 果
一、psi-LVRU6MP-DEC1-shRNA的构建和鉴定
将合成的三对寡核苷酸链经退火后形成三条双链DNA片段,将上述片段从BamHⅠ和EcoRⅠ两个酶切位点克隆到质粒载体psi-LVRU6MP中,新的质粒命名:psi-LVRU6MP-DEC1-shRNA1、psi-LVRU6MP-DEC1-shRNA-2和psi-LVRU6MP-Negative。用限制性核酸内切酶EcoRⅠ和NheⅠ对上述3个质粒载体进行双酶切,得到3463 bp和4894 bp 两条片段,用1%琼脂糖凝胶电泳,初步证明克隆正确(图2)。将阳性克隆送DNA测序证实干扰载体中特异性干扰序列完全正确(图3)。
二、慢病毒感染T98G胶质瘤细胞荧光显微镜下观察
本实验选用的干扰质粒载体psi-LVRU6MP含有报告基因mCherry,其表达的mCherry蛋白能在荧光显微镜下显现肉眼可见的红色荧光。慢病毒感染的T98G胶质瘤细胞系,其DEC1-shRNA-1组和DEC1-shRNA- 2组以及Negative-shRNA阴性对照组细胞在倒置荧光显微镜下均可见明显的红色荧光,而未感染的T98G细胞组未见到红色荧光,表明这3种病毒均能高效感染胶质瘤细胞T98G (图4)。
三、Western Blot检测T98G各组细胞DEC1蛋白的表达量
Western Blot 结果(重复3次)显示:DEC1-shRNA-1和DEC1-shRNA-2组T98G细胞的DEC1表达量明显低于未处理组和阴性对照组Negative-shRNA。DEC1-shRNA-1组是未处理组的39.47%±0.69%(t=87.27,P=0.0001),DEC1-shRNA-2组是未处理组的41.17%±0.89%(t=66.29,P=0.0002),而DEC1-shRNA-1组和DEC1-shRNA-2组之间无显著性差异(t=3.09,P=0.09);阴性对照组与未处理组相比亦无明显差异(t=2.47,P=0.13)(图5),说明DEC1基因表达的敲减效果明显,敲减DEC1基因表达的T98G胶质瘤稳转细胞系建立成功。
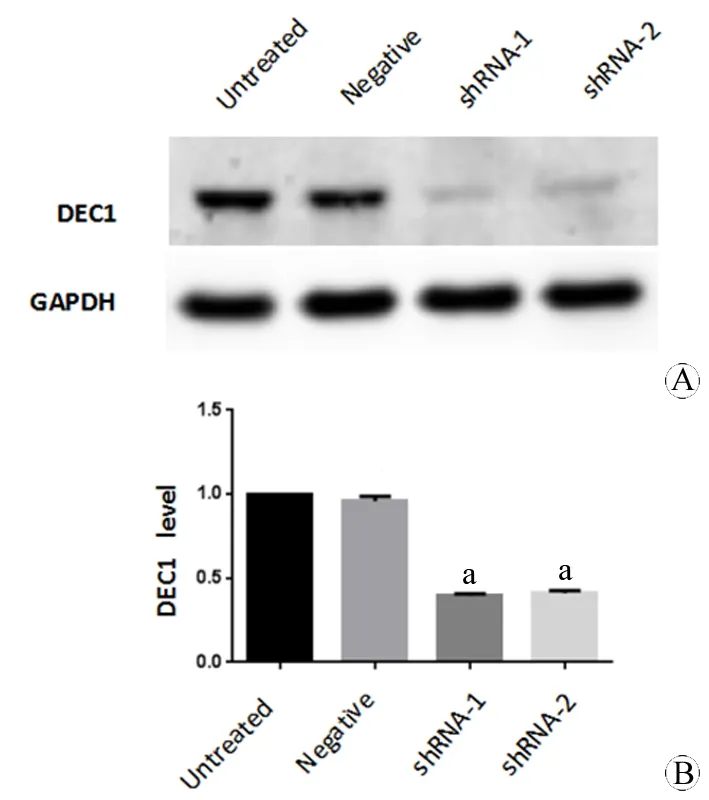
图5 Western Blot 检测DEC1蛋白在T98G各组细胞中的表达
Fig 5 Detection of DEC1 protein expression in T98G cells of each group by Western Blot
A: DEC1 protein expression in each group of T98G cells by Western Blot; B: Statistical analysis of DEC1 protein expression in T98G cells of each group of (n=3)
aPlt;0.05,vsuntreated group.
讨 论
DEC1基因在不同的哺乳动物中有不同的命名。Shen[1]在人类软骨细胞分化的原代培养中诱导的人类基因命名为DEC1,Boudjelal[2]在小鼠胚胎癌性细胞诱导表达的同源基因命名为Stral3,Rossner在大鼠分化的神经元中发现DEC1 的同源基因命名为SHARP-2[3]。DEC1基因位于人类染色体3p25.3-26 上,大约5.7 Kb,包含5个外显子和4个内含子,DEC1启动子区域包括多个GC盒而非TATA盒,在转录因子数据库中发现其5'端区域有多个转录因子结合位点,其中包括cAMP应答元件及多个 E-box[12]。DEC1蛋白由412个氨基酸组成,可被血清饥饿、缺氧、全反维甲酸等细胞外刺激诱导表达,参与细胞分化和凋亡、 周期调节等。研究发现DEC1在很多肿瘤如白血病、结肠癌、肺腺癌、肾癌等组织中存在高表达,可调控肿瘤生长、凋亡相关因子[13]的表达,因而参与肿瘤的发生发展。
由于神经细胞的不可分裂特性,使基因导入系统的选择受到了限制,传统的逆转录病毒不能感染非分裂细胞,而腺病毒不能整合入宿主细胞基因组,因此都存在一定的缺陷,而慢病毒载体可以有效解决以上问题。慢病毒属于逆转录病毒的一种,病毒能在体内、外高效感染分裂细胞和非分裂细胞并整合入基因组,故由慢病毒载体介导而导入的目的基因可以在神经细胞[14-15]中得到长期而稳定的表达。这些优势是其它病毒介导的外源基因传递系统所无可比拟的。因此,慢病毒载体目前被认为是神经系统比较适合的载体系统。本实验成功构建了慢病毒干扰质粒载体psi-LVRU6MP-DEC1-shRNA,与辅助质粒共转染293T细胞获得病毒上清,感染T98G胶质瘤细胞系后,DEC1-shRNA-1组和DEC1-shRNA-2组以及Negative-shRNA阴性对照组细胞在倒置荧光显微镜下均可见明显的红色荧光,表明该慢病毒系统能高效感染神经胶质瘤细胞。Western Blot结果显示DEC1-shRNA-1组和DEC1-shRNA-2组细胞中DEC1表达水平较对照组明显降低,说明稳定敲减DEC1的T98G细胞系建立成功。
1SHEN M, KAWAMOTO T, YAN W, et al. Molecular characterization of the novel basic helix-loop-helix protein DEC1 expressed in differentiated human embryo chondrocytes [J]. Biochem Biophys Res Commun, 1997, 236(2): 294-298.
2BOUDJELAL M, TANEJA R, MATSUBARA S, et al. Overexpression of Stra13, a novel retinoic acid-inducible gene of the basic helix-loop-helix family, inhibits mesodermal and promotes neuronal differentiation of P19 cells [J]. Genes Dev, 1997, 11(16): 2052-2065.
3ROSSNER M J, DORR J, GASS P, et al. SHARPs: mammalian enhancer-of-split- and hairy-related proteins coupled to neuronal stimulation [J]. Mol Cell Neurosci, 1997, 10(3-4): 460-475.
4JINHUA H, ZHAO M, WEI S, et al. Down regulation of differentiated embryo-chondrocyte expressed gene 1 is related to the decrease of osteogenic capacity [J]. Curr Drug Targets, 2014, 15(4): 432-441.
5MIYAZAKI K, MIYAZAKI M, GUO Y, et al. The role of the basic helix-loop-helix transcription factor Dec1 in the regulatory T cells [J]. J Immunol, 2010, 185(12): 7330-7339.
6NAKASHIMA A, KAWAMOTO T, HONDA K K, et al. DEC1 modulates the circadian phase of clock gene expression [J]. Mol Cell Biol, 2008, 28(12): 4080-4092.
7SHEN L, CUI A, XUE Y, et al. Hepatic differentiated embryo-chondrocyte-expressed gene 1 (Dec1) inhibits sterol regulatory element-binding protein-1c (Srebp-1c) expression and alleviates fatty liver phenotype [J]. J Biol Chem, 2014, 289(34): 23332-23342.
8ZHENG Y, JIA Y, WANG Y, et al. The hypoxia-regulated transcription factor DEC1 (Stra13, SHARP-2) and its expression in gastric cancer [J]. OMICS, 2009, 13(4): 301-306.
9SHI X H, ZHENG Y, SUN Q, et al.DEC1 nuclear expression: a marker of differentiation grade in hepatocellular carcinoma [J]. World J Gastroenterol, 2011, 17(15): 2037-2043.
10ZHENG Y, SHI X, WANG M, et al. The increased expression of DEC1 gene is related to HIF-1alpha protein in gastric cancer cell lines [J]. Mol Biol Rep, 2012, 39(4): 4229-4236.
11DAVIDSON B L, BOUDREAU R L. RNA interference: a tool for querying nervous system function and an emerging therapy [J]. Neuron, 2007, 53(6): 781-788.
12TERAMOTO M, NAKAMASU K, NOSHIRO M, et al. Gene structure and chrom- osomal Location of a Human bHLH Transcriptional Factor DECIxStral3xSHARP-2/BHLHB2 [J]. J Biochem, 2001, 129(3): 391-396.
13LI Y, ZHANG H, XIE M, et al. Abundant expression of Dec1/stra13/sharp2 in colon carcinoma: its antagonizing role in serum deprivation-induced apoptosis and selective inhibition of procaspase activation [J]. Biochem J, 2002, 367(Pt 2): 413-422.
14CAO S, WU C, YANG Y, et al. Lentiviral vector-mediated stable expression of sTNFR-Fc in human macrophage and neuronal cells as a potential therapy for neuro AIDS [J]. J Neuroinflammation, 2011, 8(5): 48-54.
15李晓明, 林伟, 章翔, 等. 基于GatewayTM系统Dec1慢病毒表达载体的构建 [J]. 中华神经外科疾病研究杂志, 2013, 12(2): 126-129.
ConstructionofshRNAlentiviralexpressionvectorofDEC1geneandestablishmentofitsstabletransfectiongliomacellline
WEIXuehui1,LIXiaoming1,ZHANGYao1,RUYi2,WANGQinhao2,LIXia2,LINWei1
1DepartmentofNeurosurgery,XijingHospital;2DepartmentofBiochemistryandMolecularBiology,SchoolofBasicMedicine,FourthMilitaryMedicalUniversity,Xi'an710032, China
ObjectiveThe differentiated embryo-chondrocyte expressed gene 1(DEC1)-short hairpin RNA (shRNA) lentiviral expression vector and stably knock down DEC1 in human glioma cell line T98G were constructed.MethodsAccording to the cDNA sequence of the DEC1 gene provided by the GenBank, two specific shRNA sequences were designed and synthesized. A non-specific sequence was designed as negative control. They were cloned into the Psi-LVRU6MP plasmid vector. After the recombinant vectors were identified by enzyme digestion and DNA sequencing, they were packaged to obtain the lentiviral particles. Then T98G glioma cell line was infected with the three groups of lentivirus. After screening with puromycin, the expression of mCherry was observed under the fluorescence microscope, and the expression level of DEC1 protein in each group was detected by Western Blot.ResultsshRNA lentiviral expression vector targeting DEC1 gene was confirmed by restriction endonuclease digestion and DNA sequencing. Fluorescence microscopy showed that each group of cells had been efficiently infected by shRNA lentivirus. Western Blot results showed that DEC1 protein levels in the two DEC1-shRNA groups were significantly down-regulated compared with the untreated group and the negative control group, accounting for 39.47%±0.69% and 41.17%±0.89% of the untreated group, respectively (Plt;0.05). But there were no significant differences either between two DEC1-shRNA groups or between the negative control and the untreated group (Pgt;0.05).ConclusionThe DEC1-shRNA lentiviral expression vector was successfully constructed and stably infected into human glioma T98G cell line. Both groups of DEC1-shRNA lentivirus could markedly suppressed the expression of target gene DEC1. The results provide the experimental basis for further study of DEC1 function and mechanism in the human glioma cell line T98G.
DEC1 gene; Short hairpin RNA; Lentiviral expression vector; Glioma
1671-2897(2017)16-015-05
R 739.41
A
国家自然科学基金资助项目(81572469)
魏学辉,硕士研究生,E-mail: wxh296@sohu.com
*通讯作者: 林伟,副教授、副主任医师,E-mail: linwei@fmmu.edu.cn;李霞,副教授,E-mail: lixia@fmmu.edu.cn
2016-08-18;
2016-10-30)
