1/2,5-二取代吲唑-3-甲酰胺衍生物的合成及其抗肿瘤活性
2017-09-16何华龙高添桃朱永霞张强胜刘志昊张力丹余洛汀
奉 强, 何华龙, 高添桃, 朱永霞, 张强胜, 刘志昊, 何 冰, 张力丹, 余洛汀*
(1. 华西医院 药物化学实验室、肿瘤中心,四川大学协同创新中心,四川 成都 610041;2. 成都师范学院 化学与生命科学学院,四川 成都 611130)
·研究论文·
1/2,5-二取代吲唑-3-甲酰胺衍生物的合成及其抗肿瘤活性
奉 强1,2, 何华龙1, 高添桃1, 朱永霞1, 张强胜1, 刘志昊1, 何 冰2, 张力丹1, 余洛汀1*
(1. 华西医院 药物化学实验室、肿瘤中心,四川大学协同创新中心,四川 成都 610041;2. 成都师范学院 化学与生命科学学院,四川 成都 611130)
以吲唑-3-羧酸为原料,依次经溴代和酯化反应制得中间体5-溴吲唑-3-羧酸甲酯(2);2经N-烷基化并原位水解生成1/2-取代-5-溴吲唑-3-羧酸(3);3与吡啶酮甲胺类化合物经缩合反应制得酰胺(4);4与芳基频哪醇硼酸酯发生Suzuki偶联反应合成了14个新型二取代吲唑-3-甲酰吡啶酮甲胺衍生物(5a~5n),收率26%~32%,其结构经1H NMR,13C NMR和HR-MS(ESI-TOF)表征。通过NOE差谱确证了取代基在吲唑氮原子上的取代位置。采用MTT法研究了5a~5n对人B淋巴瘤细胞(Ramos)、人黑色素瘤细胞(CHL-1, WM-266-4)和乳腺癌细胞(BT-474)的体外抗肿瘤活性。结果表明:5a,5b,5m对Ramos细胞、5a,5b,5l对WM-266-4细胞、5a,5b,5d,5e,5h,5j,5m,5n对CHL-1细胞和5a,5b,5d,5h,5m对BT-474细胞具有较好的抑制活性(IC50<10.0 μmol.L-1)。
吲唑-3-甲酸; 1/2,5-二取代吲唑-3-甲酰胺衍生物; Suzuki偶联反应; 合成; 抗肿瘤活性
吲唑衍生物种类繁多,是一类重要的杂环化合物,在农药、医药领域有着广泛用途[1-5]。吲唑-4-甲酰胺类化合物因其具有良好的抗肿瘤生物活性而得以开发研究。2013年美国北卡罗莱纳州大学和结构基因联盟公司报道的抗肿瘤小分子抑制剂UNC1999[6]、比作用于其他组蛋白甲基转移酶选择性高1 000倍以上的选择性EZH2抑制剂GSK343[7]以及抗肿瘤临床药物EPZ005687[8],均含有1,6-二取代吲唑-4-甲酰胺结构。
吡啶酮甲胺结构在抗肿瘤药物中广泛存在[9]。在前期研究中本课题组以取代四氢异喹啉酮甲胺为原料合成了系列含吡啶酮结构的新型苯甲酰胺衍生物,体外和大鼠实验结果表明:该类苯甲酰吡啶酮甲胺衍生物对黑色素瘤和乳腺癌的生长具有很好的抑制作用[10-12]。
为满足活性筛选和新药研究开发的需要,依据药物设计原理,本文对1,6-二取代吲唑-4-甲酰吡啶酮甲胺进行结构改造,合成系列1/2,5-二取代吲唑-3-甲酰吡啶酮胺。以吲唑-3-羧酸为原料,经溴代反应制得5-溴吲唑-3-羧酸(1);1经酯化反应制得5-溴吲唑-3-羧酸甲酯(2);2经N-烷基化并原位水解生成1/2-取代-5-溴吲唑-3-羧酸(3);3在缩合剂作用下与吡啶酮甲胺类化合物缩合制得酰胺(4);4与芳基频哪醇硼酸酯发生Suzuki偶联反应合成了14个新型二取代吲唑-3-甲酰吡啶酮甲胺衍生物(5a~5n, Scheme 1),其结构经1H NMR,13C NMR和HR-MS(ESI-TOF)表征。通过NOE差谱确证了取代基在吲唑N原子上的取代位置,并采用MTT法对目标化合物5a~5n进行抑制人B淋巴瘤细胞(Ramos)、人黑色素瘤细胞(CHL-1, WM-266-4)和乳腺癌细胞(BT-474)体外抗肿瘤活性测试。
1 实验部分
1.1 仪器与试剂
Bruker-400 MHz型核磁共振仪(CDCl3为溶剂,TMS为内标);MicroTMQ-TOF型高分辨质谱仪。
中间体1和2按文献[13-14]方法制备;吡啶酮甲胺类化合物,纯度≥97%(HPLC);硼酸酯化合物,纯度≥98%(HPLC);硅胶,200~300目,青岛海洋化工有限公司;其余所用试剂均为分析纯,使用前未经进一步处理,直接使用。
1.25a~5n的合成(以5a为例)
(1) 6-溴-2-环己基吲唑羧酸(3a)的合成
在反应瓶中加入214.7 g(57.6 mmol),无水碳酸钠9.8 g(92.4 mmol),溴代环己烷15.1 g(92.5 mmol)和DMF 80 mL,缓慢升温至60 ℃,反应24 h(TLC监测)。冷却至室温,倒入碎冰中,搅拌至冰块融化,用乙酸乙酯(2×300 mL)萃取,合并萃取液,依次用水(400 mL)和饱和食盐水(300 mL)洗涤,无水硫酸钠干燥,减压蒸除溶剂后经硅胶柱层析(洗脱剂:石油醚 ∶乙酸乙酯=8 ∶1,V∶V)纯化得白色固体组分A8.7 g和组分B5.0 g。
将组分A8.7 g溶于甲醇50 mL中,搅拌下一次性加入一水合氢氧化锂2.0 g(48 mmol)的水(25 mL)溶液,升温至50 ℃,反应2 h(TLC监测)。减压蒸除甲醇,残余物用水(50 mL)稀释,冷却至0 ℃,小心加入稀盐酸调至pH 2~3(析出白色固体),陈化1 h。过滤,固体用甲醇重结晶得白色固体3a7.38 g,收率40%;1H NMR(400 MHz, DMSO-d6)δ:13.86(s, 1H), 8.18(d,J=1.8 Hz, 1H), 7.77(d,J=9.0 Hz, 1H), 7.45(dd,J=9.1 Hz, 1.9 Hz, 1H), 6.20~5.94(m, 1H), 1.89(d,J=12.2 Hz, 2H), 1.64(dd,J=38.0 Hz, 12.4 Hz, 2H), 1.48~0.99(m, 6H);13C NMR(101 MHz, DMSO-d6)δ: 161.04, 145.27, 129.54, 124.84, 124.66, 123.82, 120.73, 117.93, 62.72, 32.57, 25.84, 25.00。
(2) 5-溴-2-环己基-2H-吲唑-3-甲酰(4,6-二甲基-2-氧代-1,2-二氢吡啶-3-甲基)甲胺(4a)的合成
在反应瓶中加入3a3.23 g(10 mmol)和无水二甲亚砜50 mL,搅拌10 min,滴加N-甲基吗啉5.06 g(50 mmol),冰水浴冷却下一次性加入EDCI 3.45 g(18 mmol)和HOAt 2.46 g(18 mmol),控制温度不高于20 ℃,搅拌反应1 h。冷却至5 ℃,加入4,6-二甲基吡啶酮-3-甲胺1.82 g(12 mmol),加毕,自然升至室温,反应过夜(TLC监测)。反应液倒入水/乙酸乙酯(300 mL,V/V=1/1)中,搅拌15 min后静置分液,水层用乙酸乙酯(2×50 mL) 萃取,合并萃取液,依次用水100 mL和饱和食盐水100 mL洗涤,无水硫酸钠干燥,减压蒸除溶剂得化合物4a粗品,经硅胶柱层析[洗脱剂:以DCM/Pet(V/V=1/1)洗去小极性杂质,再用DCM洗脱]纯化得4a4.08 g,收率86%;1H NMR(400 MHz)δ: 12.35(s, 1H), 7.88(t,J=6.2 Hz, 1H), 7. 65(dd,J=8.8 Hz, 1.7 Hz, 1H), 7.51(d,J=1.7 Hz, 1H), 6.80(d,J=8.8 Hz, 1H), 5.96(s, 1H), 4.76(d,J=6.1 Hz, 2H), 4.62~4.40(m, 1H), 2.47(s, 3H), 2.39(s, 3H), 2.10~1.76(m, 6H), 1.46(m, 2H), 1.38~1.24(m, 2H);13C NMR(101 MHz, CDCl3)δ: 165.64, 157.38, 142.74, 136.72, 132.48, 127.00, 125.66, 123.32, 122.85, 119.80, 109.87, 109.73, 106.28, 65.79, 34.80, 32.04, 25.69, 25.34, 19.57, 18.64。
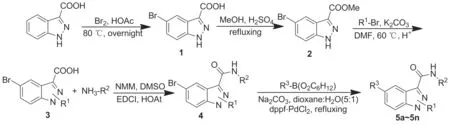

Scheme 1
(3) 2-环己基-5-[(6-吗啡啉-4-基)吡啶-3-基]-2H-吲唑-3-甲酰(4,6-二甲基-2-氧代-1,2-二氢吡啶酮-3-基)甲胺(5a)的合成
氮气保护下,在100 mL三口瓶中加入二氧六环/水(V/V=5/1)36 mL,搅拌下依次加入4a0.474 g(1 mmol)、6-(吗啉-4-基)吡啶-3-硼酸频呐醇酯0.32 g(1.1mmol)和无水碳酸钠0.212 g(2 mmol),氮气充分置换,快速加入催化剂dppf·PdCl240 mg(0.05 mmol),再次用氮气充分置换反应体系,快速升温至回流,反应3 h(TLC监测)。减压蒸除二氧六环,将反应混合物转移至烧杯中,加入50 mL水,搅拌10 min,用氯仿(3×30 mL)萃取,合并萃取液,依次用水(2×50 mL)和饱和食盐水(50 mL)洗涤,无水硫酸钠干燥,减压蒸出溶剂得5a粗品,经硅胶柱层析[洗脱剂:用DCM洗去小极性杂质,再用DCM/甲醇(V/V=15/1)洗脱]纯化得5a0.48 g。
用上述类似方法合成5b~5n。
5a: 收率89%;1H NMR(400 MHz)δ: 12.57(s, 1H, Pyridone-NH), 8.52(dd,J=8.0 Hz, 2.0 Hz, 2H, Indazole and Pyridine-ArH), 7.94(t,J=6.2 Hz, 1H, CONH), 7.86(dd,J=8.8, 2.6 Hz, 1H, Pyridine-ArH), 7.56(dd,J=8.8, 1.7 Hz, 1H, Indazole-ArH), 7.49(d,J=8.7 Hz, 1H, Indazole-ArH), 6.71(d,J=8.8 Hz, 1H, Pyridine-ArH), 5.96(s, 1H, Pyridone-ArH), 4.64(d,J=6.1 Hz, 2H), 4.48~4.30(m, 1H, Indazole-CH), 4.00~3.76(m, 4H), 3.55(dd,J=5.7 Hz, 4.0 Hz, 4H), 2.44(s, 3H), 2.35(s, 3H), 2.11~1.74(m, 6H), 1.46(m, 2H), 1.38~1.21(m, 2H);13C NMR(101 MHz)δ: 165.46, 162.50, 158.60, 150.64, 146.32, 142.67, 139.22, 137.15, 136.66, 132.84, 127.00, 125.49, 123.72, 122.58, 119.88, 109.83, 109.71, 106.76, 66.79, 58.77, 45.76, 34.84, 32.44, 25.66, 25.32, 19.75, 18.88; HR-MS(ESI-TOF)m/z: Calcd for C31H37N6O3{[M+H]+} 541.292 7, found 541.293 4。
2-环己基-5-[6-(4-甲基哌嗪-1-基)吡啶-3-基]-2H-吲唑-3-甲酰(4,6-二甲基-2氧代-1,2-二氢吡啶-3-基)甲胺(5b): 收率30.3%;1H NMR(400 MHz, CDCl3)δ: 11.86(s, 1H, Pyridone-NH), 8.50(dd,J=8.8 Hz, 2.1 Hz, 2H, Indazole-, Pyridine-ArH), 7.93(t,J=6.2 Hz, 1H, CONH), 7.84(dd,J=8.8 Hz, 2.6 Hz, 1H, Pyridine-ArH), 7.56(dd,J=8.8 Hz, 1.7 Hz, 1H, Indazole-ArH), 7.48(d,J=8.8 Hz, 1H, Indazole-ArH), 6.73(d,J=8.8 Hz, 1H, Pyridine-ArH), 5.94(s, 1H, Pyridone-ArH), 4.63(d,J=6.2 Hz, 2H), 4.40(m, 1H, Indazole-CH), 3.64(t,J=5.0 Hz, 4H), 2.59(t,J=5.0 Hz, 4H), 2.44(s, 3H), 2.39(s, 3H), 2.33(s, 3H), 2.12~1.87(m, 7H), 1.47(q,J=12.7 Hz, 2H);13C NMR(101 MHz)δ: 165.16, 162.52, 158.43, 150.58, 146.33, 142.33, 139.19, 137.11, 136.61, 132.94, 126.51, 125.47, 123.75, 122.76, 119.78, 109.80, 109.60, 106.92, 58.76, 54.82, 46.12, 45.18, 34.83, 32.44, 29.71, 25.67, 19.75, 18.95; HR-MS(ESI-TOF)m/z: Calcd for C32H40N7O2{[M+H]+} 554.324 3, found 554.321 5。
1-(4-甲氧基-1-丁基)-5-[(4-吗啡啉-4-基)甲基吡啶基]-1H-吲唑-3-甲酰(1-甲基-3-氧-2,3,5,6,7,8-六氢喹啉-4-基)甲胺(5c): 收率31.2%;1H NMR(400 MHz)δ: 13.42(s, 1H, Pyridone-NH), 8.53(d,J=1.6 Hz, 1H, Indazole-ArH), 8.00(t,J=6.0 Hz, 1H, CONH), 7.61~7.51(m, 3H, Indazole-, benzene-ArH), 7.35(d,J=8.8 Hz, 1H, Indazole-ArH), 7.30(d,J=7.9 Hz, 2H, benzene-ArH), 4.60(d,J=6.0 Hz, 2H), 4.29(t,J=7.1 Hz, 2H, Indazole-CH2), 3.65(t,J=4.6 Hz, 4H), 3.45(s, 2H), 3.26(t,J=6.2 Hz, 2H), 3.18(s, 3H), 2.91(t,J=6.0 Hz, 2H), 2.38(dt,J=11.2 Hz, 5.1 Hz, 6H), 2.26(s, 3H), 1.91(t,J=7.2 Hz, 2H), 1.73~1.57(m, 4H), 1.47(dq,J=12.6 Hz, 6.4 Hz, 2H);13C NMR(101 MHz)δ: 163.97, 162.24, 150.95, 140.89, 140.20, 140.00, 137.83, 136.44, 135.53, 129.59, 127.31, 126.53, 123.57, 121.61, 120.77, 114.59, 109.46, 72.03, 67.02, 63.14, 58.59, 53.65, 49.28, 34.48, 26.92, 26.78, 25.04, 22.41, 22.34, 16.69; HR-MS(ESI-TOF)m/z: Calcd for C35H44N5O4{[M+H]+} 598.339 3, found 598.336 4。
1-环己基-5-[6-(4-甲基哌嗪-1-基)吡啶-3-基]-1H-吲唑-3-甲酰(1-甲基-3-氧-2,3,5,6,7,8-六氢喹啉-4-基)甲胺(5d): 收率30.7%;1H NMR(400 MHz)δ: 13.61(s, 1H, Pyridone-NH), 8.58(t,J=5.8 Hz, 1H, CONH), 8.50(d,J=2.4 Hz, 1H, Pyridine-ArH), 8.10(s, 1H, Indazole-ArH), 7.83(d,J=8.9 Hz, 1H, Indazole-ArH), 7.71(dd,J=8.8 Hz, 2.6 Hz, 1H, Pyridine-ArH), 7.50(dd,J=9.0 Hz, 1.4 Hz, 1H, Indazole-ArH), 6.39(d,J=8.8 Hz, 1H, Pyridine-ArH), 5.67~5.37(m, 1H, Indazole-CH), 4.68(d,J=5.7 Hz, 2H), 3.31(t,J=5.0 Hz, 4H), 2.94(t,J=6.3 Hz, 2H), 2.62(s, 2H), 2.27(t,J=5.3 Hz, 6H), 2.22(s, 3H), 2.16(dd,J=11.2 Hz, 3.4 Hz, 2H), 1.99~1.92(m, 2H), 1.90(s, 3H), 1.82~1.66(m, 5H), 1.64~1.50(m, 2H), 1.36(m, 1H);13C NMR(101 MHz)δ: 163.92, 159.54, 158.20, 150.38, 146.43, 145.96, 141.34, 135.72, 133.70, 127.37, 125.81, 125.08, 120.90, 120.35, 119.09, 114.99, 114.82, 106.51, 60.90, 54.54, 46.05, 45.05, 35.99, 33.68, 29.70, 27.52, 25.71, 25.41, 24.85, 22.25, 16.50; HR-MS(ESI-TOF)m/z: Calcd for C35H44N7O2{[M+H]+}594.355 6, found 594.352 6。
1-(4-甲氧基-1-丁基)-5-[(6-吗啡啉-4-基)吡啶-3-基]-1H-吲唑-3-甲酰(4,6-二甲基-2-氧代-1,2-二氢吡啶酮-3-基)甲胺(5e): 收率28.9%;1H NMR(400 MHz)δ: 13.07(s, 1H, Pyridone-NH), 8.51(dd,J=4.7, 2.0 Hz, 2H, Indazole-, Pyridine-ArH), 8.05(t,J=6.1 Hz, 1H, CONH), 7.85(dd,J=8.8, 2.6 Hz, 1H, Pyridine-ArH), 7.57(dd,J=8.8 Hz, 1.7 Hz, 1H, Indazole-ArH), 7.44(d,J=8.7 Hz, 1H, Indazole-ArH), 6.71(d,J=8.8 Hz, 1H, Pyridine-ArH), 5.97(s, 1H, Pyridone-ArH), 4.64(d,J=6.0 Hz, 2H), 4.37(t,J=7.1 Hz, 2H, Indazole -CH), 3.91~3.75(m, 4H), 3.60~3.49(m, 4H), 3.35(t,J=6.2 Hz, 2H), 3.28(s, 3H), 2.44(s, 3H), 2.38(s, 3H), 2.06~1.91(m, 2H), 1.62~1.47(m, 2H);13C NMR(101 MHz)δ: 165.63, 162.29, 158.61, 150.50, 146.32, 143.00, 140.01, 137.57, 136.61, 132.89, 126.87, 125.89, 123.63, 122.34, 119.83, 109.78, 109.70, 106.75, 72.02, 66.77, 58.60, 49.30, 45.73, 34.95, 26.90, 26.77, 19.71, 18.81; HR-MS(ESI-TOF)m/z: Calcd for C30H37N6O4{[M+H]+} 545.287 6, found 545.288 6。
2-环己基-5-[4-(吗啡啉-4-基)甲基]苯基-1H-吲唑-3-甲酰(4,6-二甲基-2-氧代-1,2-二氢吡啶酮-3-基)甲胺(5f): 收率31.7%;1H NMR(400 MHz)δ: 12.88(s, 1H, Pyridone-NH), 8.61(d,J=1.6 Hz, 1H, Indazole-ArH), 7.95(t,J=6.2 Hz, 1H, CONH), 7.66~7.60(m, 3H, Indazole-, benzene-ArH), 7.48(d,J=8.9 Hz, 1H, Indazole-ArH), 7.38(d,J=7.9 Hz, 2H, benzene-ArH), 5.96(s, 1H, Pyridone-ArH), 4.64(d,J=6.1 Hz, 2H), 4.49~4.31(m, 1H, Indazole-CH), 3.73(t,J=4.6 Hz, 4H), 3.54(s, 2H), 2.48(t,J=4.6 Hz, 4H), 2.44(s, 3H), 2.36(s, 3H), 2.03(dt,J=11.7 Hz, 4.2 Hz, 3H), 1.98~1.88(m, 3H), 1.78(dt,J=13.2 Hz, 3.3 Hz, 1H), 1.46(m, 2H), 1.37~1.19(m, 3H);13C NMR(101 MHz)δ: 165.59, 162.49, 150.71, 142.85, 140.10, 139.41, 137.33, 136.35, 135.54, 129.60, 127.34, 126.18, 123.63, 122.51, 120.76, 109.78, 109.58, 67.02, 63.15, 58.73, 53.64, 34.84, 32.45, 25.67, 25.33, 19.76, 18.84; HR-MS(ESI-TOF)m/z: Calcd for C33H40N5O3{[M+H]+} 554.313 1, found 554.313 2。
1-环己基-5-[4-(吗啡啉-4-基)甲基]苯基-1H-吲唑-3-甲酰(1-甲基-3-氧-2,3,5,6,7,8-六氢喹啉-4-基)甲胺(5g): 收率30.8%;1H NMR(400 MHz)δ: 13.76(s, 1H, Pyridone-NH), 8.42(t,J=5.8 Hz, 1H, CONH), 8.07(s, 1H, Indazole-ArH), 7.77(d,J=9.0 Hz, 1H, Indazole-ArH), 7.45(d,J=9.0 Hz, 1H, Indazole-ArH), 7.37(d,J=7.7 Hz, 2H, benzene-ArH), 6.98(d,J=7.7 Hz, 2H, benzene-ArH), 5.48(tt,J=10.1 Hz, 5.0 Hz, 1H, Indazole-CH), 4.65(d,J=5.7 Hz, 2H), 3.39(t,J=4.5 Hz, 4H), 3.04(s, 2H), 2.88(t,J=6.3 Hz, 2H), 2.18(t,J=6.3 Hz, 2H), 2.10(q,J=5.2 Hz, 4.8 Hz, 8H), 1.88(d,J=13.4 Hz, 2H), 1.78(s, 3H), 1.74~1.58(m, 4H), 1.49(m, 2H), 1.29(m, 2H);13C NMR(101 MHz)δ: 164.07, 159.63, 150.20, 146.64, 141.57, 140.02, 136.48, 129.44, 127.66, 126.79, 126.08, 120.80, 120.44, 118.86, 116.46, 114.88, 66.73, 62.96, 60.96, 53.34, 35.89, 33.68, 27.55, 25.73, 25.41, 24.83, 22.29, 16.35; HR-MS(ESI-TOF)m/z: Calcd for C36H44N5O3{[M+H]+}594.344 4, found 594.342 8。
1-(4-甲氧基-1-丁基)-5-[6-(4-甲基哌嗪-1-基)吡啶-3-基]-1H-吲唑-3-甲酰(1-甲基-3-氧-2,3,5,6,7,8-六氢喹啉-4-基)甲胺(5h): 收率29.6%;1H NMR(400 MHz)δ: 13.32(s, 1H, Pyridone-NH), 8.43(d,J=1.6 Hz, 1H, Indazole-ArH), 8.41(d,J=2.6 Hz, 1H, Pyridine-ArH), 7.99(t,J=6.0 Hz, 1H, CONH), 7.75(dd,J=8.8 Hz, 2.6 Hz, 1H, Pyridine-ArH), 7.49(dd,J=8.8 Hz, 1.7 Hz, 1H, Indazole-ArH), 7.35(d,J=8.8 Hz, 1H, Indazole-ArH), 6.64(d,J=8.8 Hz, 1H, Pyridine-ArH), 4.59(d,J=6.0 Hz, 2H), 4.29(t,J=7.1 Hz, 2H, Indazole-CH2), 3.55(t,J=4.9 Hz, 4H), 3.25(t,J=6.2 Hz, 2H), 3.18(s, 3H), 2.91(t,J=6.0 Hz, 2H), 2.50(t,J=5.0 Hz, 4H), 2.37(t,J=6.0 Hz, 2H), 2.29(s, 3H), 2.25(s, 3H), 1.90(p,J=7.3 Hz, 2H), 1.78~1.56(m, 4H), 1.47(m, 2H);13C NMR(101 MHz)δ: 163.88, 162.26, 158.44, 151.00, 146.27, 140.88, 139.97, 137.60, 136.54, 132.94, 126.38, 125.84, 123.62, 121.56, 119.70, 114.61, 109.68, 106.92, 72.01, 58.58, 54.80, 49.27, 46.10, 45.16, 34.49, 27.35, 26.88, 26.76, 25.02, 22.38, 22.32, 16.68; HR-MS(ESI-TOF)m/z: Calcd for C34H44N7O3{[M+H]+}598.350 6, found 598.354 1。
1-(4-甲氧基-1-丁基)-5-[6-(4-甲基哌嗪-1-基)吡啶-3-基]-1H-吲唑-3-甲酰(4,6-二甲基-2-氧代-1,2-二氢吡啶酮-3-基)甲胺(5i): 收率28.1%;1H NMR(400 MHz)δ: 12.98(s, 1H, Pyridone-NH), 8.51~8.50(m, 1H, Indazole-ArH), 8.49(d,J=2.6 Hz, 1H, Pyridine-ArH), 8.04(t,J=6.1 Hz, 1H, CONH), 7.83(dd,J=8.8 Hz, 2.6 Hz, 1H, Pyridine-ArH), 7.57(dd,J=8.8 Hz, 1.7 Hz, 1H, Indazole-ArH), 7.43(d,J=8.7 Hz, 1H, Indazole-ArH), 6.72(d,J=8.8 Hz, 1H, Pyridine-ArH), 5.96(s, 1H, Pyridone-ArH), 4.63(d,J=6.1 Hz, 2H), 4.37(t,J=7.1 Hz, 2H, Indazole-CH2), 3.63(t,J=5.1 Hz, 4H), 3.35(t,J=6.2 Hz, 2H), 3.28(s, 3H), 2.58(t,J=5.0 Hz, 4H), 2.43(s, 3H), 2.37(s, 6H), 2.05~1.92(m, 2H), 1.64~1.46(m, 2H);13C NMR(101 MHz)δ: 165.57, 162.30, 158.45, 150.53, 146.28, 142.97, 139.97, 137.54, 136.56, 132.99, 126.38, 125.88, 123.62, 122.34, 119.70, 109.79, 109.68, 106.93, 72.02, 58.59, 54.81, 49.29, 46.12, 45.17, 34.94, 26.89, 26.76, 19.71, 18.81; HR-MS(ESI-TOF)m/z: Calcd for C31H40N7O3{[M+H]+} 558.319 3, found 558.317 3。
1-环己基-5-[6-(吗啡啉-4-基)吡啶-3-基]-1H-吲唑-3-甲酰(1-甲基-3-氧-2,3,5,6,7,8-六氢喹啉-4-基)甲胺(5j): 收率29.4%;1H NMR(400 MHz)δ: 13.63(s, 1H, Pyridone-NH), 8.43(t,J=5.9 Hz, 1H, CONH), 8.41(d,J=2.5 Hz, 1H, Pyridine-ArH), 8.05(s, 1H, Indazole-ArH), 7.76(d,J=8.9 Hz, 1H, Pyridine-ArH), 7.67~7.56(m, 1H, Indazole-ArH), 7.41(dd,J=9.0 Hz, 1.5 Hz, 1H, Indazole-ArH), 6.22(d,J=8.7 Hz, 1H, Pyridine-ArH), 5.50(m, 1H, Indazole-CH), 4.61(d,J=5.8 Hz, 2H), 3.46(t,J=4.7 Hz, 4H), 3.04(t,J=4.8 Hz, 4H), 2.89(t,J=6.4 Hz, 2H), 2.23(t,J=6.3 Hz, 2H), 2.15~1.98(m, 4H), 1.86(s, 4H), 1.74~1.60(m, 6H), 1.49(m, 2H), 1.37~1.20(m, 1H);13C NMR(101 MHz)δ: 162.95, 158.49, 157.35, 149.35, 145.39, 145.01, 140.39, 134.78, 132.75, 130.46, 126.25, 125.42, 124.16, 119.81, 119.55, 118.14, 113.93(d,J=2.6 Hz), 105.30, 68.65, 65.35, 59.92, 44.41, 34.89, 32.63, 26.50, 24.70, 24.39, 23.85, 21.26, 15.48; HR-MS(ESI-TOF)m/z: Calcd for C34H41N6O3{[M+H]+} 581.324 0, found 581.322 9。
1-环戊基-5-[6-(4-甲基哌嗪-1-基)吡啶-3-基]-1H-吲唑-3-甲酰(1-甲基-3-氧-2,3,5,6,7,8-六氢喹啉-4-基)甲胺(5k): 收率29.1%;1H NMR(400 MHz)δ: 13.72(s, 1H, Pyridone-NH), 8.42(d,J=6.0 Hz, 1H, CONH), 8.40(d,J=2.3 Hz, 1H, Pyridine-ArH), 8.01(s, 1H, Indazole-ArH), 7.73(d,J=8.9 Hz, 1H, Indazole-ArH), 7.62(dd,J=8.8 Hz, 2.5 Hz, 1H, Pyridine-ArH), 7.41(d,J=9.0 Hz, 1H, Indazole-ArH), 6.25(d,J=8.7 Hz, 1H, Pyridine-ArH), 5.96(p,J=7.7 Hz, 1H, Indazole-CH), 4.59(d,J=5.7 Hz, 2H), 3.18(t,J=4.9 Hz, 4H), 2.88(t,J=6.3 Hz, 2H), 2.17(dt,J=9.8 Hz, 6.1 Hz, 10H), 2.13(s, 3H), 1.95(dq,J=13.4 Hz, 7.4 Hz, 2H), 1.80(s, 3H), 1.75~1.53(m, 6H);13C NMR(101 MHz)δ: 163.99, 159.62, 158.23, 150.35, 146.44, 145.95, 141.43, 135.66, 133.76, 128.04, 125.76, 125.10, 121.11, 120.41, 119.10, 114.98, 114.65, 106.42, 62.42, 54.53, 46.08, 45.01, 35.91, 33.77, 27.46, 24.93, 24.83, 22.27, 16.43; HR-MS(ESI-TOF)m/z: Calcd for C34H42N7O2{[M+H]+}580.340 0, found 580.339 3。
1-环戊基-5-[4-(吗啡啉-4-基)甲基]苯基-1H-吲唑-3-甲酰(1-甲基-3-氧-2,3,5,6,7,8-六氢喹啉-4-基)甲胺(5l): 收率30.2%;1H NMR(400 MHz)δ: 13.88(s, 1H, Pyridone-NH), 8.34(t,J=5.9 Hz, 1H, CONH), 8.06(s, 1H, Indazole-ArH), 7.74(d,J=8.9 Hz, 1H, Indazole-ArH), 7.44(d,J=9.0 Hz, 1H, Indazole-ArH), 7.37(d,J=7.6 Hz, 2H, Benzene-ArH), 6.95(d,J=7.7 Hz, 2H, Benzene-ArH), 5.97(p,J=7.7 Hz, 1H, Indazole-CH), 4.64(d,J=5.8 Hz, 2H), 3.37(t,J=4.5 Hz, 4H), 3.00(s, 2H), 2.90(d,J=6.6 Hz, 2H), 2.28~2.13(m, 6H), 2.08(t,J=4.4 Hz, 4H), 2.00~1.89(m, 2H), 1.77(s, 3H), 1.73~1.56(m, 6H);13C NMR(101 MHz)δ: 163.08, 158.68, 149.17, 145.62, 140.58, 138.99, 135.43, 135.15, 128.41, 127.35, 125.74, 125.06, 119.97, 119.54, 117.82, 115.29, 113.82, 65.68, 61.92, 61.41, 52.28, 34.79, 32.77, 28.66, 26.44, 23.93, 23.79, 21.27, 15.25; HR-MS(ESI-TOF)m/z: Calcd for C35H42N5O3{[M+H]+}580.328 8, found 580.326 2。
1-(4-甲氧基-1-丁基)-5-[6-(吗啡啉-4-基)吡啶-3-基]-1H-吲唑-3-甲酰(1-甲基-3-氧-2,3,5,6,7,8-六氢喹啉-4-基)甲胺(5m): 收率26.4%;1H NMR(400 MHz)δ: 13.18(s, 1H, Pyridone-NH), 8.53(d,J=1.6 Hz, 1H, Indazole-ArH), 8.51(d,J=2.5 Hz, 1H, Indazole-ArH), 8.06(t,J=6.1 Hz, 1H, CONH), 7.85(dd,J=8.8 Hz, 2.6 Hz, 1H, Indazole-ArH), 7.58(dd,J=8.7 Hz, 1.7 Hz, 1H, Indazole-ArH), 7.44(d,J=8.7 Hz, 1H, Indazole-ArH), 6.71(d,J=8.8 Hz, 1H, Indazole-ArH), 4.67(d,J=6.0 Hz, 2H), 4.38(t,J=7.1 Hz, 2H, Indazole-CH2), 3.86(t,J=4.8 Hz, 4H), 3.56(t,J=4.8 Hz, 4H), 3.35(t,J=6.2 Hz, 2H), 3.27(s, 3H), 3.00(t,J=6.0 Hz, 2H), 2.46(t,J=5.9 Hz, 2H), 2.34(s, 3H), 1.99(p,J=7.3 Hz, 2H), 1.75(tt,J=11.8 Hz, 6.5 Hz, 4H), 1.56(dq,J=12.7 Hz, 6.4 Hz, 2H);13C NMR(101 MHz)δ: 163.86, 162.27, 158.62, 150.99, 146.34, 140.71, 140.02, 137.65, 136.62, 132.87, 126.91, 125.86, 123.66, 121.68, 119.88, 114.57, 109.69, 106.75, 72.04, 66.79, 58.61, 49.30, 45.74, 34.49, 27.36, 26.91, 26.78, 25.05, 22.41, 22.33, 16.76; HR-MS(ESI-TOF)m/z: Calcd for C33H41N6O4{[M+H]+}585.318 9, found 585.320 1。
1-环己基-5-[6-(吗啡啉-4-基)吡啶-3-基]-1H-吲唑-3-甲酰(1-甲基-3-氧-2,3,5,6,7,8-六氢喹啉-4-基)甲胺(5n): 收率28.7%;1H NMR(400 MHz)δ: 13.55(s, 1H, Pyridone-NH), 8.48(d,J=2.5 Hz, 1H, Pyridine-ArH), 8.40(t,J=5.9 Hz, 1H, CONH), 8.11(s, 1H, Indazole-ArH), 7.82(d,J=8.9 Hz, 1H, Indazole-ArH), 7.69(dd,J=8.8, 2.6 Hz, 1H, Pyridine-ArH), 7.47(dd,J=9.0, 1.6 Hz, 1H, Indazole-ArH), 6.29(d,J=8.8 Hz, 1H, Pyridine-ArH), 6.05(p,J=7.6 Hz, 1H, Indazole-CH), 4.67(d,J=5.9 Hz, 2H), 3.55(t,J=4.8 Hz, 4H), 3.11(t,J=4.8 Hz, 4H), 2.99(t,J=6.4 Hz, 2H), 2.38~2.19(m, 4H), 2.03(dt,J=10.6 Hz, 4.4 Hz, 2H), 1.95(s, 3H), 1.85~1.66(m, 4H);13C NMR(101 MHz)δ: 163.92, 159.63, 158.39, 150.36, 146.45, 146.05, 141.32, 135.84, 133.76, 128.00, 126.51, 125.20, 121.09, 120.84, 119.15, 114.89, 106.33, 66.39, 62.48, 45.43, 35.85, 33.76, 27.45, 24.92, 24.90, 22.31, 16.52; HR-MS(ESI-TOF)m/z: Calcd for C33H39N6O3{[M+H]+}567.308 4, found 567.310 6。
1.3 体外抗肿瘤活性测试
采用MTT法测试了5a~5n对人B淋巴瘤细胞(Ramos)、人黑色素瘤细胞(CHL-1, WM-266-4)及乳腺癌细胞(BT-474)的体外抗肿瘤活性。
收集处于对数生长期的肿瘤细胞,将细胞重悬成浓度为1~3×103个/mL的细胞悬液,按照每孔100 μL细胞悬液将细胞接种到96孔板中,并置于37 ℃、 5%CO2细胞培养箱内孵育过夜。次日,加入100 μL含有不同梯度浓度药物的培养基处理,每个药物浓度设置3个复孔,并用含有0.1%DMSO的培养基处理细胞作为阴性对照。将细胞培养板在细胞培养箱内继续孵育4 d后,每个孔内加入20 μL 5 mg·mL-1MTT溶液,对于贴壁生长的细胞,于37 ℃孵育1~4 h,形成甲臜结晶后弃去上清,再向每个孔内加入150 μL DMSO溶解甲臜结晶;对于悬浮生长的细胞,于37 ℃孵育24 h,形成甲臜后加入50 μL 20%的SDS溶液,放置过夜。最后,用酶标仪检测570 nm波长下的吸光度值(A)(A值与活细胞数成正比),并计算药物对肿瘤细胞的生长抑制率[抑制率/%=(A570对照组-A570实验组)/A570对照组×100%]。最后,用Graphpad Prism计算拟合药物的生长抑制曲线并计算半数抑制浓度值IC50。
2 结果与讨论
2.1 合成
(1)3的合成
吲唑-N-烷基化反应选择性不强,常常同时发生于1-或2-氮原子上,生成两种取代产物的混合物。本实验以DMF为溶剂,在碳酸钠或碳酸钾等无机碱作用下,2与卤代烃于60~80 ℃反应,生成1-或2-吲唑N-烷基化产物。经柱层析纯化分别得到1-, 2-N-烷基化产物,纯度可达90%。经碱性水解、酸化得到对应酸。将所得酸用甲醇重结晶,能以约40%收率获得吲唑2-烃基化羧酸3a和约20%收率得到吲唑1-烃基化羧酸3b。
(2)4的合成
羧酸与胺的缩合,可选择的缩合试剂较多。本路线多种缩合试剂在毫摩尔规模反应合成4时收率一般都较高,柱层析纯化反应收率能达到60~90%。在后期将反应规模放大至10 g量时,发现以EDCI和HOAt为催化剂时反应效果最好:副反应少,反应体系易于纯化,经简单洗涤后即以93%收率得到纯度高于95%以上产品,无需再经柱层析纯化步骤即可用于下步反应。
(3)3的结构确证
3a和3b的1H NMR谱中,吲唑芳环上质子峰的裂分形状完全相同,氢质子化学位移几乎相等,差别仅在与吲唑氮原子相接的C—H上,虽然二者均显示出多重峰,但前者吸收峰位于δ6.20~5.94,后者吸收峰位于δ5.23~5.47。
由于3a,3b分子中与吲唑氮原子相接的C—H与吲唑7-H距离差别很大,表现出较大NOE效应差异,为此,通过NOE差谱(图1)确定二者的结构。激发该C—H,发现在3a中它与吲唑芳氢仅有极弱NOE效应,而在3b中观察到它与吲唑芳氢7-H具有强烈NOE效应,故知在3a中取代烷基位于距吲唑芳氢7-H较远处,3b中取代烷基位于距吲唑芳氢7-H较近处,即3a中烷基在吲唑环N2上取代,3b中烷基在吲唑环N1上取代。
2.2 生物活性
以MTT法测定5a~5n体外对人B淋巴瘤细胞(Ramos)、人黑色素瘤细胞(CHL-1, WM-266-4)以及乳腺癌细胞(BT-474)的抑制作用,其IC50结果见表1。
由表1可知,当吲唑氮原子上取代基为链状取代基时,仅5m对Ramos,5e,5h,5m对CHL-1和5h对BT-474具有较好的肿瘤细胞抑制活性,最高达到7. 25 μmol·L-1(5m对CHL-1肿瘤细胞);当吲唑氮原子上取代基换为环状烃基时,其抗肿瘤活性最高可提高近1倍(5h与5d对CHL-1肿瘤细胞抑制活性IC50值分别为8.73 μmol·L-1和4.29 μmol·L-1)。

δ

CompIC50/μmol·L-1RamosCHL-1WM-266-4BT-4745a9.023.083.777.115b3.190.990.740.865c26.317.7>3021.45d10.24.2925.15.605e14.87.8813.420.05f>3011.710.6>305g>30>30>30>305h10.18.7311.610.05i11.118.76.87>305j11.97.2214.0>305k15.211.214.720.65l31.316.70.31>305m9.307.2526.310.05m20.26.2016.7>30EPZ00568717.668>80>30
当吡啶酮片段上取代基为两甲基时,比其并环类似物活性要好一些,但5l对WM-266-4具有优异的抑制活性,达到0.37 μmol·L-1;同时,当吲唑环上取代芳基为哌嗪吡啶时,其抗肿瘤活性往往由于取代苯基和吗啉吡啶,如5g对所给肿瘤细胞几乎没有抑制活性。5c和5f仅有中等抑制活性(5l对WM-266-4例外)。
当将吲唑氮原子上取代基由1-改至2-时,对肿瘤细胞抗肿瘤活性普适性更广,活性更高。如5a,5b对所给肿瘤细胞均有较好抗肿瘤活性,达到低浓度抗肿瘤活性。因此,在今后的进一步结构优化中,应当保留吲唑N2上环状取代基,改变吡啶酮结构上取代基,探索5-芳杂环对药物活性的影响,找到活性优异的新药分子。
综上,所合成的14个化合物中,部分化合物体外抗肿瘤活性好于阳性对照化合物EPZ005687,这说明二取代吲唑-3-羧酸酰胺类化合物可以作为先导化合物进一步研究。
以吲唑-3-甲酸为起始原料,合成了14个新型的1/2-烃基-5-芳香基二取代吲唑-3-甲酰吡啶酮甲胺衍生物(5a~5n)并用MTT法研究了他们对人B淋巴瘤细胞(Ramos)、人黑色素瘤细胞(CHL-1, WM-266-4)、乳腺癌细胞(BT-474)的体外抗肿瘤活性。结果表明:5a,5b,5m对Ramos具有较好的抑制活性;5a,5b,5l对WM-266-4具有较好的抑制活性;5a,5b,5d,5e,5h,5j,5m,5n对CHL-1具有较好的抑制活性;5a,5b,5d,5h,5m对BT-474具有较好的抑制活性;IC50值均小于10.0 μmol·L-1,优于阳性对照物EPZ005687,说明这类1/2-烃基-5-芳香基二取代吲唑-3-甲酰吡啶酮甲胺类化合物可以作为先导化合物进行进一步的抗肿瘤活性研究。
[1] Jakupec M A, Reisner E, Keppler B K,etal. Redox-active antineoplastic ruthenium complexes with indazole:Correlation ofinVitropotency and reduction potential[J].J Med Chem,2005,48(8):2831-2837.
[2] Wong TW, Lee FY, Vite GD,etal. Preclinical antitumor activity of BMS-599626,a pan-HER Kinase inhibitor that inhibits HER1/HER2 homodimer and heterodimer Signaling[J].Clin Cancer Res,2006,12(20):6186-6193.
[3] Duan JX, Cai XH, Matteucci M,etal. Potent antitubulin tumor cell cytotoxins based on 3-aroyl indazoles[J].J Med Chem,2007,50(5):1001-1006.
[4] 龙伟,邱文革,何宏,等. 新型吲唑类PARP-1抑制剂的合成及其生物活性评价[J].合成化学,2016,24(4):277-282.
[5] 周云鹏,何畅,王洋,等. 新型吲唑类化合物的合成及其抗肿瘤活性[J].合成化学,2016,24(4):293-296.
[6] Konze K D, Ma A, Li F,etal. An orally bioavailable chemical probe of the lysine methyltransferases EZH2 and EZH1[J].ACS chemical biology,2013,8(6):1324-1334.
[7] Verma S K, Tian X, LaFrance L V,etal. Identification of potent,selective,cell-active inhibitors of the histone lysine methyltransferase EZH2[J].ACS medicinal chemistry letters,2012,3(12):1091-1096.
[8] Knutson S K, Warholic N M, Wigle T J,etal. Durable tumor regression in genetically altered malignant rhabdoid tumors by inhibition of methyltransferase EZH2[J].Proceedings of the National Academy of Sciences,2013,110(19):7922-7927.
[9] Diaz E, Machutta C A, Chen S,etal. Development and validation of reagents and assays for EZH2 peptide and nucleosome high-throughput screens[J].Journal of biomolecular screening,2012,17(10):1279-1292.
[10] Zhang L D, Song X J, Yu L T,etal. Design,synthesis and biological evaluation of novel 1-methyl-3-oxo-2,3,5,6,7,8-hexahydroisoquinolins as potential EZH2 inhibitors[J].RSC Adv,2015(5):25967-25978.
[11] Song X J, Gao T T, Yu L T,etal. Selective inhibition of EZH2 by ZLD1039 blocks H3K27methylation and leads to potent anti-tumor activity in breast cancer[J].Sci Rep,2016(6):24893.
[12] Gao T T, Zhang L D, Yu L T,etal. ZLD1122,a novel EZH2 and EZH1 small molecular inhibitor, blocks H3K27 methylation and diffuse large B cell lymphoma cell growth[J].RSC Adv,2016,(6):28512-28521.
[13] Schmidt A, Snovydovych B, Habeck T,etal.N-heterocyclic carbenes of 5-haloindazoles generated by decarboxylation of 5-haloindazolium-3-carboxylates[J].Eu J Org Chem,2007(29):4909-4916.
[14] Migliorini A, Oliviero C, Gasperi T,etal. The Suzuki reaction applied to the synthesis of novel pyrrolyl and thiophenyl indazoles[J].Molecules,2012,(17):4508-4521.
Synthesis and Antitumor Activities of 1/2,5-Disubstituted Indazole-3-carboxamide Derivatives
FENG Qiang1,2, HE Hua-long1, GAO Tian-tao1, ZHU Yong-xia1, ZHANG Qiang-sheng1, LIU Zhi-hao1, HE Bing2, ZHANG Li-dan1, YU Luo-ting1*
(1. Lab of Medicinal Chemistry, Cancer Center, West China Hospital, Sichuan University and Collaborative Innovation Center, Chengdu 610041, China; 2. College of Chemistry and Life Science, Chengdu Normal University, Chengdu 611130, China)
Fourteen novel disubsitituted indazole-3-carboxamide derivatives(5a~5n) were synthesized from indazole-3-carboxylic acidviabromination, esterification,N-alkylation, amidation and Suzuki-coupling reactions in overall yields of 26%~32%. These products were characterized by1H NMR,13C NMR and HR-MS(ESI-TOF). We also examined the1H-1H NOE difference spectroscopy of3aand3band confirmed the exact substitution position of R on the N of indazole. Theinvitroantitumor activities against human B lymphoma cells(Ramos), human melanoma cells(CHL-1, WM-266-4) and breast cancer cells(BT-474) have been demonstrated by MTT assays. The results showed that5a,5band5mexhibited good inhibition against Ramos;5a,5band5lexhibited excellent inhibition against WM-266-4;5a,5b,5d,5e,5h,5j,5mand5nshowed advanced inhibition against CHL-1;5a,5b,5d,5hand5mexhibited good inhibition against BT-474 with IC50<10.0 μmol·L-1, respectively.
indazole-3-carboxylic acid; disubsitituted indazole-3-carboxamide derivative; Suzuki coupling reaction; synthesis; antitumor activity
2017-05-09;
: 2017-07-28
国家自然科学基金资助项目(81602398); 中国博士后基金资助项目(2016T90861)
奉强(1974-),男,四川内江人,讲师,博士研究生,主要从事药物合成化学研究。 E-mail: 592744998@qq.com
余洛汀,教授,博士生导师, Tel. 028-85502796, E-mail: yuluot@scu.edu.cn
O626.21; O626.32
: ADOI: 10.15952/j.cnki.cjsc.1005-1511.2017.09.17108
