3,3-二取代3-吲哚-3′-基氧化吲哚的立体选择性合成
2017-09-16崔宝东袁昌伦陈永正
崔宝东, 汪 微, 袁昌伦, 单 静, 陈永正
(遵义医学院 药学院, 贵州 遵义 563000)
·快递论文·
3,3-二取代3-吲哚-3′-基氧化吲哚的立体选择性合成
崔宝东, 汪 微, 袁昌伦, 单 静, 陈永正*
(遵义医学院 药学院, 贵州 遵义 563000)
以10 mol%(R,R)-环己二胺衍生的手性双功能硫脲叔胺(4b)为催化剂,3′-吲哚-3-氧化吲哚与α-氨基砜为原料,经3′-吲哚-3-氧化吲哚与原位生成的N-Boc芳香醛亚胺的不对称Mannich反应,合成了20个3,3-二取代3-吲哚-3′-基氧化吲哚类化合物(3a~3t),分离收率54%~98%,dr值90 ∶10~>99 ∶1,其结构经1H NMR,13C NMR和HR-MS(ESI-TOF)表征。
3′-吲哚-3-氧化吲哚;α-氨基砜; 有机催化; Mannich反应; 芳香醛亚胺; 3,3-二取代3-吲哚-3′-基氧化吲哚; 合成
3,3′-双吲哚杂环骨架广泛存在于天然产物与药物活性分子中,是构成天然生物碱的重要结构单元,此类化合物一般具有较好的生物活性和潜在的药用价值[1-3]。因此,发展简便、高效的合成方法,用于合成含3,3′-双吲哚骨架的杂环类化合物具有重要意义。
通过文献调研发现,目前已经发展了一些有效合成含3,3′-双吲哚骨架类化合物的方法[4-8],其中,3′-吲哚-3-氧化吲哚作为亲核试剂与亲电受体的催化不对称反应是一种最为直接、有效的合成方法。这些反应类型包括不对称烯丙基化反应[9]、不对称Michael反应[10]和多组分反应[11]。然而,对于有机小分子催化的3′-吲哚-3-氧化吲哚与N-Boc醛亚胺的不对称Mannich反应还未见报道。
基于以上研究背景,我们预测在手性双功能硫脲叔胺的催化下,3′-吲哚-3-氧化吲哚能够与原位生成的N-Boc醛亚胺发生不对称Mannich反应,可用于高选择性地合成含3,3′-双吲哚的化合物(Scheme 1)。本文以10 mol%(R,R)-环己二胺衍生的手性双功能硫脲叔胺(4b)为催化剂,3′-吲哚-3-氧化吲哚与α-氨基砜为原料,经3′-吲哚-3-氧化吲哚与原位生成的N-Boc芳香醛亚胺的不对称Mannich反应,合成了20个3,3-二取代3-吲哚-3′-基氧化吲哚类化合物(3a~3t, Scheme 2和表1),分离收率54%~98%,dr值90 ∶10~>99 ∶1,产物结构经1H NMR,13C NMR和HR-MS(ESI-TOF)表征。

Scheme1
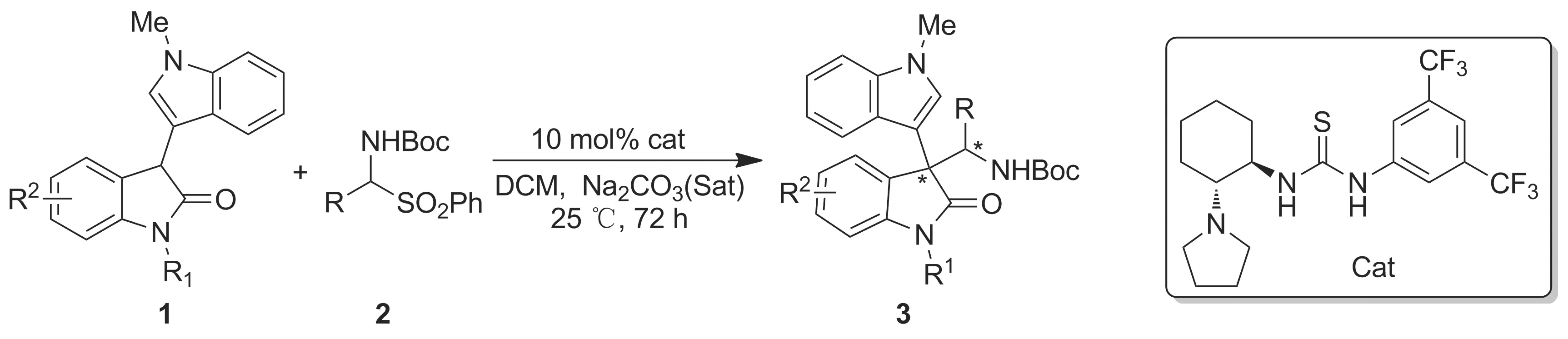
Scheme 2

Entry12Yieldof3/%adrb1R1=Me,R2=H(1a)R=C6H5(2a)3a/98>99∶12R1=Me,R2=H(1a)R=3-MeC6H4(2b)3b/9390∶103R1=Me,R2=H(1a)R=4-MeC6H4(2c)3c/8192∶84R1=Me,R2=H(1a)R=3-OMeC6H4(2d)3d/8292∶85R1=Me,R2=H(1a)R=3,4-(OMe)2C6H3(2e)3e/9493∶76R1=Me,R2=H(1a)R=3-NO2C6H4(2f)3f/7692∶87R1=Me,R2=H(1a)R=4-FC6H4(2g)3g/9392∶88R1=Me,R2=H(1a)R=3-ClC6H4(2h)3h/8192∶89R1=Me,R2=H(1a)R=4-ClC6H4(2i)3i/8192∶810R1=Me,R2=H(1a)R=3-BrC6H4(2j)3j/7093∶711R1=Me,R2=H(1a)R=4-BrC6H4(2k)3k/8393∶712R1=Me,R2=H(1a)R=1-naphthyl(2l)3l/81>99∶113R1=Me,R2=H(1a)R=2-furyl(2m)3m/7390∶1014R1=Me,R2=H(1a)R=2-thienyl(2n)3n/9690∶1015R1=Bn,R2=H(1b)R=C6H5(2a)3o/5490∶1016R1=Bn,R2=H(1b)R=3-OMeC6H4(2d)3p/8490∶1017R1=Bn,R2=H(1b)R=4-FC6H4(2g)3q/7292∶818R1=Bn,R2=H(1b)R=4-BrC6H4(2k)3r/7090∶1019R1=Me,R2=5-Cl(1c)R=C6H5(2a)3s/6893∶720R1=Me,R2=7-Cl(1d)R=C6H5(2a)3t/8397∶3
ayield表示产物3的分离收率;bdr值由1H NMR确定。
1 实验部分
1.1 仪器与试剂
SGW X-4型显微熔点仪;Agilent-400 M型核磁共振仪(CDCl3或DMSO-d6为溶剂,TMS为内标);Waters Xevo G2 QTOF型质谱分析仪。
所用试剂均为分析纯。
1.23a~3t的合成(以3a为例)
在4 mL硬质反应管里中依次加入手性双功能硫脲叔胺催化剂(4b)4.4 mg(10 mol%)、 3′-吲哚-3-氧化吲哚(1a)27.6 mg(0.1 mmol)、α-氨基砜(2a)52.1 mg(0.15 mmol)、 CH2Cl2(1.0 mL)与饱和Na2CO3水溶液(0.1 mL),搅拌下于25 ℃反应72 h(TLC监测)。混合物经硅胶柱层析[洗脱剂:V(乙酸乙酯) ∶V(石油醚)=1 ∶5~1 ∶3)纯化得3a47.2 mg。
用类似方法合成3b~3t。
[(1-甲基-3-(1-甲基-1H-吲哚-3-基)-2-氧化吲哚啉-3-基)]-苯甲氨基甲酸叔丁酯(3a): 白色泡沫状固体,收率98%,dr>99 ∶1, m.p.204.5~205.2 ℃;1H NMRδ(major): 1.40(d,J=1.4 Hz, 9H), 3.08(d,J=1.1 Hz, 3H), 3.80(s, 3H), 5.76(dd,J=1.4 Hz, 8.8 Hz, 1H), 6.50(d,J=7.8 Hz, 1H), 6.91~6.98(m, 6H), 7.01~7.05(m, 3H), 7.08(d,J=7.7 Hz, 1H), 7.14~7.17(m, 2H), 7.28(d,J=8.2 Hz, 1H), 7.44(d,J=1.2 Hz, 1H);13C NMRδ(major): 26.1, 28.5, 33.0, 56.0, 58.5, 79.5, 107.9, 109.6, 111.4, 119.1, 119.5, 121.8, 123.0, 124.5, 126.1, 127.1, 127.3, 127.7, 128.1, 128.2, 131.7, 137.3, 137.6, 142.6, 155.6, 177.9; HR-MS(ESI-TOF)m/z: Calcd for C30H32N3O3{[M+H]+}482.244 4, found 482.247 9。
[(1-甲基-3-(1-甲基-1H-吲哚-3-基)-2-氧化吲哚啉-3-基)]-3-甲基苯甲氨基甲酸叔丁酯(3b): 白色泡沫状固体,收率93%,dr90 ∶10, m.p.170.2~170.9 ℃;1H NMRδ(major): 1.41(s, 9H), 2.12(s, 3H), 3.08(s, 3H), 3.80(s, 3H), 5.71(d,J=8.9 Hz, 1H), 6.50(d,J=7.8 Hz, 1H), 6.76~6.79(m, 2H), 6.84(d,J=7.3 Hz, 1H), 6.88~6.95(m, 5H), 7.05~7.08(m, 1H), 7.13~7.16(m, 2H), 7.28(d,J=8.4 Hz, 1H), 7.45(s, 1H);13C NMRδ(major): 21.2, 26.1, 28.5, 33.0, 56.0, 58.5, 79.5, 107.9, 109.6, 111.4, 119.1, 119.5, 121.8, 122.9, 124.6, 124.8, 126.1, 126.9, 127.9, 128.1, 128.2, 128.6, 131.8, 136.6, 137.2, 137.5, 142.6, 155.6, 178.0; HR-MS(ESI-TOF)m/z: Calcd for C31H34N3O3{[M+H]+}496.260 0, found 496.260 2。
[(1-甲基-3-(1-甲基-1H-吲哚-3-基)-2-氧化吲哚啉-3-基)]-4-甲基苯甲氨基甲酸叔丁酯(3c): 白色泡沫状固体,收率81%,dr92 ∶8, m.p.171.4~172.2 ℃;1H NMRδ(major): 1.41(s, 9H), 2.12(s, 3H), 3.08(s, 3H), 3.80(s, 3H), 5.71(d, 8.9 Hz, 1H), 6.50(d,J=7.8 Hz, 1H), 6.76~6.79(m, 2H), 6.84(d,J=7.3 Hz, 1H), 6.88~6.95(m, 5H), 7.05~7.08(m, 1H), 7.13~7.16(m, 2H), 7.28(d,J=8.4 Hz, 1H), 7.45(s, 1H);13C NMRδ(major): 21.2, 26.1, 28.5, 33.0, 56.0, 58.5, 79.5, 107.9, 109.6, 111.4, 119.1, 119.5, 121.8, 122.9, 124.6, 124.8, 126.1, 126.9, 127.9, 128.1, 128.2, 128.6, 131.8, 136.6, 137.2, 137.5, 142.6, 155.6, 178.0; HR-MS(ESI-TOF)m/z: Calcd for C31H34N3O3{[M+H]+}496.260 0, found 496.260 2。
[(1-甲基-3-(1-甲基-1H-吲哚-3-基)-2-氧化吲哚啉-3-基)]-3-甲氧基苯甲氨基甲酸叔丁酯(3d): 白色泡沫状固体,收率82%,dr92 ∶8, m.p.180.2~180.9 ℃;1H NMRδ(major): 1.41(s, 9H), 2.12(s, 3H), 3.09(s, 3H), 3.55(s, 3H), 3.79(s, 3H), 5.75(d,J=8.8 Hz, 1H), 6.42(s, 1H), 6.55(d,J=7.7 Hz, 1H), 6.60(d,J=8.1 Hz, 1H), 6.66(d,J=7.6 Hz, 1H), 6.88(d,J=8.6 Hz, 1H), 6.92~6.99(m, 3H), 7.03(d,J=7.9 Hz, 1H), 7.08~7.12(m, 1H), 7.14~7.17(m, 2H), 7.28(d,J=8.4 Hz, 1H), 7.40(s, 1H);13C NMRδ(major): 26.1, 28.4, 33.0, 55.1, 56.0, 58.5, 79.5, 108.1, 109.6, 111.4, 112.8, 113.9, 119.2, 119.5, 119.8, 121.8, 122.8, 124.5, 126.1, 128.1, 128.2, 128.3, 131.7, 137.2, 139.2, 142.8, 155.6, 158.5, 177.8; HR-MS(ESI-TOF)m/z: Calcd for C31H34N3O4{[M+H]+}512.254 9, found 512.254 0。
[(1-甲基-3-(1-甲基-1H-吲哚-3-基)-2-氧化吲哚啉-3-基)]-3,4-二甲氧基苯甲氨基甲酸叔丁酯(3e): 白色泡沫状固体,收率94%,dr93 ∶7, m.p.101.2~102.1 ℃;1H NMRδ(major): 1.39(s, 9H), 3.08(s, 3H), 3.55(s, 3H), 3.76(s, 3H), 3.78(s, 3H), 5.72(d,J=8.8 Hz, 1H), 6.32(d,J=1.6 Hz, 1H), 6.54~6.59(m, 2H), 6.64~6.67(m, 1H), 6.80(d,J=8.3 Hz, 1H), 6.92~6.96(m, 2H), 7.05(d,J=7.9 Hz, 1H), 7.07~7.11(m, 1H), 7.13~7.16(m, 2H), 7.27(d,J=7.9 Hz, 1H), 7.35(s, 1H);13C NMRδ(major): 26.2, 28.5, 33.0, 55.6, 55.8, 56.3, 58.1, 79.5, 108.2, 109.6, 109.8, 111.1, 111.5, 119.3, 119.6, 121.9, 122.8, 124.5, 126.1, 128.1, 128.3, 130.3, 131.8, 137.3, 142.9, 147.4, 147.9, 155.5, 177.9; HR-MS(ESI-TOF)m/z: Calcd for C32H36N3O5{[M+H]+}542.265 5, found 542.265 0。
[(1-甲基-3-(1-甲基-1H-吲哚-3-基)-2-氧化吲哚啉-3-基)]-3-硝基苯甲氨基甲酸叔丁酯(3f): 淡黄色泡沫状固体,收率76%,dr92 ∶8, m.p.105.6~107.3 ℃;1H NMRδ(major): 1.39(s, 9H), 3.09(s, 3H), 3.80(s, 3H), 5.86(d,J=8.2 Hz, 1H), 6.52(d,J=7.8 Hz, 1H), 6.93~7.01(m, 4H), 7.06~7.10(m, 1H), 7.15~7.22(m, 3H), 7.29(d,J=8.2 Hz, 1H), 7.34~7.36(m, 2H), 7.83(s, 1H), 7.92(d,J=8.1 Hz, 1H);13C NMRδ(major): 26.2, 28.4, 33.0, 55.6, 58.2, 80.1, 108.3, 109.7, 110.6, 119.1, 119.8, 122.1, 122.4, 122.8, 123.4, 124.6, 125.9, 128.0, 128.1, 128.8, 130.7, 133.8, 137.3, 140.3, 142.3, 147.2, 155.6, 177.3; HR-MS(ESI-TOF)m/z: Calcd for C30H31N4O5{[M+H]+}527.229 4, found 527.227 8。
[(1-甲基-3-(1-甲基-1H-吲哚-3-基)-2-氧化吲哚啉-3-基)]-4-氟苯甲氨基甲酸叔丁酯(3g): 白色固体,收率93%,dr92 ∶8, m.p.207.6~208.8 ℃;1H NMRδ(major): 1.39(s, 9H), 3.09(s, 3H), 3.79(s, 3H), 5.74(d,J=8.4 Hz, 1H), 6.54(d,J=7.6 Hz, 1H), 6.68~6.72(m, 2H), 6.89~6.99(m, 6H), 7.08~7.12(m, 1H), 7.14~7.16(m, 2H), 7.28(d,J=9.0 Hz, 1H), 7.39(s, 1H);13C NMRδ(major): 26.2, 28.4, 33.0, 56.0, 57.9, 79.7, 108.1, 109.6, 111.2, 114.0(d,J=21.2 Hz, 1C), 119.1, 119.6, 121.9, 123.1, 124.5, 126.1, 128.0, 128.4, 129.3(d,J=8.0 Hz, 1C), 131.5, 133.6(d,J=3.1 Hz, 1C), 137.3, 142.5, 155.6, 162.0(d,J=244.1 Hz, 1C), 177.8; HR-MS(ESI-TOF)m/z: Calcd for C30H31N3O3F{[M+H]+}500.234 9, found 500.232 5。
[(1-甲基-3-(1-甲基-1H-吲哚-3-基)-2-氧化吲哚啉-3-基)]-3-氯苯甲氨基甲酸叔丁酯(3h): 白色泡沫状固体,收率81%,dr92 ∶8, m.p.194.4~195.6 ℃;1H NMRδ(major): 1.40(s, 9H), 3.12(s, 3H), 3.79(s, 3H), 5.71(d,J=8.6 Hz, 1H), 6.55(d,J=7.7 Hz, 1H), 6.82(d,J=7.6 Hz, 1H), 6.91-6.96(m, 5H), 6.99~7.03(m, 2H), 7.09~7.17(m, 3H), 7.28(d,J=9.3 Hz, 1H), 7.38(s, 1H);13C NMRδ(major): 26.2, 28.4, 33.0, 55.8, 57.9, 79.7, 108.2, 109.6, 111.1, 119.1, 119.6, 121.9, 123.1, 124.5, 126.0, 127.3, 128.0, 128.5, 129.1, 131.3, 133.1, 136.5, 137.3, 142.5, 155.5, 177.7; HR-MS(ESI-TOF)m/z: Calcd for C30H31N3O3Cl{[M+H]+}516.205 4, found 516.201 4。
[(1-甲基-3-(1-甲基-1H-吲哚-3-基)-2-氧化吲哚啉-3-基)]-4-氯苯甲氨基甲酸叔丁酯(3i): 白色泡沫状固体,收率81%,dr92 ∶8, m.p.195.9~196.8 ℃;1H NMRδ(major): 1.39(s, 9H), 3.10(s, 3H), 3.79(s, 3H), 5.74(d,J=8.5 Hz, 1H), 6.56(d,J=7.7 Hz, 1H), 6.88~7.01(m, 8H), 7.09~7.18(m, 3H), 7.28(d,J=8.3 Hz, 1H), 7.37(s, 1H);13C NMRδ(major): 26.2, 28.5, 33.0, 55.8, 58.2, 79.8, 108.2, 109.7, 111.1, 119.1, 119.7, 122.0, 123.2, 124.6, 126.1, 126.3, 127.5, 127.7, 128.1, 128.3, 128.5, 131.3, 133.2, 137.3, 139.9, 142.5, 155.6, 177.7; HR-MS(ESI-TOF)m/z: Calcd for C30H31N3O3Cl{[M+H]+}516.205 4, found 516.206 1.
[(1-甲基-3-(1-甲基-1H-吲哚-3-基)-2-氧化吲哚啉-3-基)]-3-溴苯甲氨基甲酸叔丁酯(3j): 白色泡沫状固体,收率70%,dr93 ∶7, m.p.105.2~106.5 ℃;1H NMRδ(major): 1.40(s, 9H), 3.13(s, 3H), 3.79(s, 3H), 5.70(d,J=8.6 Hz, 1H), 6.56(d,J=7.7 Hz, 1H), 6.83~6.88(m, 2H), 6.91~6.96(m, 4H), 7.09~7.18(m, 5H), 7.28(d,J=8.2 Hz, 1H), 7.39(s, 1H);13C NMRδ(major): 26.2, 28.4, 33.0, 55.8, 58.2, 79.8, 108.2, 109.6, 111.0, 119.1, 119.6, 121.3, 122.0, 123.2, 124.5, 126.0, 126.7, 128.0, 128.6, 130.4, 130.6, 131.2, 137.3, 140.2, 142.5, 155.6, 177.6; HR-MS(ESI-TOF)m/z: Calcd for C30H31N3O3Br{[M+H]+}560.154 9, found 560.153 7。
[(1-甲基-3-(1-甲基-1H-吲哚-3-基)-2-氧化吲哚啉-3-基)]-4-溴苯甲氨基甲酸叔丁酯(3k): 白色固体,收率83%,dr93 ∶7, m.p.199.6~200.5 ℃;1H NMRδ(major): 1.39(s, 9H), 3.10(s, 3H), 3.78(s, 3H), 5.72(d,J=8.6 Hz, 1H), 6.57(d,J=7.7 Hz, 1H), 6.84~6.89(m, 3H), 6.92~6.96(m, 2H), 7.00(d,J=7.8 Hz, 1H), 7.09~7.17(m, 5H), 7.28(d,J=8.2 Hz, 1H), 7.36(s, 1H);13C NMRδ(major): 26.2, 28.4, 33.0, 55.8, 58.0, 79.7, 108.3, 109.6, 111.1, 119.1, 119.6, 121.3, 121.9, 123.1, 124.5, 126.0, 128.0, 128.5, 129.4, 130.2, 131.2, 137.0, 137.3, 142.5, 155.5, 177.7; HR-MS(ESI-TOF)m/z: Calcd for C30H31N3O3Br{[M+H]+}560.154 9, found 560.158 6.
[(1-甲基-3-(1-甲基-1H-吲哚-3-基)-2-氧化吲哚啉-3-基)]-1-萘甲氨基甲酸叔丁酯(3l): 红色固体,收率81%,dr>99 ∶1, m.p.198.6~199.8 ℃;1H NMRδ(major): 1.33(s, 9H), 3.16(s, 3H), 3.82(s, 3H), 6.43~6.46(m, 1H), 6.57(d,J=7.6 Hz, 1H), 6.72~6.88(m, 4H), 7.08~7.12(m, 1H), 7.18(d,J=7.2 Hz, 1H), 7.29~7.49(m, 6H), 7.64~7.71(m, 3H), 8.45(d,J=8.2 Hz, 1H);13C NMRδ(major): 26.2, 28.1, 32.8, 51.2, 55.3, 79.1, 108.2, 110.2, 111.6, 118.5, 119.3, 121.5, 122.0, 123.8, 124.0, 124.6, 125.0, 125.4, 125.5, 125.6, 128.0, 128.1, 128.2, 128.3, 130.1, 131.0, 132.6, 135.5, 136.9, 141.9, 154.8, 178.1; HR-MS(ESI-TOF)m/z: Calcd for C34H34N3O3{[M+H]+}532.260 0, found 532.260 7。
[(1-甲基-3-(1-甲基-1H-吲哚-3-基)-2-氧化吲哚啉-3-基)]-2-呋喃甲氨基甲酸叔丁酯(3m): 淡黄色固体,收率73%,dr90 ∶10, m.p. 193.3~194.2 ℃;1H NMRδ(major): 1.41(s, 9H), 3.26(s, 3H), 3.75(s, 3H), 5.83(d,J=2.6 Hz, 1H), 5.92(d,J=9.4 Hz, 1H), 6.04(d,J=1.8 Hz, 1H), 6.62(d,J=9.2 Hz, 1H), 6.73(d,J=7.8 Hz, 1H), 6.92~6.96(m, 2H), 7.06~7.12(m, 3H), 7.14~7.19(m, 2H), 7.25(d,J=7.6 Hz, 1H), 7.34(s, 1H);13C NMRδ(major): 26.5, 28.4, 32.9, 53.0, 55.1, 79.7, 107.4, 108.0, 109.5, 110.0, 111.0, 119.4, 119.5, 121.8, 123.0, 124.5, 126.0, 127.9, 128.4, 131.0, 137.3, 141.3, 143.1, 151.7, 155.4, 177.9; HR-MS(ESI-TOF)m/z: Calcd for C28H30N3O4{[M+H]+}472.223 6, found 472.224 8。
[(1-甲基-3-(1-甲基-1H-吲哚-3-基)-2-氧化吲哚啉-3-基)]-2-噻吩甲氨基甲酸叔丁酯(3n): 白色泡沫状固体,收率96%,dr90 ∶10, m.p.83.3~84.2 ℃;1H NMRδ(major): 1.41(s, 9H), 3.16(s, 3H), 3.76(s, 3H), 6.12(d,J=9.0 Hz, 1H), 6.50(d,J=3.1 Hz, 1H), 6.64~6.71(m, 3H), 6.96~7.00(m, 3H), 7.11~7.22(m, 4H), 7.27~7.30(m, 2H);13C NMRδ(major): 26.4, 28.4, 33.0, 54.7, 56.3, 79.8, 108.2, 109.6, 111.2, 119.6, 121.9, 123.1, 124.3, 124.6, 125.5, 125.9, 126.1, 128.1, 128.6, 131.3, 137.3, 141.7, 143.4, 155.4, 177.6; HR-MS(ESI-TOF)m/z: Calcd for C28H30N3O3S{[M+H]+}488.200 8, found 488.204 7。
[(1-苄基-3-(1-甲基-1H-吲哚-3-基)-2-氧化吲哚啉-3-基)]-苯甲氨基甲酸叔丁酯(3o): 淡黄色固体,收率54%,dr90 ∶10, m.p.90.6~91.8 ℃;1H NMRδ(major): 1.40(s, 9H), 3.78(s, 3H), 4.70(d,J=15.5 Hz, 1H), 4.90(d,J=15.5 Hz, 1H), 5.87(d,J=8.7 Hz, 1H), 6.49(d,J=6.9 Hz, 1H), 6.82~6.86(m, 1H), 6.90~7.03(m, 8H), 7.10~7.11(m, 3H), 7.15(d,J=7.9 Hz, 1H), 7.20(d,J=7.3 Hz, 1H), 7.28(s, 4H), 7.40(s, 1H);13C NMRδ(major): 28.5, 33.0, 44.2, 56.2, 58.2, 79.5, 109.2, 109.5, 112.0, 119.5, 119.6, 121.8, 123.0, 124.9, 126.0, 127.3, 127.5, 127.7, 127.9, 128.0, 128.1, 128.2, 128.8, 131.6, 135.3, 137.3, 137.8, 142.1, 155.6, 178.1; HR-MS(ESI-TOF)m/z: Calcd for C36H36N3O3{[M+H]+}558.275 1, found 558.276 7。
[(1-苄基-3-(1-甲基-1H-吲哚-3-基)-2-氧化吲哚啉-3-基)]-3-甲氧基苯甲氨基甲酸叔丁酯(3p): 白色泡沫状固体,收率84%,dr90 ∶10, m.p.78.6~79.8 ℃;1H NMRδ(major): 1.41(s, 9H), 3.51(s, 3H), 3.78(s, 3H), 4.79(d,J=15.5 Hz, 1H), 4.88(d,J=15.5 Hz, 1H), 5.89(d,J=8.8 Hz, 1H), 6.51(d,J=10.1 Hz, 2H), 6.67~6.72(m, 2H), 6.86~6.97(m, 3H), 6.99~7.09(m, 5H), 7.13~7.17(m, 1H), 7.22(d,J=7.4 Hz, 1H), 7.26~7.28(m, 4H), 7.36(s, 1H);13C NMRδ(major): 28.5, 33.0, 44.2, 55.1, 56.3, 58.1, 79.6, 109.3, 109.5, 112.0, 113.0, 114.0, 119.5, 119.7, 120.1, 121.8, 122.8, 124.9, 126.0, 127.3, 127.7, 128.1, 128.2, 128.5, 128.8, 131.5, 135.2, 137.3, 139.4, 142.3, 155.5, 158.6, 178.0; HR-MS(ESI-TOF)m/z: Calcd for C37H38N3O4{[M+H]+}588.286 2, found 588.287 6.
[(1-苄基-3-(1-甲基-1H-吲哚-3-基)-2-氧化吲哚啉-3-基)]-4-氟苯甲氨基甲酸叔丁酯(3q): 白色泡沫状固体,收率72%,dr92 ∶8, m.p.95.4~96.8 ℃;1H NMRδ(major): 1.39(s, 9H), 3.78(s, 3H), 4.76(d,J=15.4 Hz, 1H), 4.88(d,J=15.5 Hz, 1H), 5.85(d,J=8.6 Hz, 1H), 6.55(d,J=7.8 Hz, 1H), 6.67~6.71(m, 2H), 6.84~7.06(m, 7H), 7.10~7.17(m, 3H), 7.20(d,J=7.3 Hz, 1H), 7.30(d,J=3.2 Hz, 4H), 7.35(s, 1H);13C NMRδ(major): 28.5, 33.0, 44.2, 56.2, 57.6, 79.7, 109.3, 109.6, 111.8, 114.3(d,J=21.3 Hz, 1C), 119.6, 121.9, 123.1, 124.8, 126.0, 127.8, 127.9, 128.0, 128.4, 128.8, 129.6(d,J=8.1 Hz, 1C), 131.4, 133.7(d,J=3.0 Hz, 1C), 135.2, 137.3, 142.1, 155.5, 162.0(d,J=244.2 Hz, 1C), 177.9; HR-MS(ESI-TOF)m/z: Calcd for C36H35N3O3F{[M+H]+}576.266 2, found 576.268 6.
[(1-苄基-3-(1-甲基-1H-吲哚-3-基)-2-氧化吲哚啉-3-基)]-4-溴苯甲氨基甲酸叔丁酯(3r): 白色
泡沫状固体,收率70%,dr90 ∶10, m.p.99.8~110.0 ℃;1H NMRδ(major): 1.39(s, 9H), 3.78(s, 3H), 4.81(d,J=15.5 Hz, 1H), 4.89(d,J=15.5 Hz, 1H), 5.84(d,J=8.5 Hz, 1H), 6.56(d,J=7.8 Hz, 1H), 6.86~6.96(m, 5H), 7.01~7.06(m, 4H), 7.12~7.17(m, 3H), 7.22(d,J=7.4 Hz, 1H), 7.26~7.35(m, 5H);13C NMRδ(major): 28.4, 33.0, 44.2, 56.0, 57.6, 79.7, 109.4, 109.6, 111.7, 119.5, 119.6, 121.4, 121.9, 123.1, 124.8, 125.9, 127.5, 127.8, 128.0, 128.5, 128.8, 129.7, 130.6, 131.2, 135.0, 137.1, 137.3, 142.1, 155.4, 177.9; HR-MS(ESI-TOF)m/z: Calcd for C36H35N3O3Br{[M+H]+}636.186 2, found 636.184 6。
[(5-氯-1-甲基-3-(1-甲基-1H-吲哚-3-基)-2-氧化吲哚啉-3-基)]-苯甲氨基甲酸叔丁酯(3s): 白色固体,收率68%,dr93 ∶7, m.p.200.2~201.6 ℃;1H NMRδ(major): 1.40(s, 9H), 3.06(s, 3H), 3.81(s, 3H), 5.72(d,J=8.9 Hz, 1H), 6.41(d,J=8.3 Hz, 1H), 6.86~6.90(m, 1H), 6.92~6.99(m, 4H), 7.03~7.07(m, 4H), 7.10~7.11(m, 1H), 7.15~7.19(m, 1H), 7.30(d,J=8.2 Hz, 1H), 7.45(s, 1H);13C NMRδ(major): 26.3, 28.5, 33.0, 56.3, 58.4, 79.7, 108.9, 109.7, 110.7, 118.8, 119.7, 122.0, 125.0, 126.0, 127.3, 127.5, 127.6, 127.7, 128.1, 128.2, 128.3, 133.5, 137.3, 141.2, 155.5, 177.6; HR-MS(ESI-TOF)m/z: Calcd for C30H31N3O3Cl{[M+H]+}516.204 8, found 516.205 2。
[(7-氯-1-甲基-3-(1-甲基-1H-吲哚-3-基)-2-氧化吲哚啉-3-基)]-苯甲氨基甲酸叔丁酯(3t): 白色固体,收率83%,dr97 ∶3, m.p.96.8~98.2 ℃;1H NMRδ(major): 1.41(s, 9H), 3.44(s, 3H), 3.80(s, 3H), 5.71(d,J=8.9 Hz, 1H), 6.81~6.85(m, 1H), 6.88(d,J=8.9 Hz, 1H), 6.93~7.12(m, 9H), 7.15~7.18(m, 1H), 7.29(d,J=8.2 Hz, 1H), 7.45(s, 1H);13C NMRδ(major): 28.5, 29.6, 33.0, 56.0, 58.7, 79.6, 109.7, 111.0, 115.3, 119.0, 119.7, 122.0, 123.2, 123.7, 126.0, 127.2, 127.6, 127.7, 128.1, 130.4, 134.6, 137.2, 137.3, 138.6, 155.5, 178.3; HR-MS(ESI-TOF)m/z: Calcd for C30H31N3O3Cl{[M+H]+}516.204 8, found 516.204 7。
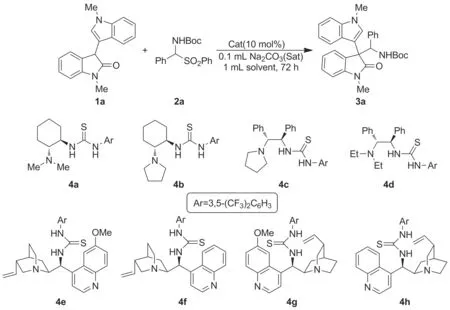
Scheme 3
2 结果与讨论
2.1 反应条件优化
以N1-位甲基取代的3′-吲哚-3-氧化吲哚(1a)和苯基取代的α-氨基砜(2a)的不对称Mannich反应作为模板反应,分别考察了不同骨架衍生的手性双功能硫脲催化剂(4)的催化性能,期望获得光学纯的3,3-二取代3-吲哚-3′-基氧化吲哚类化合物(3a)(Scheme 3,表2)。
遗憾的是,无论是手性的环己二胺骨架、1,2-二苯基乙二胺骨架还是金鸡纳碱骨架衍生的双功能硫脲作为催化剂,均几乎产生消旋化的产物(3a),而产物的非对映选择性均很高(dr>99∶1)(Entries 1~8)。综合考虑产物收率和非对映选择性,我们选择(R,R)-环己二胺衍生且含吡咯单元的双功能硫脲4b作为理想的催化剂用于合成消旋化的3,3-二取代3-吲哚-3′-基氧化吲哚类化合物。
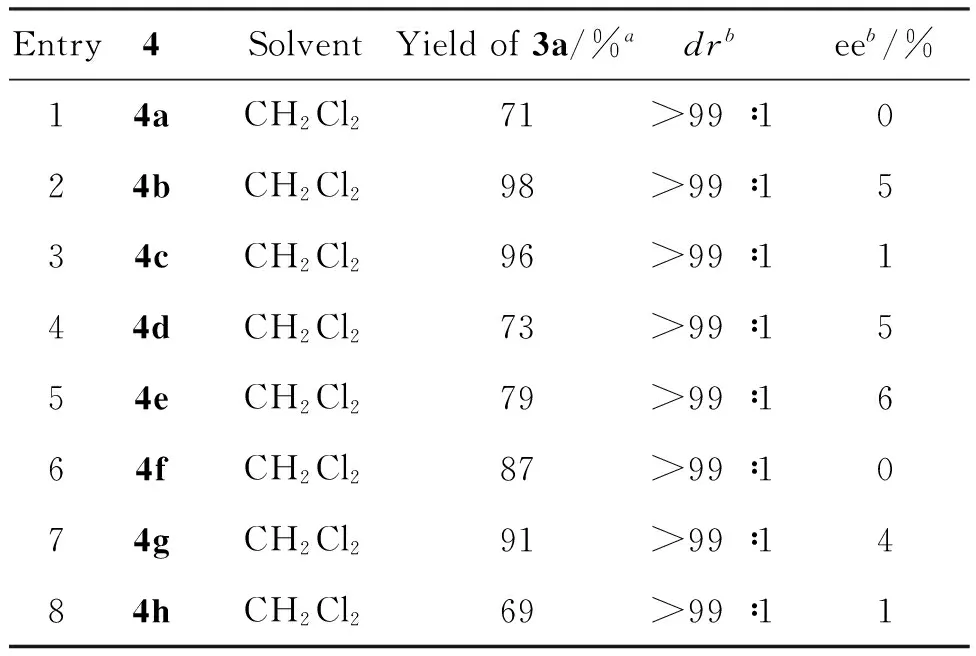
表2 反应条件优化
ayield表示产物3a的分离收率;bdr和ee值由手性的HPLC分析测定。
2.2 底物的适用性
由表1可见,该反应体系适用于芳香环上含有各种取代基团的α-氨基砜(2)。无论是给电子基团还是吸电子基团取代以及不同位置取代的α-氨基砜底物均能很好地与3′-吲哚-3-氧化吲哚底物(1a)反应,得到较好收率与高非对映选择性的产物(3b~3k)(Entries 2~11)。当α-氨基砜的芳香取代基由苯基换为大位阻的1-萘基、2-呋喃基或2-噻吩取代基时,反应也能很好的进行,得到中等以上收率和高达>99∶1dr值的相应产物(3l~3n)(Entries 12~14)。此外,我们还考察了氧化吲哚N1-位取代基对该反应的影响。当氧化吲哚N1-位的取代基由甲基换为苄基时,反应的活性有所降低。在同样时间内,3′-吲哚-3-氧化吲哚底物(1b)不能完全消耗,相应产物(3o)的分离收率只有54%(Entry 15)。为进一步探索该反应体系的底物适应范围,我们以3′-吲哚-3-氧化吲哚(1b)作为亲核试剂与不同取代的α-氨基砜(2)的不对称Mannich反应,也能够以较好的收率和dr值得到相应的加成产物(3p~3r)(Entries 16~18)。最后,我们还考察了氧化吲哚苯环上取代基对反应的影响。当氧化吲哚苯环的C5位或C7位分别引入氯原子取代基时,产物(3s)和(3t)的收率分别为68%和83%,dr值分别为93 ∶7和97 ∶3(Entries 19~20)。
2.3 反应机理
根据文献报道[12-13],我们提出了该不对称Mannich反应的可能过渡态(Scheme 4)。首先,在手性双功能硫脲叔胺催化剂催化作用下,催化剂的叔胺部分作为碱促使3′-吲哚-3-氧化吲哚(1)的C3-位失去质子生成烯醇式负离子,并与催化剂的叔胺部分形成氢键,用于活化3′-吲哚-3-氧化吲哚的C3-位;催化剂的硫脲部分以双氢键的形式活化原位生成的N-Boc芳香醛亚胺;在这种立体空间环境下,3′-吲哚-3-氧化吲哚负离子对原位生成的N-Boc芳香醛亚胺的C=N双键进行选择性亲核进攻,高非对映选择性生成产物(3)。
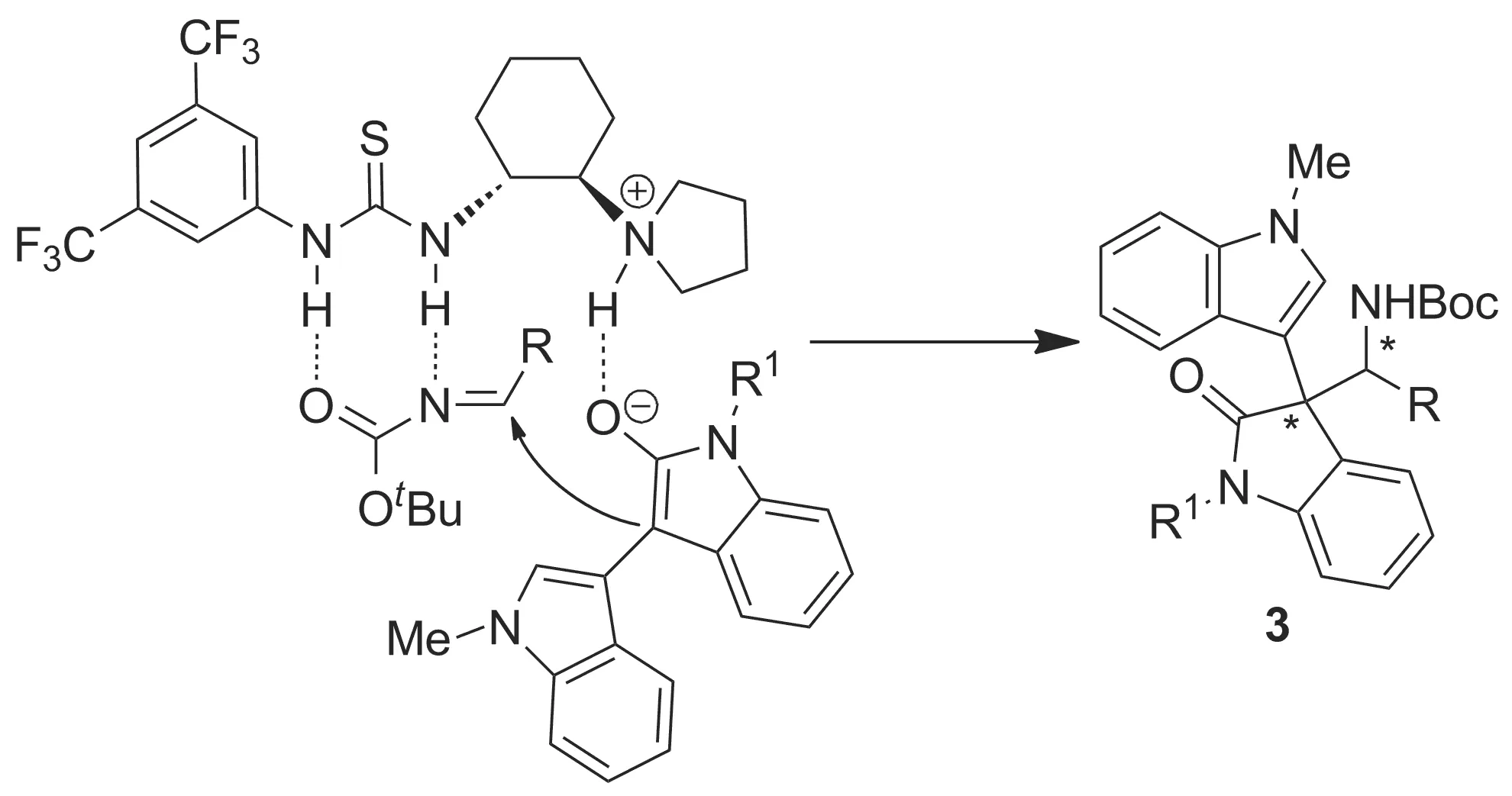
Scheme 4
通过(R,R)-环己二胺衍生的手性双功能硫脲叔胺催化剂催化3′-吲哚-3-氧化吲哚与原位生成的N-Boc芳香醛亚胺的不对称Mannich反应,实现了一系列3,3-二取代3-吲哚-3′-基氧化吲哚类化合物的有效合成,产物的收率和dr值分别高达98%与大于99 ∶1。本研究为双吲哚类化合物的立体选择性合成提供了一种可供选择的有效途径。
[1] Ruiz-Sanchis P, Savina S A, Albericio F,etal. Structure, bioactivity and synthesis of natural products with hexahydropyrrolo[2,3-b]indole [J].Chem Eur J,2011,17(5):1388-1408.
[2] Usami Y, Yamaguchi J, Numata A. Gliocladins A-C and glioperazine: Cytotoxic dioxo- or trioxopiperazine metabolites from aGliocladiumSp. Separated from a Sea Hare [J].Heterocycles,2004,63(5):1123-1129.
[3] Arai T, Yamamoto Y, Awata A,etal. Catalytic asymmetric synthesis of mixed 3,3′-bisindoles and their evaluation as Wnt signaling inhibitors [J].Angew Chem Int Ed,2013,52(9):2486-2490.
[4] Steven A, Overman L E. Total synthesis of complex cyclotryptamine alkaloids: Stereocontrolled construction of quaternary carbon stereocenters [J].Angew Chem Int Ed,2007,46(29):5488-5508.
[5] Schmidt M A, Movassaghi M. New strategies for the synthesis of hexahydropyrroloindole alkaloids inspired by biosynthetic hypotheses [J].Synlett,2008,3:313-324.
[6] Link J T, Overman L E. Stereocontrolled total syntheses ofmeso-chimonanthine andmeso-calycanthineviaa novel samarium mediated reductive dialkylation [J].J Am Chem Soc,1996,118(34):8166-8167.
[7] Overman L E, Watson D A. Diastereoselection in the formation of spirocyclic oxindoles by the intramolecular Heck reaction[J].J Org Chem,2006,71(7):2587-2599.
[8] Kieffer M E, Chuang K V, Reisman S E. Copper-catalyzed diastereoselective arylation of tryptophan derivatives: Total synthesis of (+)-naseseazines A and B[J].J Am Chem Soc,2013,135(15):5557-5560.
[9] Ghosh S, Chaudhuri S, Bisai A. Catalytic enantioselective decarboxylative allylations of a mixture of allyl carbonates and allyl esters: Total synthesis of (-)- and (+)-folicanthine[J].Chem Eur J,2015,21(48):17479-17484.
[10] Awata A, Wasai M, Masu H,etal. An imidazoline-aminophenol(IAP) nickel catalyst: Structure and catalytic activity in the enantioselective 1,4-addition of 3′-indolyl-3-oxindoles to nitroethylene [J].Chem Eur J,2014,20(6):2470-2477.
[11] Jing C, Xing D, Hu W. Catalytic asymmetric four-component reaction for the rapid construction of 3,3-disubstituted 3-indol-3′-yloxindoles[J].Org Lett,2015,17(17):4336-4339.
[12] Shan J, Cui B, Wang Y,etal. Organocatalytic asymmetric Mannich reaction of 3-hydroxyoxindoles/3-aminooxindoles withinsitugeneratedN-Boc protected aldimines for the synthesis of vicinal oxindole-diamines/aminoalcohols[J].J Org Chem,2016,81(13):5270-5277.
[13] Wang H Y, Zhang K, Zheng C W,etal. Asymmetric dual-reagent catalysis:Mannich-type reactions catalyzed by ion pair[J].Angew Chem Int Ed,2015,54(6):1775-1779.
Stereoselective Synthesis of 3,3-Disubstituted 3-Indol-3′-yloxindoles
CUI Bao-dong, WANG Wei, YUAN Chang-lun, SHAN Jing, CHEN Yong-zheng*
(College of Pharmacy, Zunyi Medical University, Zunyi 563000, China)
An asymmetric Mannich reaction of 3′-indole-3-oxindoles andinsitugeneratedN-Boc protected aldimines with the chiral bifunctional thiourea-tertiary amine catalyst(4b) derived from (R,R)-cyclohexyl diamine was realized. Twenty 3,3-disubstituted 3-indol-3′-yloxindoles(3a~3t) were obtained in 54%~98% yields withdrof 90∶10~>99∶1. The structures were characterized by1H NMR,13C NMR and HR-MS(ESI-TOF).
3′-indole-3-oxindole;α-amidosulfone; organocatalysis; Mannich reaction; aldimine; 3,3-disubstituted 3-indol-3′-yloxindole; synthesis
2017-04-04;
: 2017-07-20
贵州省高校优秀科技创新人才支持计划项目(黔教合KY字[2015]480)
崔宝东(1984-),男,汉族,山东菏泽人,博士研究生,主要从事不对称催化与手性药物合成研究。 E-mail: cuibaodong85@163.com
陈永正,博士,教授,硕士生导师, E-mail: yzchen@zmc.edu.cn
O621.3; O626
: ADOI: 10.15952/j.cnki.cjsc.1005-1511.2017.09.17079
