胃腺癌组织中GDF-15和p53表达特征及其临床意义
2017-07-31王文军何雷张帆李佳嘉徐国祥徐五琴
王文军,何雷,张帆,李佳嘉,徐国祥,徐五琴
(皖南医学院弋矶山医院病理科,芜湖 241000)
胃腺癌组织中GDF-15和p53表达特征及其临床意义
王文军*,何雷,张帆,李佳嘉,徐国祥,徐五琴
(皖南医学院弋矶山医院病理科,芜湖 241000)
目的探讨胃腺癌及癌旁正常组织中生长分化因子15 (grow th dif erentiation factor -15, GDF-15)和p53表达的特征及临床意义。方法采用免疫组织化学Polink-1两步法检测91例胃癌手术标本,相应91例癌旁正常组织、64例肠化腺体及36例淋巴结转移癌组织中GDF-15和p53的表达特征,并结合临床资料分析其表达与临床病理参数的关系。结果GDF-15在肠上皮化生腺体、胃腺癌和淋巴结转移癌组织中的阳性表达率均显著低于癌旁组织。GDF-15的阳性表达率与患者年龄、浸润深度、淋巴结转移及pTNM分期呈正相关,而与其它临床病理参数无关。p53在癌旁正常胃粘膜腺体和肠上皮化生腺体中均不表达,在胃腺癌和淋巴结转移癌组织中的过表达阳性率均高于癌旁组织和肠上皮化生腺体。p53过表达的阳性率与患者年龄、Lauren分型(肠型与弥漫型)和分化程度呈正相关,而与其它临床病理参数无关。GDF-15与p53免疫反应性在胃癌组织中呈正相关。结论GDF-15可作为胃腺癌的胃壁浸润能力、淋巴结转移能力及临床病理分期的重要评价指标,而p53过表达可能与Lauren分型的肠型胃癌有关。尤其在老年患者中,GDF-15和p53过表达在胃腺癌的发生、进展中扮演重要角色,并起协同作用。
胃腺癌;生长分化因子-15;p53
胃癌在我国肿瘤的发病率及死亡率中均居第二位[1],是我国危害极大的恶性肿瘤之一,迄今为止尚缺乏特异性的肿瘤标志物。生长分化因子-15(grow th dif erentiation factor-15, GDF-15)作为一种应激反应蛋白在肿瘤发生的早期即被激活,是目前唯一所知的p53基因调节的分泌蛋白。野生型p53基因起抑癌作用,而p53突变或过表达可引起恶性肿瘤的发生及发展。目前,有关GDF-15表达与p53过表达在胃癌中的特征、关系及其意义的研究尚不多见。本文采用免疫组织Polink-1两步法检测GDF-15表达和p53过表达在人胃腺癌及相应癌旁组织中的表达特征,并结合临床资料分析其表达与临床病理参数间的关系。
材料和方法
1 材料
收集2013年12月至2014年5月间皖南医学院弋矶山医院病理科存档石蜡包埋组织,均为手术部分胃或全胃切除标本。实验组为91例胃腺癌组织,其中对应的91例癌旁组织(距离肿瘤边缘约5cm)、64例肠上皮化生腺体(癌旁不伴不典型增生或异型增生的慢性胃炎背景中的普通腺上皮肠上皮化生)及36例淋巴结转移癌组织作为对照组。所有病例均由两位高年资病理医师根据2010年第四版WHO消化系统胃肿瘤分类标准进行病理诊断,术前均未行任何放疗及化疗。
2 试剂
浓缩型兔抗人GDF-15多克隆抗体(ab14586)购自Abcam公司,即用型兔抗人p53单克隆抗体(EP95)、PV-6000免疫组织化学试剂盒、PBS缓冲液、枸橼酸缓冲液和DAB显色液等均购自北京中杉金桥生物技术有限公司。
3 免疫组织化学染色
上述存档蜡块组织均经10%中性福尔马林固定,常规脱水,石蜡包埋,4μm厚切片。采用Polink-1两步法免疫组织化学检测胃腺癌、淋巴结转移癌、肠上皮化生腺体组织及癌旁正常组织中的GDF-15免疫反应阳性表达和p53过表达特征。所用抗体采用pH6.0的枸橼酸钠缓冲液高压修复,DAB显色。以PBS代替一抗作空白对照,以已知阳性病例作阳性对照。操作严格按产品说明书进行。
4 结果判读
GDF-15免疫组织化学染色细胞膜和细胞质出现清晰淡黄色至棕褐色颗粒者为阳性表达细胞。GDF-15结果判定结合染色强度和阳性细胞数进行综合分级。两人双盲法分别观察切片,选择10个具有代表性的高倍视野(10×40倍),评分后取平均值。染色强度评分标准:不着色为0 分,淡黄色为1 分,黄褐色为2分,棕褐色为3 分。阳性细胞所占整张切片比例评分标准:阳性细胞数<5%为 0分,5%~25%为 1分,26%~50%为 2分,51%~75%为 3分,> 75 %为 4分。两种评分相乘,0分为阴性,≥2分为阳性;阳性率按阳性例数除以总例数计算。若两人观察结果相差3分则重新评定。p53免疫组织化学染色细胞核出现局灶清晰淡黄色,为正常表达,判为阴性;若呈弥漫棕褐色核染色且细胞数≥10%为过表达,判为阳性。
5 统计学分析
应用SPSS17.0统计软件对实验数据进行处理,两两组间比较采用χ2Fisher精确检验 ,采用Pearson进行相关性分析,P<0.05为差异有统计学意义。
结 果
1 胃腺癌患者临床病理资料
91例胃腺癌中,男性65例,女性26例;年龄31岁~81岁,中位年龄63岁。瘤体位于贲门胃底23例、胃体7例、胃窦胃角59例、幽门2例;瘤体最大径>5cm的17例、≤5cm的74例;高中分化腺癌51例、低分化腺癌40例;Lauren分型肠型54例、弥漫型26例、混合型11例;有神经侵犯的34例;有脉管侵犯的15例;浸润深度为粘膜层到粘膜下层的(T1) 45例、浸润至肌层的(T2)10例、浸润至浆膜下层的(T3)10例、浸润穿过浆膜的(T4)26例;有淋巴结转移的36例,有远处转移的2例;pTNM分期,Ⅰ期47例、Ⅱ期16例、Ⅲ期26例、Ⅳ期2例。
2 GDF-15和p53在各组织中的免疫反应阳性表达特征
GDF-15在癌旁正常胃粘膜表面粘液上皮及固有层腺体(图1A)均可呈免疫反应阳性,阳性表达率为75%。其在肠上皮化生腺体(图1B)、胃腺癌(图1C)和淋巴结转移癌(图1D)组织中的阳性表达率分别为53%、48%、31%,均低于癌旁正常组织的75%,且均有显著统计学差异(P<0.01)(表1)。GDF-15阳性表达率在肠上皮化生腺体组高于淋巴结转移癌组(0.01<P<0.05);肠上皮化生腺体组与胃腺癌组、胃腺癌组与淋巴结转移癌组阳性表达率均无统计学差异(P>0.05)。p53在癌旁胃粘膜腺体和肠上皮化生腺体中均为阴性表达。在胃腺癌(图1E)和淋巴结转移癌(图1F)组织中均为过表达,阳性表达率均为33%,均高于癌旁组织和肠上皮化生腺体(P<0.01)(表1)。胃腺癌组与淋巴结转移癌组p53阳性表达率无统计学差异(P>0.05)。
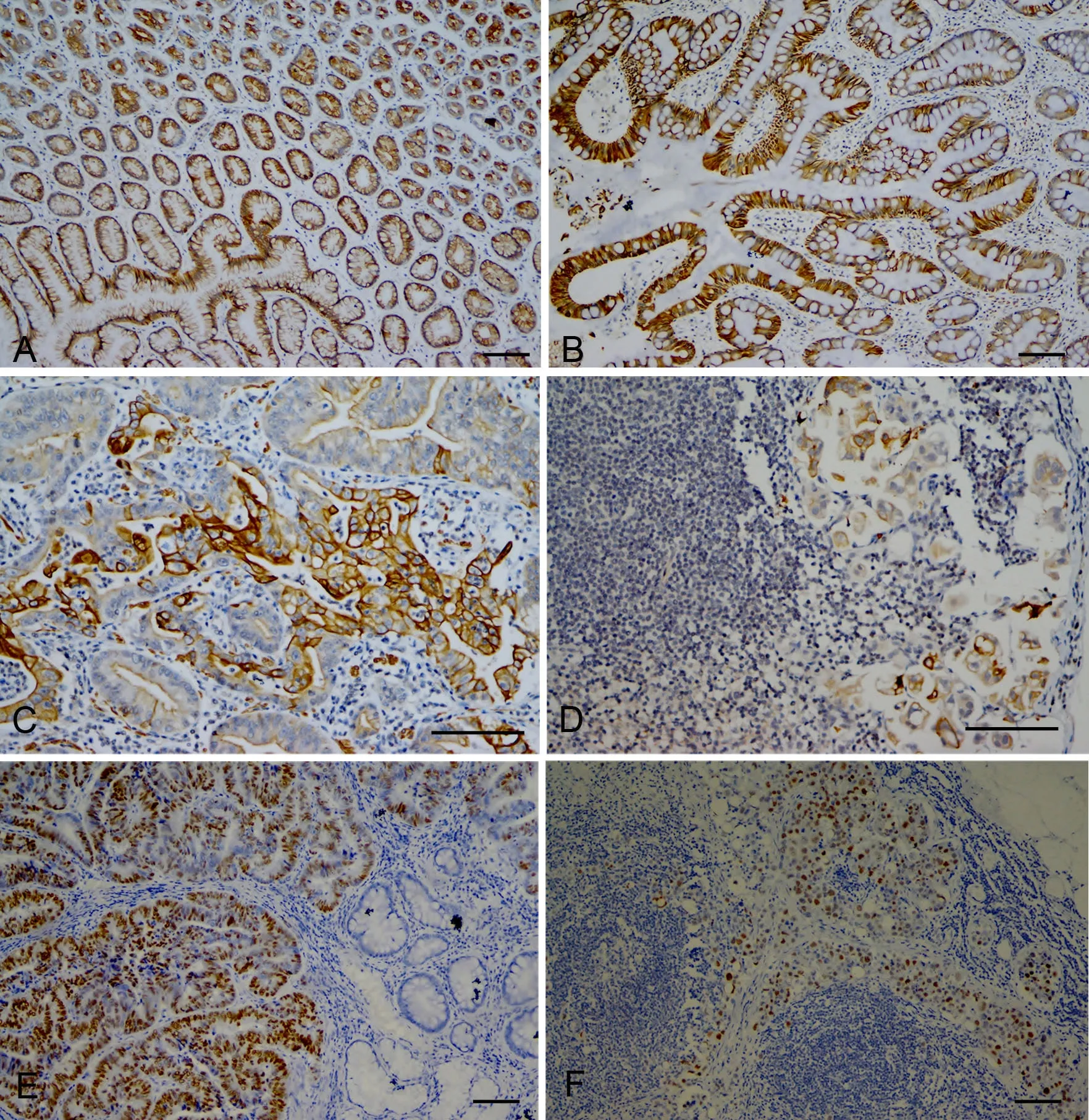
图1 胃正常粘膜、肠上皮化生腺体、腺癌及淋巴结转移癌的GDF-15和p53免疫组织化学表达特征。A, GDF-15在正常胃粘膜腺体组织中阳性表达;B, GDF-15在胃肠上皮化生腺体组织中阳性表达;C, GDF-15在胃腺癌组织中阳性表达;D, GDF-15在胃淋巴结转移癌组织中阳性表达;E, p53在胃腺癌中的过表达,其邻近正常组织及肠上皮化生腺体均不表达;F, p53在胃淋巴结转移癌组织中过表达;比例尺,100μmFig. 1 Immunohistochem ical features of normal gastric tissue, intestinal metaplasia, adenocarcinoma and lymph node metastasis adenocarcinoma tissue. A, positive expression of GDF-15 in normal gastric tissue; B, positive expression of GDF-15 in gastric intestinal metaplasia tissue; C, positive expression of GDF-15 in gastric adenocarcinoma tissue; D, positive expression of GDF-15 in gastric lymph node metastasis adenocarcinoma tissue; E, p53 overexpression in gastric adenocarcinoma tissue, and no expression in adjacent normal tissue and intestinal metaplasia tissue; F, p53 overexpression in gastric lymph node metastasis adenocarcinoma tissue; scale bar, 100μm
3 GDF-15和p53免疫反应性与胃癌临床病理参数的关系
GDF-15的阳性表达率与患者年龄、浸润深度、淋巴结转移及pTNM分期呈正相关,即GDF-15阳性表达率在年龄>60岁、浸润深度超过粘膜下层、有淋巴结转移和pTNM分期(Ⅲ+Ⅳ)组高于年龄≤60岁、浸润深度不超过粘膜下层、无淋巴结转移和pTNM分期(Ⅰ+Ⅱ)组(P<0.05),而与其它临床病理参数如性别、肿瘤大小、部位、分化程度、Lauren分型、神经侵犯及脉管侵犯均无关(P>0.05)(表2)。p53过表达的阳性率与患者年龄、Lauren分型(肠型与弥漫型)及分化程度呈正相关(P<0.05),即p53过表达阳性率在年龄>60岁、肠型胃癌、高中分化胃癌组高于年龄≤60岁、弥漫型胃癌和低分化胃癌组(P<0.05),而与其它临床病理参数如患者性别、肿瘤部位、肿瘤大小、神经侵犯、脉管侵犯、浸润深度、淋巴结转移及TNM分期均无关(P>0.05)(表2)。
4 胃癌组织中GDF-15与p53免疫反应性呈正相关
91例胃腺癌组织中GDF-15与p53免疫反应共同阳性20例,共同阴性37例;44例GDF-15表达阳性中p53阴性24例,47例GDF-15表达阴性中p53过表达10例。经Pearson相关分析显示,GDF-15表达和p53过表达呈正相关(表3)。

表1 胃腺癌组织GDF-15和p53免疫性表达特征Tab. 1 Characteristics of GDF-15 expression and p53 overexpression in gastric adenocarcinoma
讨 论
生长分化因子-15(GDF-15),又名M IC-1、PTGFB、NAG-1、PLAB、PDF,属转化生长因子β(transform ing grow th factor-beta, TGF-β)超家族成员之一[2],在大鼠神经元发育和小脑颗粒细胞成熟[3]、食物的摄入和体重调节[4]、能量代谢调节以及延长哺乳动物寿命[5]等过程中发挥重要作用。此外,GDF-15在前列腺癌[6]、结直肠癌[7]、卵巢癌[8]、胰腺癌[9]、胶质瘤[10]和子宫内膜癌[11]等多种肿瘤组织及体液中均有表达,其在调节肿瘤细胞增殖、分化、凋亡、侵袭和转移中起着双重作用,究竟是抑癌还是促癌,目前仍有争议。
GDF-15在胃癌中的研究报道较少,其作用尚有争议。本研究结果与Park JY[12]相似,显示GDF-15在正常胃组织中有表达,而在肠上皮化生腺体、胃癌及淋巴结转移癌组织中表达逐渐下降,提示GDF-15在胃癌发生、发展的过程中抑癌作用逐渐减弱,导致胃癌的发生及进展。而随着患者年龄、浸润深度、淋巴结转移及pTNM分期的进展,GDF-15阳性表达率又呈升高趋势,提示GDF-15在进展期胃癌中促癌作用逐渐增强,导致老年患者胃癌的进一步侵袭和转移。据此,GDF-15具有双重作用,即在胃癌早期发挥抑癌作用,而在胃癌进展期发挥促癌作用,与Husaini 等[13]在前列腺癌研究中的结论类似。其可能机制为:①GDF-15启动子甲基化水平在肿瘤发生早期较高,导致其蛋白表达下降,抑癌作用减弱,而肿瘤晚期启动子甲基化水平下降,导致其蛋白表达升高,促癌作用增强[14]。②钙结合蛋白calumenin 15与GDF-15启动子区域结合导致后者转录增强,诱发细胞伪足形成,促进肿瘤细胞迁移[15]。③肿瘤微环境中肿瘤相关纤维母细胞源性的GDF-15表达升高,促进肿瘤的生长、侵袭和转移[16]。④ GDF -15通过抑制树突细胞的成熟及作用,抑制肿瘤特异性的免疫反应,导致肿瘤细胞发生免疫逃逸[17]。

表2 胃腺癌组织中GDF-15和p53表达与临床病理参数之间的关系Tab. 2 The relationship of GDF-15 expression and p53 overexpression w ith clinicopathological characteristics in gastric adenocarcinoma

表3 胃腺癌组织中GDF-15与p53免疫反应性的相关性Tab. 3 Correlation between GDF-15 expression and p53 overexpression in gastric adenocarcinom a
野生型p53是细胞生长的负调节因子,能够监视细胞基因组的完整性、修复各种因素导致的DNA损伤以及清除各种有癌变倾向的细胞等,起抑癌作用。突变p53失去对细胞生长、凋亡、DNA修复等的调控作用,引起细胞的转化和癌变。野生型p53的半衰期约15m in,且极不稳定,常规免组织化学方法为不着色或仅为局灶细胞核淡黄色染色,突变型p53蛋白稳定性增加,半衰期延长,常表现为弥漫的细胞核强阳性染色,故目前免疫组织化学检测出的弥漫强阳性表达模式为p53蛋白过表达。本研究中p53在癌旁正常胃粘膜腺体和肠上皮化生腺体中均不显色为阴性,只在胃腺癌和淋巴结转移癌中呈强阳性表达。Kawata 等[18]的研究显示,p53在胃良性腺瘤中无表达,而在早期胃癌中呈升高趋势,且与胃癌粘膜下浸润有关,提示正常基因状态即野生型p53作为抑癌基因有利于防止胃癌的发生,一旦p53突变而过表达则导致胃癌的发生及进展。p53过表达的阳性率与患者年龄、Lauren分型(肠型与弥漫型)及分化程度有关,提示p53过表达与老年患者、高中分化胃癌及肠型胃癌的发生、发展有关。这与Waraya 等[19]的研究结论中p53突变过表达胃癌的临床病理特征相似。
GDF-15的启动子区有两个p53的结合位点,包括RE1和RE2,其中RE2最为主要且与p53激活GDF-15最为相关[20]。p53对于GDF-15属于一过性的、短暂的转录调控,有利于在生理及特定条件下的转录调节[21]。 GDF-15表达与p53过表达呈正相关,提示GDF15与野生型p53共同发挥抑癌作用,及时发现受损基因,促使S期停滞[22],防止胃癌的发生进展。而当胃癌进展后,尤其在老年患者,p53过表达与GDF-15共同促进了胃癌的进一步侵袭和转移。其机制之一可能与GDF-15通过介导p53泛素化抑制p53功能,导致肿瘤血管生成有关[23]。
综上所述,GDF-15可作为胃癌胃壁浸润能力、淋巴结转移能力及临床病理分期的重要评价指标,而p53过表达可能与Lauren分型肠型胃癌有关。尤其在老年患者[24-25]中,GDF-15表达和p53过表达在胃癌的发生、进展中扮演重要角色,并起协同作用。而GDF-15在胃腺上皮不同程度上皮内瘤变中的表达特征,其在不同肠上皮化生类型中的表达差异及与相应胃癌的关系和作用机制,其和非p53依赖的信号通路及与胃癌的关系有待进一步研究。
[1] ChenWQ, Zheng RS, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin, 2016, 66(2): 115-132.
[2] Böttner M, Suter-Crazzolara C, Schober A, et al. Expression of a novel member of the TGF-beta superfamily, grow th/ dif erentiation factor-15/macrophage-inhibiting cytokine-1 (GDF-15/M IC- 1) in adult rat tissues. Cell Tissue Res, 1999, 297(1): 103-110.
[3] Wang CY, Huang AQ, Zhou MH, et al. GDF15 regulates Kv2.1-mediated outward K+ current through the Akt/ mTOR signalling pathway in rat cerebellar granule cells. Biochem J, 2014, 460 (1): 35-47.
[4] Tsai VW, Manandhar R, Jørgensen SB, et al. The anorectic actions of the TGFβ cytokine M IC-1/GDF15 require an intact brainstem area postrema and nucleus of the solitary tract. PLOS One, 2014, 9(6): 1-10.
[5] Wang X, Chrysovergis K, Kosak J, et al. hNAG-1 increases lifespan by regulating energy metabolism and insulin/ IGF-1/mTOR signaling. Aging, 2014, 6(8): 690-704.
[6] Nakamura T, Scorilas A, Stephan C, et al. Quantitative analysis of macrophage inhibitory cytokine-1(M IC-1) gene expression in human prostatic tissues. Br J Cancer, 2003, 88(7): 1101-1104.
[7] Wallin U, Glimelius B, Jirström K, et al. Grow th dif erentiation factor 15: a prognostic marker for recurrence in colorectal cancer. Br J Cancer, 2011, 104(10): 1619-1627.
[8] Bock AJ, Stavnes HT, Kempf T, et al. Expression and clinical role of grow th dif erentiation factor-15 in ovarian carcinoma ef usions. Int J Gynecol Cancer, 2010, 20(9): 1448-1455.
[9] Wang X, Li Y, Tian H, et al. Macrophage inhibitory cytokine 1 (M IC-1/GDF15) as a novel diagnostic serum biomarker in pancreatic ductal adenocarcinoma. BMC Cancer, 2014, 14(578): 1-11.
[10] Roth P, Junker M, Tritschler I, et al. GDF-15 contributes to proliferation and immune escape of malignant gliomas. Clin Cancer Res, 2010, 16(15): 3851-3859.
[11] Staff AC, Trovik J, Eriksson AG, et al. Elevated plasma grow th dif erentiation factor-15 correlates w ith lymph node metastases and poor survival in endometrial cancer. Clin Cancer Res, 2011, 17(14): 4825-4833.
[12] Park JY, Park KH, Bang S, et al. Expression of nonsteroidal anti-infammatory drug-activated gene-1(NAG-1) inversely correlates w ith tumor progression in gastric adenomas and carcinomas. J Cancer Res Clin Oncol, 2008, 134(9): 1029-1035.
[13] Husaini Y, Lockwood GP, Nguyen TV, et al. Macrophage inhibitory cytokine-1 (M IC-1 / GDF15) gene deletion promotes cancer grow th in TRAMP prostate cancer prone m ice. PLOS One, 2015, 10(2): 1-12.
[14] Costa VL, Henrique R, Danielsen SA, et al. Three epigenetic biomarkers, GDF15, TMEFF2, and VIM, accurately predict bladder cancer from DNA-based analyses of urine samples. Clin Cancer Res, 2010, 16 (23): 5842-5851.
[15] Feng H, Chen L, Wang Q, et al. Calumenin-15 facilitates filopodia formation by promoting TGF-β superfam ily cytokine GDF-15 transcription. Cell Death Dis, 2013, 4(10): 1-10.
[16] Bruzzese F, Hägglöf C, Leone A, et al. Local and systemic protumorigenic ef ects of cancer-associated fbroblast-derived GDF15. Cancer Res, 2014, 74(13): 3408-3417.
[17] Zhou Z, Li W, Song Y, et al. Grow th dif erentiation factor-15 suppresses maturation and function of dendritic cells and inhibits tumor-specif c immune response. PLOS One, 2013, 8(11): 1-13.
[18] Kawata S, Yashima K, Yamamoto S, et al. AID, p53 and MLH1 expression in early gastric neoplasms and the correlation w ith the background mucosa. Oncol Lett, 2015, 10 (2): 737-743.
[19] Waraya M, Yamashita K, Ema A, et al. Exclusive association of p53 mutation w ith super-high methylation of tumor suppressor genes in the p53 pathway in a unique gastric cancer phenotype. PLOS One, 2015, 10(10): 1-17.
[20] Osada M, Park HL, Park MJ, et al. A p53-type response element in the GDF15 promoter confers high specif city for p53 activation. Biochem Biophys Res Commun, 2007, 354 (4): 913-918.
[21] Meanson BD, Bose R, Hamill JD, et al. The role of mRNA decay in p53-induced gene expression. RNA, 2011, 17(12): 2222-2234.
[22] Agarwal MK, Hastak K, Jackson MW, et al. Macrophage inhibitory cytokine 1 mediates a p53-dependent protective arrest in S phase in response to starvation for DNA precursors. Proc Natl Acad Sci USA, 2006, 103(44): 16278-16283.
[23] Song H, Yin D, Liu Z. GDF-15 promotes angiogenesis through modulating p53/HIF-1α signaling pathway in hypoxic human umbilical vein endothelial cells. Mol Biol Rep, 2012, 39(4): 4017-4022.
[24] Skipworth RJ, Deans DA, Tan BH, et al. Plasma M IC-1 correlates w ith systemic infammation but is not an independent determ inant of nutritional status or survival in oesophago-gastric cancer. Br J Cancer, 2010, 102(4): 665-672.
[25] Seo JY, Jin EH, Jo HJ, et al. Clinicopathologic and molecular features associated w ith patient age in gastric cancer. World J Gastroenterol, 2015, 21(22): 6905-6913.
The characteristics and clinical signif cance of GDF-15 expression and p53 overexpression in gastric cancer
Wang Wenjun*, He Lei, Zhang Fan, Li Jiajia, Xu Guoxiang, Xu Wuqin
(Department of Pathology,Yijishan Hospital of Wannan Medical College,Wuhu 241000,China)
ObjectiveTo investigate the characteristics and clinical signif cance of the expression of grow th dif erentiation factor 15 (GDF-15) and the overexpression of p53 in gastric adenocarcinoma and adjacent normal tissue.M ethodsThe expression of GDF-15 and p53 were detected by Polink-1 two-step immunohistochem ical method in 91 samples from gastric adenocarcinoma and adjacent normal tissue, also in 64 samples w ith intestinal metaplasia and 36 lymph node tissue w ith metastasis adenocarcinoma. The relationship between the expression of these proteins and clinicopathologic features was analyzed.ResultsThe positive rate of GDF-15 expression was obviously lower in intestinal metaplasia tissue, gastric adenocarcinoma tissue and lymph node metastasis adenocarcinoma tissue than that in normal gastric tissue. GDF-15 expression was positively correlated w ith age, depth of tumor invasion, lymph node metastasis and pTNM staging, while not related to other clinicopathologic features. There was no p53 expression in normal gastric tissue and intestinal metaplasia tissue. The positive rate of p53 overexpression was signif cantly higher in gastric adenocarcinoma tissue and lymph node metastasis adenocarcinoma tissue than that in normal gastric tissue and intestinal metaplasia tissue. P53 overexpression was positively correlated w ith age, Lauren’s type ( intestinal pattern and dif used pattern) and dif erentiation degree, while not related to other clinicopathologic features. GDF-15 expression and p53 overexpression were positively correlated in gastric cancer tissue.ConclusionGDF-15 is a potential index for assessing the ability of gastric wall invasion, lymph node metastasis and pTNM staging of gastric adenocarcinoma. P53 overexpression may be related to the intestinal pattern of Lauren’s type of gastric adenocarcinoma. GDF-15 expression and p53 overexpression cooperatively play an important role in the occurrence and development of gastric cancer, especially among elder patients.
Gastric adenocarcinoma; GDF-15; p53
R735.2; R365
ADOI:10.16705/ j. cnki. 1004-1850.2017.03.010
2017-02-09
2017-06-05
安徽省高校自然科学研究项目(KJ2011Z394)
王文军,男(1976年),汉族,副主任医师,副教授
*通讯作者(To whom correspondence should be addressed):dyww j5@163.com
