LKB1抑制人巨细胞肺癌细胞的增殖和迁移侵袭、m TOR磷酸化与VEGF和MM P9表达
2017-07-31陈逸轩刘燕娄丽丽时妍梅陈文莉陈新年
陈逸轩,刘燕,娄丽丽,时妍梅,陈文莉,陈新年*
(兰州大学1第一临床医学院,2基础医学院病理生理研究所,兰州 730000)
LKB1抑制人巨细胞肺癌细胞的增殖和迁移侵袭、m TOR磷酸化与VEGF和MM P9表达
陈逸轩1Δ,刘燕2Δ,娄丽丽1,时妍梅2,陈文莉2,陈新年2*
(兰州大学1第一临床医学院,2基础医学院病理生理研究所,兰州 730000)
目的分析肝激酶B1(liver kinase B1, LKB1)对人巨细胞肺癌(the people giant cell lung cancer,PGCL3)细胞增殖、迁移、侵袭的影响及其机制。方法构建真核表达载体pCMV-LKB1,转染人巨细胞肺癌细胞。转染后48h,用免疫印迹法检测各组细胞LKB1蛋白表达水平,明确转染PGCL3效率。转染后每隔12h、连续72h检测LKB1对细胞增殖能力的影响;Transwell小室检测转染后各组细胞迁移、侵袭力。提取各组细胞蛋白检测基质金属蛋白酶2(matrix metalloproteinase 2,MMP2)、基质金属蛋白酶9(matrix metalloproteinase 9,MMP9)、雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)和血管内皮生长因子(vascular endothelial grow th factor, VEGF)的表达水平。结果pCMV-LKB1经PCR、双酶切及DNA测序鉴定后,证实目的基因片段插入方向正确,核酸序列与NCBI公布的LKB1的核酸序列一致;免疫印迹分析显示,转染pCMV-LKB1的PGCL3细胞中LKB1表达水平明显增高,表明pCMV-LKB1构建成功。增殖曲线分析和5-乙炔基-2,脱氧嘧啶核苷(5-ethynyl-2,-deoxyuridine,EdU)检测显示,转染pCMV-LKB1能显著抑制PGCL3细胞的增殖;Transwell小室检测显示,过表达LKB1可显著抑制PGCL3细胞的迁移与侵袭;免疫印迹分析显示,过表达LKB1的PGCL3细胞其LKB1下游分子中总mTOR表达量不变,磷酸化mTOR(p-mTOR)水平降低,mTOR下游分子VEGF表达量也显著降低,迁移侵袭相关蛋白MMP2表达无变化,但MMP9水平显著降低。结论LKB1可能通过下调PGCL3细胞p-m TOR水平,降低VEGF表达,从而抑制巨细胞肺癌细胞增殖,并可能通过下调金属基质蛋白酶MMP9的表达,降低巨细胞肺癌细胞迁移侵袭能力。
肝激酶B1;增殖;迁移;侵袭;基质金属蛋白酶;雷帕霉素靶蛋白;血管内皮生长因子
人巨细胞肺癌因具极高的转移性而成为一种特殊类型的肺癌,被发现时往往已出现转移而不能施行常规的手术治疗,预后较差[1],因此如何有效控制其转移是治疗人巨细胞肺癌的关键。人巨细胞肺癌细胞株(PGCL3)具有很强的远处转移能力[2]。肝激酶B1 (liver kinase B1, LKB1) 即丝氨酸-苏氨酸激酶11(serine/threonine kinase, STK11)是一个抑癌基因,于1998年由德国科学家在Peutz-Jeghers综合征(PJ综合征)患者体内发现[3],它主要通过调节mTOR的活性来控制细胞增殖和生物能量代谢。已有研究表明,LKB1的失活与多种恶性肿瘤的发生、发展有关,表现为使细胞的迁移侵袭力增加[4-7],但对人巨细胞肺癌的作用如何尚未见报道。本文以LKB1为靶点,人高转移巨细胞肺癌细胞株(PGCL3)为研究对象,探究 LKB1对人巨细胞肺癌增殖、迁移、侵袭的影响,并对其机制进行探讨。
材料与方法
1 pCMV-LKB1真核表达载体的构建
根据基因文库中LKB1登录号(NM_000455.4)设计LKB1引物: gactgacgtgtagaacaatcg, gaaccggcaggaagactgag。引物由上海捷瑞生物工程有限公司合成。按照Trizol试剂盒(Invitrogen公司)说明书要求提取人胚肾293-T细胞的总RNA,经反转录获得cDNA,以此cDNA为模板进行PCR扩增:Prem ix Taq12.5μl、上下游引物各1μl、模板2μl、加ddH2O至25μl的反应体系。反应条件为94℃5 m in,94℃30 S,61℃ 30 S,72℃ 2m in,共30个循环,72℃延伸10 m in,电泳后可见目的条带,胶回收目的基因LKB1片段。将回收的LKB1片段与克隆载体pMD-18T(大连宝生物工程有限公司)连接,构建克隆载体pMD-18T-LKB1。用限制性内切酶SalI、EcoR I(大连宝生物工程有限公司)对克隆载体pMD-18TLKB1和真核表达载体pCMV-Blank(天根生物科技有限公司)进行双酶切,将酶切产物用T4 DNA连接试剂盒(大连宝生物工程有限公司)构建真核表达载体pCMV-LKB1。将构建的pCMV-LKB1进行PCR、双酶切鉴定,并对DNA测序。
2 细胞培养及转染
PGCL3细胞株由兰州大学病理生理学研究所保存,用含10%胎牛血清的RPM I-1640培养在37℃、5%CO2的细胞培养箱内培养,每2天换液一次。当细胞生长至90%的汇合度时用0.25%的胰酶消化,传代至新的25cm2的培养瓶中。当细胞生长处于对数期且汇合度达到80%时将pCMV-Blank、pCMV-LKB1分别与转染试剂Lipofect以2:1的比例混合转染PGCL3细胞。
3 细胞增殖检测
转染后24h,将各组细胞以2×104cells/孔种于24孔板培养,每隔12h,用胰酶消化细胞,用细胞计数板计数每孔中的细胞总数,连续计数6次,绘制细胞增殖曲线,观察各组细胞增殖情况。另取各组对数生长期细胞,以每孔2×103细胞接种于96孔板中培养24h,按照EdU试剂盒(广州瑞博)说明书提供的方法将细胞用EdU孵育2h,4%的多聚甲醛室温固定30m in,后用Apollo染细胞,再用Hoechst33342反应液染细胞核,于荧光显微镜下观测拍照。每个孔内随机选取5个区域记录,使用Image J软件将同一视野下的图片叠加,计算增殖细胞比例。绿色荧光细胞占总细胞数的百分比代表细胞增殖能力。
4 Transwell小室观察各组细胞迁移、侵袭能力
迁移实验:转染24h后,将各组细胞消化,以5×104个/孔接种至Transwell小室(Corning,美国)上室,下室加入600μl含10%FBS的RPM I-1640培养液。培养24h后取出小室,PBS冲洗2次,用棉签小心拭去上室细胞,甲醇固定10m in,风干后用0.1%的结晶紫染色15m in,PBS冲洗2次,晾干后在倒置显微镜下(20倍物镜)随机挑选5个视野拍照并计数,以穿过Transwell小室膜的细胞数表示迁移力。
侵袭实验:接种细胞前将Matrigel基质胶(BD,美国)用RPM I-1640培养液按1:8比例稀释,100μl均匀加入小室,置入37℃培养箱1h,使胶变为固态,用温热的RPM I-1640培养液清洗1次,接种细胞,其余步骤与迁移实验一样,以穿过Transwell小室基质胶及膜的细胞数表示侵袭力。
5 Western blot检测LKB1及相关蛋白表达水平
转染后48h,收集分别转染pCMV-Blank和pCMV-LKB1的PGCL3细胞及未转染的PGCL3细胞提取蛋白进行SDS-PAGE凝胶电泳、转膜,用5%的脱脂奶粉封闭1h,分别加入1:1000稀释的一抗(兔抗人LKB1、m TOR、p-m TOR、VEGF、MMP2、MMP9,Immnoway,美国),4℃过夜。次日,三乙醇胺缓冲溶液(Tris buf ered saline Tween-20,TBST)洗膜3次,加入1:5000稀释的辣根过氧化物酶标记的IgG(Immnoway),室温摇动孵育2h,再用TBST洗膜3次,在暗室加入ECL发光液后进行显影、定影,扫描胶片后保存图像。应用Image J软件测定各蛋白条带光密度,并用Oringin软件分析各蛋白的相对水平。
结 果
1 重组真核表达载体pCMV-LKB1鉴定
将重组真核表达载体pCMV-LKB1用PCR和双酶切鉴定,结果显示PCR扩增片段与双酶切条带大小相符,约1560bp,与设计的LKB1目的基因大小一致(图1)。将pCMV-LKB1送样测序,显示插入片段与NCBI公布的LKB1序列完全一致,且片段插入方向正确,表明真核表达载体pCMV-LKB1构建成功。
Western blot检测显示,将pCMV-LKB1转染PGCL3细胞后,PGCL3细胞内LKB1水平显著高于转染pCMV-LKB1和未转染的PGCL3细胞,表明pCMV-LKB1能在PGCL3细胞内有效过表达LKB1(图2)。
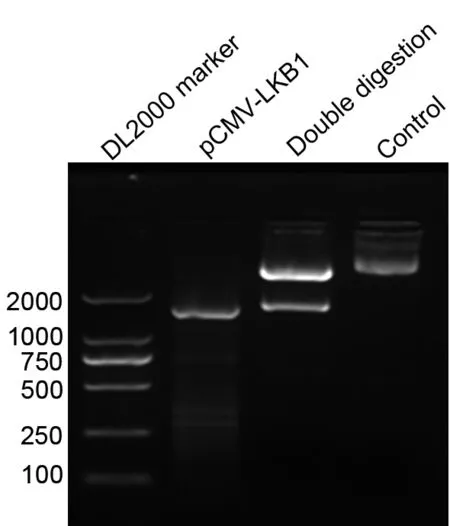
图1 重组质粒pCMV-LKB1 PCR及双酶切鉴定Fig.1 Identif cation of the recombinant plasm id pCMV-LKB1 by PCR amplif cation and double digestion

图2 重组质粒pCMV-LKB1 的Western blot鉴定。A,转染pCMV-LKB1后的PGCL3细胞中LKB1蛋白表达的Western blot检测;B, 统计学分析;**,P<0.01Fig. 2 Identif cation of the recombinant plasm id pCMV-LKB1 by Western blot. A, Western blot detection of LKB1 expression in PGCL3 cells transfected w ith pCMV-LKB1; B, statistical analysis; **, P<0.01
2 过表达LKB1抑制PGCL3细胞增殖
转染24h后,每12h计数各组细胞数,绘制分析增殖曲线,结果显示在连续72h检测中,转染过表达LKB1的PGCL3细胞增殖能力较未转染的PGCL3细胞和转染对照质粒的PGCL3细胞明显下降(图3)。
EdU实验结果显示,PGCL3组细胞增殖率为(61.7±5.6)%、PGCL3-NC组细胞增殖率为(59.3±6.6)%、PGCL3-LKB1组细胞增殖率为(29.1±5.7)%,与前两组相比,转染LKB1组的细胞增殖能力受到显著抑制(图4)。
3 过表达LKB1抑制PGCL3细胞迁移和侵袭
转染24h后用Transwell小室检测各组细胞迁移能力和侵袭力,结果显示,与转染对照质粒和未转染的PGCL3细胞相比,PGCL3-LKB1组细胞迁移力和侵袭力均显著下降(图5,图6)
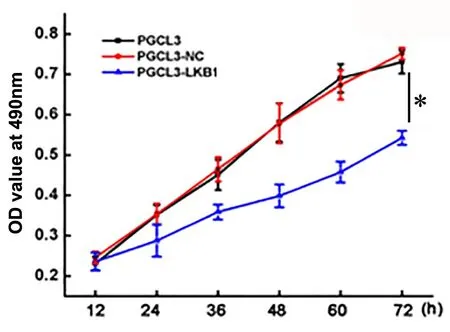
图3 LKB1对PGCL3细胞增殖力影响的增殖曲线分析。*,与PGCL3和PGCL3-NC细胞组比较,P<0.01Fig. 3 Grow th curve analysis of the ef ect of LKB1 on the proliferation of PGCL3 cells. *, P<0.01, compared w ith PGCL3 and PGCL3-NC cells

图4 EdU实验检测LKB1对PGCL3 细胞增殖的影响。比例尺,50μmFig. 4 The ef ect of LKB1 on the proliferation of PGCL3 cells detected by EdU assay. Scale bar, 50μm
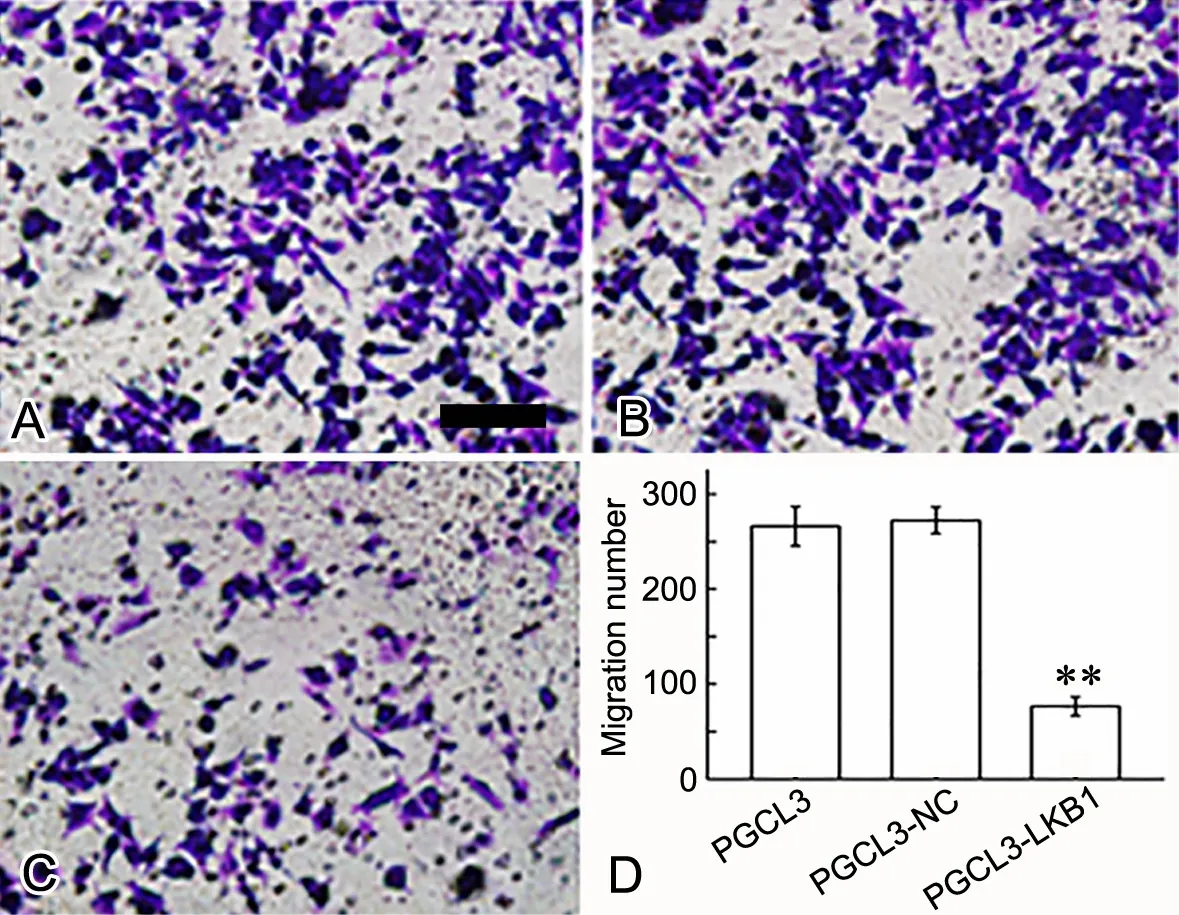
图 5 LKB1对PGCL3细胞迁移力的影响。A,PGCL3;B,PGCL3-NC;C,PGCL3-LKB1;A-C,转染过表达LKB1对PGCL3细胞迁移影响的Transwell法检测;D,PGCL3细胞迁移力统计学分析;**,与PGCL3和PGCL3-NC细胞组比较,P<0.01;比例尺,50μmFig. 5 The ef ect of LKB1 on the migration of PGCL3 cells. A, PGCL3; B, PGCL3-NC; C, PGCL3-LKB1; A to C, Transwell detection of the ef ect of LKB1 overexpression on the m igration of PGCL3 cells; D, statistical analysis of the m igration number of PGCL3 cells; **, P<0.01, compared w ith PGCL3 and PGCL3-NC cells; scale bar, 50μm
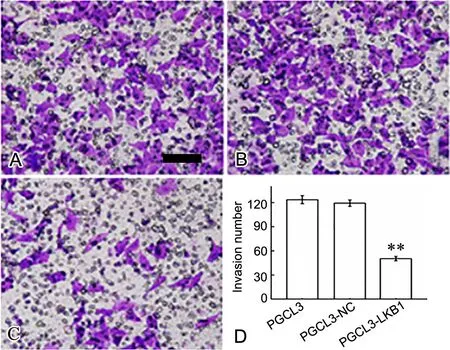
图 6 LKB1对PGCL3细胞侵袭力的影响,A,PGCL3;B,PGCL3-NC;C,PGCL3-LKB1;A-C,转染过表达LKB1对PGCL3细胞侵袭的Transwell法检测;D,PGCL3细胞侵袭力统计学分析;**,与PGCL3和PGCL3-NC细胞比较,P<0.01;比例尺,50μmFig. 6 The ef ect of LKB1 on the invasion of PGCL3 cells. A, PGCL3; B, PGCL3-NC; C, PGCL3-LKB1; A to C, Transwell detection of the ef ect of LKB1 overexpression on the invasion of PGCL3 cells; D, statistical analysis of the invasion number of PGCL3 cells; **, P<0.01, compared w ith PGCL3 and PGCL3-NC cells; scale bar, 50μm
4 过表达LKB1降低PGCL3细胞m TOR磷酸化水平和VEGF表达
为了探究LKB1抑制PGCL3细胞增殖能力的机制,应用Western blot检测过表达LKB1对LKB1下游与细胞增殖、代谢等过程密切相关的m TOR信号通路相关分子的影响。结果显示,过表达LKB1不改变总m TOR水平,但明显降低p-m TOR及VEGF水平(图7),提示LKB1可通过下调m TOR信号通路进而抑制PGCL3细胞的增殖能力。
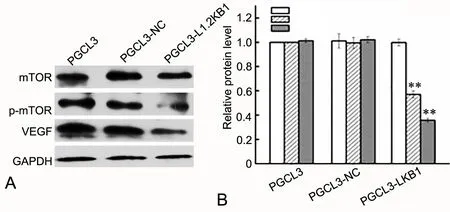
图7 过表达LKB1对PGCL3细胞增殖相关蛋白的影响。A,增殖相关蛋白的Western blot检测;B,增殖相关蛋白水平的统计学分析;**,与PGCL3和PGCL3-NC细胞比较,P<0.01Fig. 7 The ef ect of LKB1 overexpression on proliferation-related proteins in PGCL3 cells. A, Western blot detection of the levels of m TOR, p-mTOR and VEGF; B, statistical analysis of the levels of mTOR, p-mTOR and VEGF; **, P<0.01, compared w ith PGCL3 and PGCL3-NC cells
5 过表达LKB1下调PGCL3细胞MMP9水平
为了探讨LKB1抑制PGCL3迁移、侵袭的机制,应用Western blot检测过表达LKB1对PGCL3细胞中MMP2及MMP9的影响。结果显示,与PGCL3组和PGCL3-NC组比较,PGCL3-LKB1细胞中MMP2水平无明显变化,而MMP9水平显著下降(图8),提示LKB1可能通过下调MMP9抑制PGCL3细胞的迁移和侵袭。

图8 过表达LKB1对PGCL3细胞MMP2和MMP9水平的影响。A,MMP2和MMP9的Western blot检测;B,MMP2和MMP9水平的统计学分析;**,与PGCL3和PGCL3-NC细胞比较,P<0.01Fig. 8 The ef ect of LKB1 overexpression on the levels of MMP2 and MMP9 in PGCL3 cells. A, Western blot detection of MMP2 and MMP9; B, statistical analysis of the levels of MMP and MMP9; **, P<0.01, compared w ith PGCL3 and PGCL3-NC cells
讨 论
肿瘤的浸润和远处转移是恶性肿瘤的主要特征。LKB1是腺苷酸活化蛋白激酶(AMPK)的上游基因。有学者通过小鼠体内实验研究表明,LKB1可通过对腺苷酸活化蛋白激酶的磷酸化,而使AMPK转化成p-AMPK,从而使AMPK激活进而负向调节mTOR的活性[8,9,10]。m TOR是调节细胞代谢和生长的非常关键的生物大分子,由两个分管功能和生物合成的复合物组成[11]。活化的m TOR,即p-m TOR可上调多种促进细胞生长和增殖的关键蛋白,如细胞周期蛋白D1(cyclinD1)、缺氧诱导因子1a(hypoxia inducible factor 1a、HIF-1a)、癌基因c-myc、视网膜母细胞瘤易感蛋白Rb蛋白、血管内皮细胞生长因子VEGF等[11-14]。这些下游因子调控细胞周期进程、细胞生长、血管的生成等[13]。在肿瘤形成过程中,LKB1的表达降低或缺失,p-AMPK对m TOR的负性调节消失,这些因子表达增强,使细胞周期加速,细胞增殖能力增强,血管新生加快而最终加速肿瘤的形成及远处转移[14]。本研究结果显示,过表达LKB1不改变PGCL3细胞过表达LKB1使PGCL3细胞增殖能力减弱,并使其迁移侵袭力显著下降,提示高水平的LKB1可通过LKB1+/p-m TOR-/VEGF-调控使p-m TOR水平降低从而下调 VEGF的表达,进而抑制细胞增殖力和迁移侵袭力。
金属基质蛋白酶(matrix metalloproteinases, MMPs)几乎能降解细胞外基质的所有成分,进而破坏肿瘤细胞侵袭过程中的组织学屏障,在肿瘤浸润和转移中起重要作用[15,16]。本实验中,过表达LKB1对MMP2水平无明显影响,但使MMP9水平显著下调。LV Rhodes等[17]在乳腺癌细胞中也发现,LKB1高表达可以降低MMP1表达进而抑制乳腺癌细胞的转移能力。可见在PGCL3细胞中,LKB1可通过下调MMP9的表达而抑制PGCL3细胞的迁移和侵袭。
已有研究表明,LKB1低表达或缺失可使肿瘤的迁移侵袭力增强。本研究结果显示,通过转染增强PGCL3细胞中LKB1的表达可使PGCL3细胞的增殖能力、迁移、侵袭力下降。其机制可能与LKB1下调p-m TOR、VEGF、MMP9等基因的表达有关,但具体作用机制还需进一步研究。
[1] Alasio TM, Sun W, Yang GC. Giant cell carcinoma of the lung impact of diagnosis and review of cytological features. Diagn Cytopathol, 2007, 35(9): 555-559.
[2] Zhu WY, Fang WG, Zheng J. Ef ects of retinoic acid on the adhesion and motility of metastatic human lung cancer cell subline (PGCL3). Zhonghua Zhong Liu Za Zhi,1994,16(5):323-326.
[3] Hemm inki A, Avizienyte E, Roth S, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Duodecim,1998, 114(7): 667-668.
[4] Goodw in JM, Svensson RU, Lou HJ, et al. An AMPK-independent signaling pathway downstream of the LKB1 tumor suppressor controls Snail1 and metastatic potential. Mol Cell, 2014, 55(3): 436-450.
[5] Bryan BB, Schnitt SJ, Collins LC. Ductal carcinoma in situ w ith basal-like phenotype: a possible precursor to invasive basal-like breast cancer. Mod Pathol, 2006, 19(5): 617-621.
[6] Li F, Han X, Li F, et al. LKB1 Inactivation Elicits a Redox Imbalance to Modulate Non-small Cell Lung Cancer Plasticity and Therapeutic Response. Cancer Cell, 2015, 27(5): 698-711.
[7] Faubert B, Vincent EE, Griss T, et al. Loss of the tumor suppressor LKB1 promotes metabolic reprogramming of cancercells via HIF-1alpha. Proc Natl Acad Sci USA, 2014, 111(7): 2554-2559.
[8] Carretero J, M edina PP, Blanco R, et al. Dysfunctional AMPK activity, signalling through m TOR and survival in response to energetic stress in LKB1-def cient lung cancer. Oncogene, 2007, 26(11): 1616-1625.
[9] Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and grow th control in tumoursuppression. Nat Rev Cancer, 2009, 9(8): 563-575.
[10] Shaw RJ. LKB1 and AMP-activated protein kinase control of m TORsignalling and grow th. Acta Physiol (Oxf), 2009, 196(1): 65-80.
[11] Wullschleger S, Loew ith R, Hall MN. TOR signaling in grow th and metabolism. Cell, 2006, 124(3): 471-484.
[12] M ita MM, M ita A, Row insky E K. The molecular target of rapamycin (m TOR) as a therapeutic target against cancer. Cancer Biol Ther, 2003, 2(4 Suppl 1): S169-S177.
[13] Holz MK, Ballif BA, Gygi SP, et al. m TOR and S6K1 mediate assembly of the translation preinitiation complex through dynam ic protein interchange and ordered phosphorylation events. Cell, 2005, 123(4): 569-580.
[14] Guertin DA, Sabatini DM. Defining the role of m TOR in cancer. Cancer Cell, 2007, 12(1): 9-22.
[15] Lee YH, A lbig AR, Regner M, et al. Fibulin-5 initiates epithelial-mesenchymal transition (EMT) and enhances EMT induced by TGF-beta in mammary epithelial cells via a MMP-dependent mechanism. Carcinogenesis, 2008, 29(12): 2243-2251.
[16] Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor m icroenvironment. Cell, 2010, 141(1): 52-67.
[17] Rhodes LV, Tate CR, Hoang VT, et al. Regulation of triple-negative breast cancer cell metastasis by the tumor-suppressor liver kinase B1. Oncogenesis, 2015, 4: e168.
LKB1 inhibits the proliferation and invasion of human lung giant cell cancer cell line through down-regulating the phosphorylation of m TOR and the exp ression of VEGF and MMP9
Chen Yixuan1Δ, Liu Yan2Δ, Lou Lili1, Shi Yanmei2, Chen Wenli2, Chen Xinnian2*
(1The First Clinical Medical College,2Department of Pathophysiology, Basic Medical College, Lanzhou University, Lanzhou 730000, China)
ObjectiveTo study the ef ect of liver kinase B1 (LKB1) on the proliferation, migration and invasion of human lung giant cell cancer PGCL3 cell line and its mechanism.M ethodsEukaryotic expression vector pCMV-LKB1 was constructed and transfected into giant lung cell cancer cell line PGCL3. 48h after transfection, the level of LKB1 protein was detected by western blotting to determ ine the ef ciency of transfection. The ef ect of LKB1 on the proliferation of the cells was detected every 12h for a total of 72h. Transwell assay was applied to evaluate cell migration and invasion. Western blotting was performed to detect the expression of matrix metalloproteinase 2 (MMP2), matrix metalloproteinase 9 (MMP9), mammalian target of rapamycin (m TOR) and vascular endothelial grow th factor (VEGF).ResultsPCR, double enzyme digestion and DNA sequencing results conf rmed that the LKB1 fragment was inserted in the right direction into the recombinant eukaryotic vector pCMV-LKB1 and that its nucleic acid sequence was the same aspublished on NCBI. Western blot results showed that the level of LKB1 in the transfected cells increased signif cantly, indicating the success of pCMV-LKB1 construction. Grow th curve analysis and 5-Ethynyl-2’-deoxyuridine (EdU) test showed that the cell proliferative capacity was signif cantly inhibited by pCMV-LKB1 transfection. Transwell assay results showed that LKB1 overexpression obviously inhibited the migration and invasion of PGCL3 cells. Western blot results showed that of the downstream molecules of LKB1 in the transfected PGCL3 cells, the total m TOR level remained unchanged, while the expression of phosphorylated m-TOR (p-m TOR) decreased. VEGF, the downstream protein of mTOR, reduced signif cantly. The level of m igration related protein MMP2 remained unchanged, while that of MMP9 decreased signif cantly.ConclusionLKB1 inhibits the proliferation of PGCL3 cells possibly through down-regulating the levels of p-m TOR and VEGF; and also inhibit its migration by down-regulating MMP9 expression.
Liver kinase B1; proliferation; m igration; invasion; matrix metalloproteinase; mammalian target of rapamycin; vascular endothelial grow th factor
R734.2
A
10.16705/ j. cnki. 1004-1850.2017.03.001
2017-03-02
2017-06-05
甘肃省自然科学基金(1506RJZA205)
陈逸轩,男(1993年),汉族,本科;刘燕,女(1980年),汉族,讲师
Δ共同第一作者:陈逸轩 刘燕
*通讯作者(To whom correspondence should be addressed):chenxn@lzu.edu.cn
