听觉剥夺及耳蜗内电刺激幼鼠听皮层和下丘核CREB和NMDAR1蛋白表达变化*
2016-06-06樊碧云卢振东程岚杨军
樊碧云 卢振东 程岚 杨军
·实验研究·
听觉剥夺及耳蜗内电刺激幼鼠听皮层和下丘核CREB和NMDAR1蛋白表达变化*
樊碧云1卢振东1程岚1杨军1
【摘要】目的观察耳聋幼鼠及其耳聋后单耳植入电极电刺激后幼鼠听皮层和下丘核环磷酸腺苷反应元件结合蛋白(cyclic AMP response element- binding protein, CREB)和N-甲基-D-天冬氨酸受体-1(N-methyl-D-aspartic acid receptor one,NMDAR1)表达水平的变化。方法将66只12天龄SD幼鼠随机分为2大组,分别为耳聋造模后4周组(33只)及耳聋造模后6周组(33只)。将耳聋造模后4周组再分为对照1组、耳聋造模后4周组(4周组)及耳聋造模后3周耳蜗内电刺激组1(刺激时间为1周,简称“电刺激1组”),每组11只大鼠;将耳聋造模后6周组再分为对照2组、耳聋造模后6周组(6周组)及耳聋造模后5周耳蜗内电刺激组2(刺激时间为1周,简称“电刺激2组”),每组11只大鼠;对照1、2组均正常饲养。除对照1、2组外,在其余4组幼鼠颈背部、两侧下腹部皮下注射庆大霉素(总量为350 mg/kg),半小时后于相同部位注射呋塞米(总量为200 mg/kg),两周后行ABR检测,于耳聋造模成功后第3、5周分别对电刺激1、2组的幼鼠植入电极,在耳蜗内进行电刺激,每天3小时,持续7天。于耳聋造模后4、6周分别处死4、6周组大鼠取听皮层和下丘组织,通过免疫组织化学方法,观察CREB和NMDAR1表达水平的变化。结果耳聋造模成功后幼鼠ABR阈值均大于93 dB SPL,4周组听皮层、下丘CREB和NMDAR1的表达较对照1组增加,电刺激1组CREB和NMDAR1的表达较4周组增加。6周组听皮层和下丘CREB和NMDAR1的表达较对照2组下降,电刺激2组CREB和NMDAR1的表达较6周组增加。结论听觉剥夺可导致幼鼠听皮层和下丘CREB和NMDAR1早期表达增加而晚期表达下降。耳蜗植入电极电刺激可导致幼鼠听皮层和下丘CREB和NMDAR1的表达增加,反映这两个部位神经元的可塑性变化。
【关键词】听觉可塑性;环磷酸腺苷反应元件结合蛋白;N-甲基-D-天冬氨酸受体-1;电刺激;感音神经性聋
以往研究观察了耳聋幼鼠及耳聋后单耳植入电极电刺激后幼鼠听觉中枢的听皮层、下丘及耳蜗核部位的脑源性神经营养因子(BDNF)和即刻早期基因之一c-fos的基因及蛋白表达水平的变化,发现BDNF与其受体酪氨酸蛋白激酶B(T yrosine receptor kinase-B, TrkB) 结合后, 磷酸化激活的TrkB 可进一步激活核内转录调控因子环磷酸腺苷反应元件结合蛋白(cyclic AMP response element- binding protein, CREB)刺激BDNF的转录,从而促进神经元的生长、保护受损神经元[1]。C-fos作为即刻早期基因的一种,其表达与中枢神经系统的功能可塑性相关[2],而N-甲基-D-天冬氨酸受体(N-methyl-D-aspartic acid receptor,NMDA)通过其结构的变化,介导Ca2+内流的调控,调节神经元内Ca2+依赖的第二信使系统,作用于c-fos基因上的钙反应序列启动c-fos基因,诱导c-fos基因表达增高[3];因此,在BDNF、c-fos的表达过程中其传导通路中的CREB和N-甲基-D-天冬氨酸受体-1(N-methyl-D-aspartic acid receptor one,NMDAR1)发挥着重要介导作用;而听觉剥夺和耳蜗内电刺激致幼鼠听皮层和下丘核CREB和NMDAR1蛋白表达的变化目前国内外尚未见报道。氨基糖苷类抗生素联合呋塞米可损伤Corti器的外毛细胞导致听力障碍[4],SD大鼠幼鼠在出生后14天Corti器才发育成熟,才有听力[5],在此期间注射耳毒性药物可造成其类似先天性感音神经性聋;因此,本研究拟通过皮下注射耳毒性药物建立先天性感音神经性聋大鼠模型,然后通过耳蜗植入电极进行电刺激,观察不同时间模型大鼠听皮层及下丘核CREB和NMDAR1的表达变化,以进一步探讨听觉剥夺和耳蜗内电刺激后幼鼠听皮层和下丘核CREB和NMDAR1蛋白表达变化的可能机制。1材料与方法
1.1实验动物及分组取出生后12天龄SD大鼠幼鼠66只(上海交通大学附属新华医院动物实验中心提供),雌雄不拘,耳廓反应灵敏,体重23~28 g。随机分为2大组,分别为耳聋造模后4周组(33只)及耳聋造模后6周组(33只),将耳聋造模后4周组再分为对照1组、耳聋造模后4周组(4周组)及耳聋造模后3周耳蜗内电刺激1组(刺激时间为1周,简称“电刺激1组”),每组11只;将耳聋造模后6周组再分为对照2组、耳聋造模后6周组(6周组)以及耳聋造模后5周接受耳蜗内电刺激2组(刺激时间为1周,简称“电刺激2组”),每组11只。
1.2耳聋动物模型的建立对照1、2组均正常饲养,其余4组分别在幼鼠颈背部、两侧下腹部皮下注射庆大霉素(总量350 mg/kg,上海中西制药有限公司),半小时后于相同部位注射呋塞米(总量200 mg/kg,上海禾丰制药有限公司),两周后行ABR检测。
1.3耳蜗内电刺激方法于造模成功后第3、5周分别对电刺激1组、电刺激2组幼鼠行全麻后,于右侧耳后切口,听泡骨壁钻孔,暴露耳蜗。耳蜗底回鼓阶钻孔,电极贴近鼓阶蜗轴插入大约3 mm,用肌肉封住切开的圆窗;参考电极置于听泡附近的颞肌内。用超强胶将刺激电极固定于动物颞骨,头皮缝合固定电极。刺激电极使用动物用铂铱合金球形电极。使用SEN-7203刺激器(日本光电公司)进行耳蜗内电刺激,刺激电流参数为电流强度0.5 mA,脉宽20~100 μs,频率30~2 000 Hz,均为双相脉冲[6],每天3小时,持续7天。
1.4免疫组织化学染色法 分别于耳聋造模后4周、6周将4周组、6周组大鼠行心脏灌注,处死动物,断头,根据大鼠脑立体定位图[7],取左侧听皮层和下丘,以4%多聚甲醛固定,经脱水、透明、浸蜡、石蜡包埋,连续冠状位切片,片厚5 μm。分别进行免疫组织化学染色;按SABC 免疫组化检测听皮层和下丘的CREB和NMDAR1的表达,严格按试剂盒说明书操作,兔抗鼠NMDAR1抗体和兔抗鼠CREB抗体均取自Abcam公司(英国)。采集图像时均在同一光强度、同一放大倍数下进行,每个标本取5张切片,每张切片取5个不同的视野,进行积分光密度值(IOD)分析,取平均值为每张切片的平均IOD值,计算每组动物的平均IOD值。
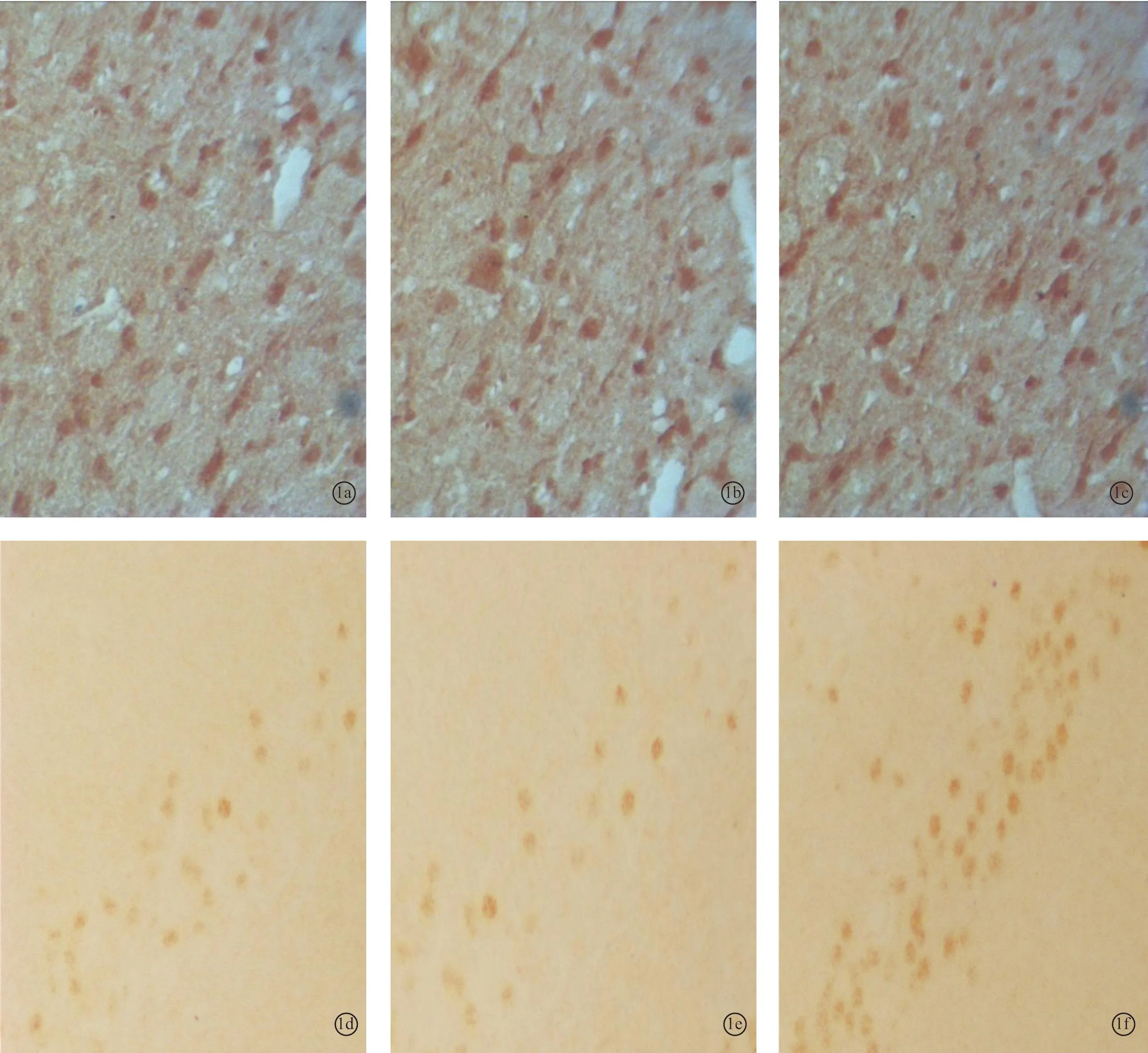
图1 耳聋造模4周后对照1组、4周组、电刺激1组下丘内NMDAR1蛋白(a~c)和CREB蛋白(d~f)的表达 (DAB×400)
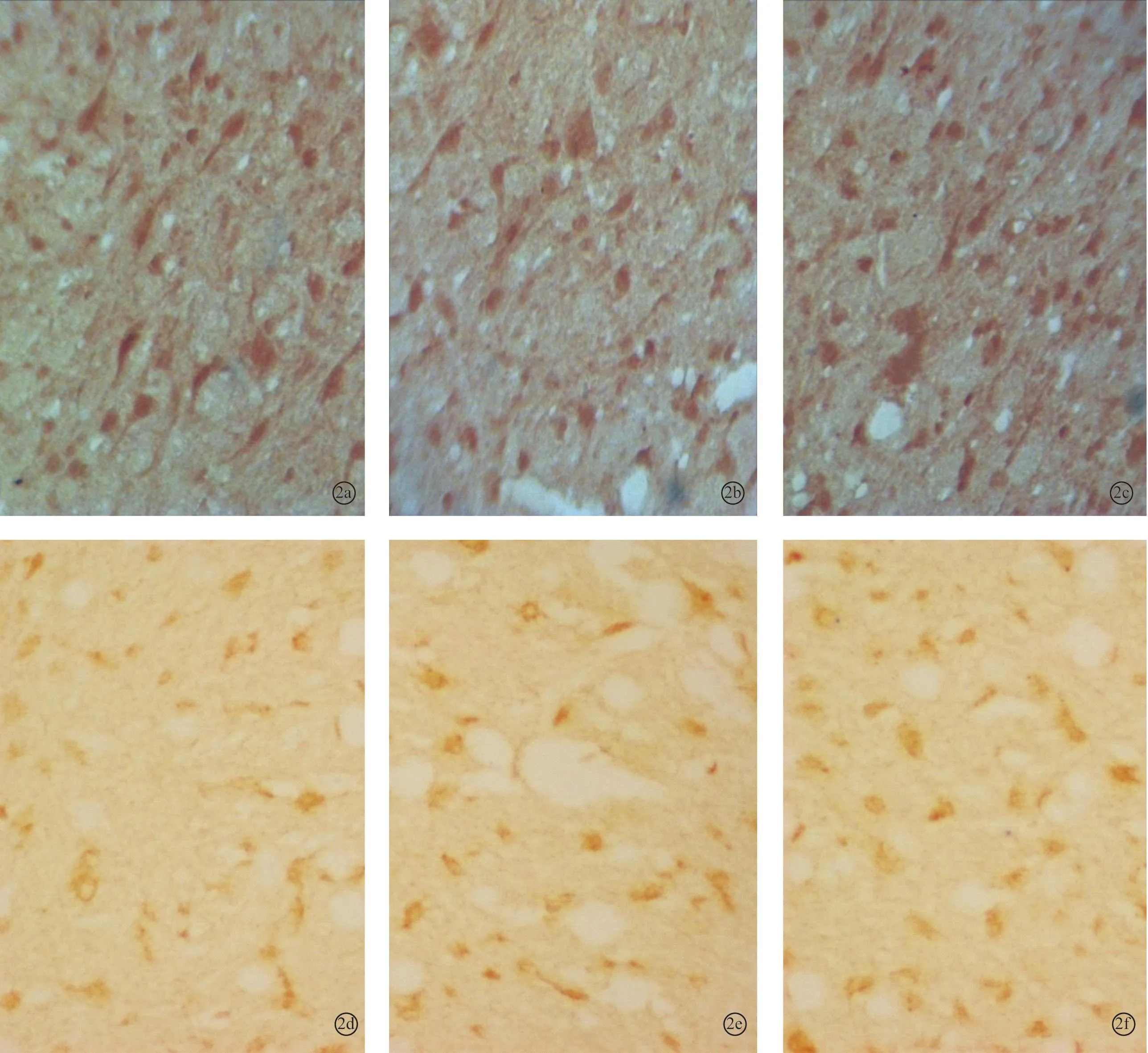
图2 耳聋造模6周后对照2组、6周组、电刺激2组下丘内NMDAR1蛋白(a~c)和CREB蛋白(d~f)的表达(DAB×400)
1.5统计学方法所有数据采用SPSS17.0 软件包进行统计学分析,组间比较采用单因素方差分析,P<0.05为差异有统计学意义。
2结果
2.1ABR检测结果耳聋造模完成后幼鼠ABR阈值均大于93 dB SPL,根据Tan等[8]建立的耳聋模型标准,本实验耳聋动物模型建立成功。
2.2各组听皮层及下丘CREB、NMDAR1蛋白表达耳聋造模后4周,在听皮层和下丘中,4周组与对照1组相比、电刺激1组与4周组相比,CREB、NMDAR1蛋白的表达量均增加(P<0.05);耳聋造模后6周,在听皮层和下丘中,6周组与对照2组相比,CREB、NMDAR1蛋白的表达量均降低(P<0.05),电刺激2组与6周组相比CREB、NMDAR1蛋白的表达量均增加(P<0.05)(表1、2,图1~4)。
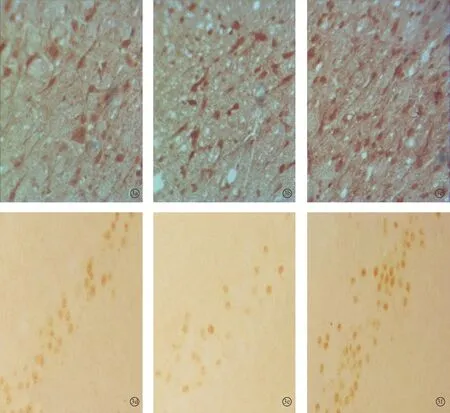
图3 耳聋造模4周后对照1组、4周组、电刺激1组听皮层内NMDAR1蛋白(a~c)和CREB蛋白(d~f)的表达 (DAB×400)
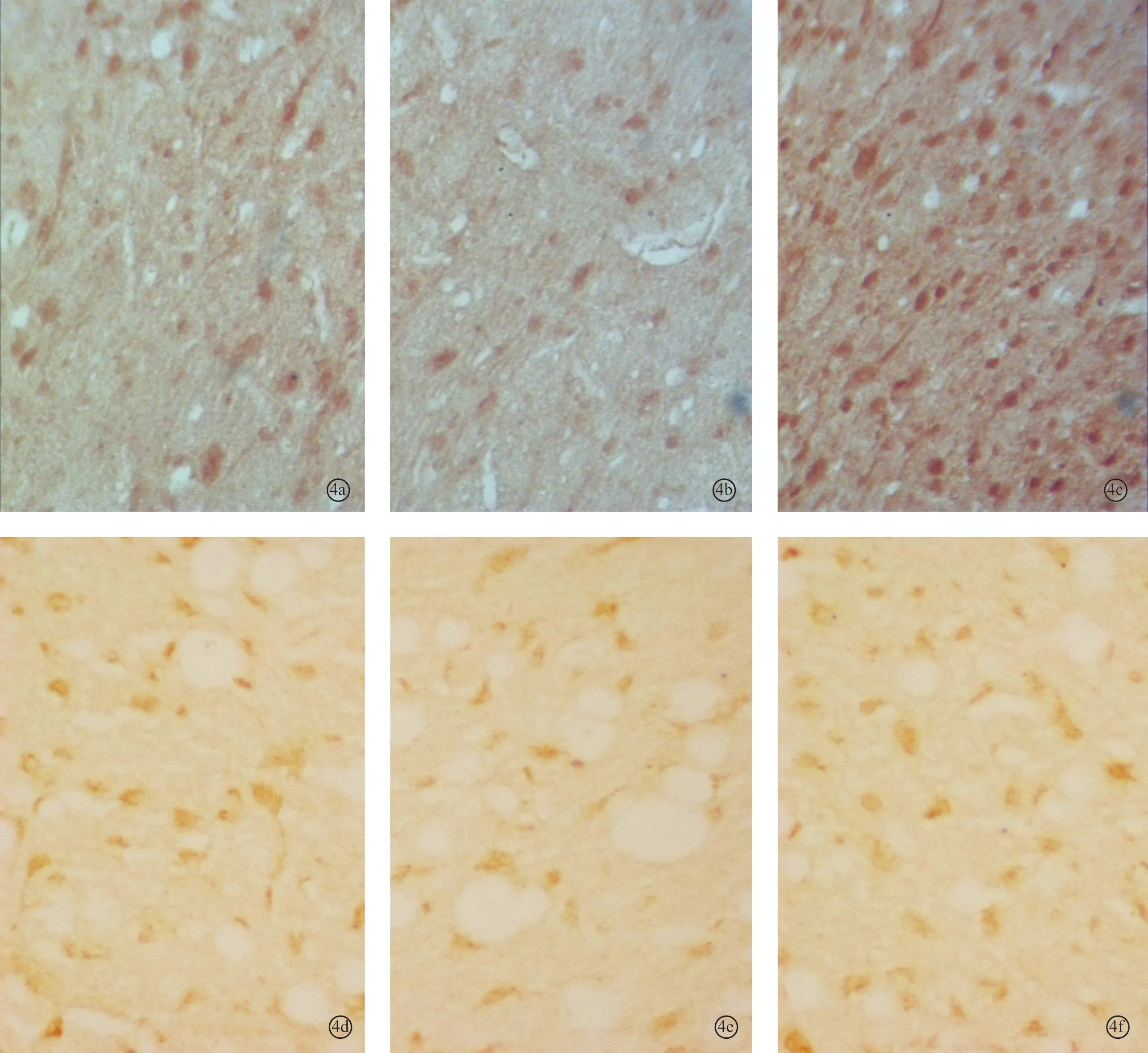
图4 耳聋造模6周后对照2组、6周组、电刺激2组听皮层内NMDAR1蛋白(a~c)和CREB蛋白(d~f)的表达 (DAB×400)
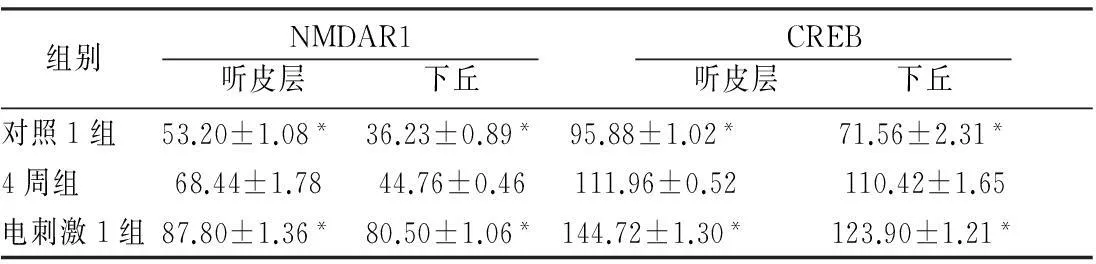
组别NMDAR1 听皮层 下丘 CREB 听皮层 下丘 对照1组53.20±1.08*36.23±0.89*95.88±1.02*71.56±2.31*4周组68.44±1.7844.76±0.46111.96±0.52110.42±1.65电刺激1组87.80±1.36*80.50±1.06*144.72±1.30*123.90±1.21*
注:*与4周组比较,P<0.05
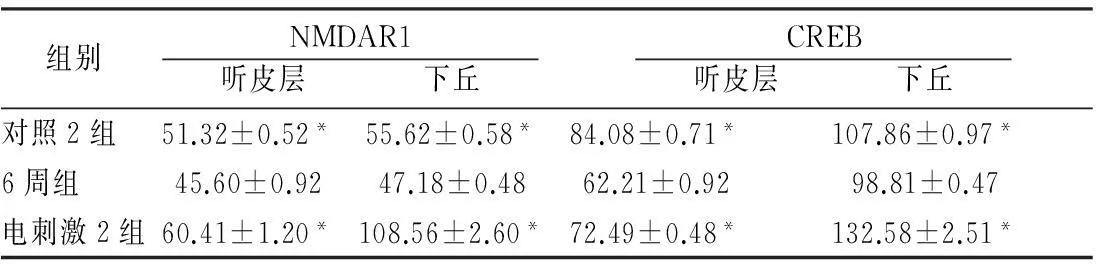
表2 耳聋造模后6周各组听皮层、下丘NMDAR1和CREB
注:*与6周组比较,P<0.05
3讨论
CREB是真核生物细胞核内的一种蛋白质,它的功能是调节基因转录,被称为调节转录的核因子,它的存在能刺激BDNF等基因的转录,所以又称之为转录增强因子[9]。此外,Alvarado研究发现BDNF为CREB的靶基因,被激活的CREB能够结合CRE并增强BDNF的转录,使其mRNA表达量增高,并且加强BDNF翻译,使BDNF蛋白表达上调[10]。BDNF作为神经营养因子家族中重要成员,可以调节神经细胞间突触的传递,修复受损神经元,维持神经元存活,促进其生长[11]。尤其在内耳神经细胞和感觉上皮细胞的发生发育过程中起重要作用,能够减少耳毒性药物、噪声等因素对毛细胞的损害,维持SGNs的存活[12~14]。前文也提到NMDAR1蛋白可以诱导c-fos基因表达增高,促进神经元修复。文中结果显示,SD幼鼠耳聋造模后4周,在听皮层和下丘中,与对照1组相比耳聋4周组CREB、NMDAR1蛋白的表达量均增加;而耳聋造模后6周,与对照2组相比耳聋6周组听皮层和下丘中,CREB、NMDAR1蛋白的表达量均降低。因此推测耳聋造模后4周CREB、NMDAR1表达增多可能是由于耳毒性药物损伤外毛细胞,导致听神经传入减少,具有活性的神经元代偿性表达CREB和NMDAR1,使其表达上升,导致BDNF和c-fos的表达上升,来维持正常的突触传递、发育;而耳聋造模后6周CREB、NMDAR1表达降低可能是由于长时间的代偿作用加剧神经元的死亡,失去神经元活动的控制后,两种蛋白的表达量下降,导致BDNF和c-fos的表达下降,神经元不能获得足够的营养支持,进一步加剧神经元的死亡。
有学者研究发现电针刺激可使慢性应激大鼠海马CREB的表达升高[15],本研究发现耳蜗内植入电极电刺激亦可使CREB在听皮层及下丘核表达增多。CREB表达的上调可作用于其靶基因BDNF,导致BDNF表达增多。BDNF对突触前兴奋性谷氨酸递质的释放及突触后膜NMDA受体均有调控作用,可上调NMDA受体的功能[16]。NMDA 受体有NMDAR1和NMDAR2两个亚单位,NMDAR1是主要的功能单位。激活后的NMDA受体可引起神经元细胞膜对Ca2+等离子的通透性增强,产生兴奋性突触后电位[17]。
耳蜗电刺激可引起NMDA的受体NMDAR1在耳蜗神经后核、腹侧核及中央核等处表达增多[18]。NMDAR1受体介导的Ca2+内流的调控,调节神经元内Ca2+依赖的第二信使系统,最终实现对中枢神经系统发育过程的复杂调控[19]。从本研究结果看,电刺激亦可引起听皮层和下丘核NMDAR1表达增多。推测是因为耳蜗内的电刺激使CREB的表达上调促使BDNF表达增多,同时,BDNF促进NMDAR1表达上调恢复神经元的兴奋性,使得电刺激在突触传递、突触发育及可塑性方面发挥重要作用[20,21]。
CREB和NMDAR1作为分子工具被用于监测听觉剥夺后听觉神经系统的变化,其表达水平反映了神经元及突触的生长发育可塑性变化,可用于监测损伤诱导的神经活动变化。本研究着重于观察幼年大鼠听觉剥夺后及电刺激后听皮层、下丘核CREB和NMDAR1的表达变化,至于CREB和NMDAR1在听觉中枢其它核团中如何变化及其变化具体机制尚待进一步研究。
4参考文献
1Ma H, Yu B, Kong L, et al. Neural stem cells over-expressing brain-derived neurotrophic factor (BDNF) stimulate synaptic protein expression and promote functional recovery following transplantation in rat model of traumatic brain injury[J].Neurochem Res, 2012,37:69.
2Adams JC. Sound stimulation induces Fos-related antigens in cells with common morphological properties throughout the auditory brainstem[J]. J Comp Neurol, 1995,361:645.
3Yakovlev AG,Faden AI.Sequential expression of c-fos protooncogene, TNF-alpha, and dynorphin genes inspinal cord following experimental traumatic injury[J]. Mol Chem Neuropathol, 1994, 23:179.
4Alvarado JC, Fuentes-Santamaria V, Franklin SR, et al. Unilateral cochlear ablation in adult ferrets results in upregulation in calretinin immunostaining in the central nucleus of the inferior colliculus[J]. Neuroscience, 2005,136: 957.
5Sobkowicz HM, Rose JE, Scott GE, et al. Ribbon synapses in the developing intact and cultured organ of Corti in the mouse[J]. Neuroscience, 1982,2:942.
6Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system[J]. Neuron, 2002,35:605.
7Paxinos G, Watson C. The rat brain in stereotaxic coordinates[M]. Third edition.San Diego, California:Academic Press Inc,1997.25~104.
8卢振东,杨军. 听觉剥夺和耳蜗内电刺激引起幼鼠听觉通路bndf和c-fos基因及蛋白改变[J].山东大学耳鼻喉眼学报,2012,26:17.
9Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing[J]. J Neurosci Methods, 1989,29:261.
10Fields RD. Regulation of neurite outgrowth and immediate early gene expression by patterned electrical stimulation[J]. Prog Brain Res,1994,103:127.
11Li L, Xu Q, Wu Y, et al. Combined therapy of methylprednisolone and brain-derived neurotrophic factor promotes axonal regeneration and functional recovery after spinal cord injury in rats[J]. Chin Med J(Engl), 2003,116:414.
12Pirvola U, Ylikoski J. Neurotrophic factors during inner ear development[J]. Curr Top Dev Biol, 2003, 57:207.
13Yagi M, Magal E,Sheng Z, et al. Hair cell protection from aminoglycoside otoxicity by adenovirus-mediated overexpression of glial cell line-derived neurotrophic factor[J]. Hum Gene Ther, 1999, 10:813.
14Chen X,Frisina RD, Bowers WJ, et al.HSV amplicon-mediated neurotrophin-3 expression protects murine spiral ganglion neurons from cisplatin-induced damage[J].Mol Ther, 2001,3:658.
15Fu WB, Liu JH, Bai YF, et al. Effect of electroacupuncture on CREB-BDNF postreceptor signal transduction pathway in hippocampus of depression rats[J]. Chinese Journal of Gerontology,2009, 23:3038.
16Levine ES. Brain-derived neurotrophic factor modulates hip-pocampal synaptic transm ission by increasing N-methyl-D-as-partic acid receptor activity[J]. Proc Natl Acad Sci USA, 1998,95:10235.
17Noguchi J, Matsuzaki M, Graham CR, et al. Spine-neck geometry determines NMDA receptor-dependt Ca2+signaling in dendrites[J]. Neuron,2005,46:609.
18Liao WH, Van Den Abbeele T, Herman P,et al.Expression of NMDA, AMPA and GABA(A) receptor subunit mRNAs in the rat auditory brainstem. II. Influence of intracochlear electrical stimulation[J].Hear Res,2000,150:12.
19Adams JC. Sound stimulation induces Fos-related antigens in cells with common morphological properties throughout the auditory brainstem[J]. J Comp Neurol, 1995,361:645.
20Ma H, Yu B, Kong L, et al. Neural stem cells over-expressing brain-derived neurotrophic factor (BDNF) stimulate synaptic protein expression and promote functional recovery following transplantation in rat model of traumatic brain injury[J]. Neurochem Res, 2012,37:69.
21Sheng M,Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system[J]. Neuron, 1990,4:477.
(2015-12-09收稿)
(本文编辑周涛)
网络出版时间:2016-4-2615:53
网络出版地址:http://www.cnki.net/kcms/detail/42.1391.R.20160426.1553.034.html
Variation of CREB and NMDAR1 Proteins Induced by Auditory Deprivation and Intracochlear Electrical Stimulation in Infant Rats
Fan Biyun, Lu Zhendong, Cheng Lan, Yang Jun
(Department of Otolaryngology-Head and Neck Surgery,Shanghai Xinhua Hospital,Affiliated to Shanghai Jiaotong University of Medicine,Ear Institute,Shanghai,200092,China)
【Abstract】ObjectiveTo observe the expression of cAMP-response element binding protein (CREB) and N-methyl-D-aspartic acid receptor (NMDA) after intracochlear electrical stimulation in the auditory cortex and inferior colliculus in infant rats with auditory deprivation. MethodsSixty six SD infant rats were randomly divided into 6 groups (11 rats each group): 4 weeks, and 6 weeks after injection of ototoxic drug, the control group, and 3 weeks and 5 weeks after injection of ototoxic drug with intra-cochlear electrical stimulation for one week. Gentamicin sulphate (350 mg/kg body weight) and frusemide (200 mg/kg body weight) were injected subcutaneously in the skin folds on the lateral abdominal side and the dorsal neck area, respectively. The expression of CREB and NMDAR1 protein were detected by immunohistological staining. ResultsThe results of immunohisto-chemistry revealed that protein expression of CREB and NMDAR1 in 4 week group of injection increased as compared to the control group, while decreasing as compared to intracochlear electrical stimulation group, significantly. However, protein expression of CREB and NMDAR1 in 6 week group of injection decreased as compared to the control group and intracochlear electrical stimulation group, significantly. ConclusionAuditory deprivation could result in the expression of protein of CREB and NMDAR1 in auditory cortex and inferior colliculus increasing in an early stage and then decreasing in infant rats. Intracochlear electrical stimulation could result in the expression of proteins of CREB and NMDAR1 in auditory cortex and inferior colliculus increasing in infant rats. The dynamic variation of CREB and NMDAR1 expression in rat auditory cortex and inferior colliculus reflects synaptic plasticity in neurons of auditory pathway.
【Key words】Auditory plasticity;Cyclic AMP response element- binding protein(CREB);N-methyl-D-aspartic acid receptor one(NMDAR1);Electrical stimulation;Sensorineural hearing loss
【中图分类号】R764.4
【文献标识码】A
【文章编号】1006-7299(2016)03-0245-06
DOI:10.3969/j.issn.1006-7299.2016.03.007
作者简介:樊碧云,女,上海人,博士研究生,主要研究方向为耳蜗听觉植入。通讯作者:杨军(Email:13764981808@126.com)
*上海市卫生局项目(2010240)、上海市科委医学引导项目(114119a6300)联合资助
1上海交通大学医学院附属新华医院耳鼻咽喉-头颈外科上海交通大学医学院耳科学研究所(上海200092)
