上皮间质转化在胃癌及相应转移淋巴结中的表达及意义
2015-01-02
(哈尔滨医科大学第一附属医院病理科,哈尔滨 150001)
上皮间质转化在胃癌及相应转移淋巴结中的表达及意义
崔苗苗,宋月佳,顾云鹤,戚基萍
(哈尔滨医科大学第一附属医院病理科,哈尔滨 150001)
目的探讨上皮间质转化(EMT)标志物在胃癌组织和转移淋巴结中的表达情况及其与恶性生物学行为的相关性。方法采用免疫组化法检测145例胃癌、25例异常增生、13例肠化、42例转移淋巴结及40例正常胃黏膜组织中EMT标志物E-cadherin、β-catenin、N-cadherin、转化生长因子β1(TGF-β1)及Snail的表达情况。结果胃癌组织中E-cadherin、β-catenin、N-cadherin、TGF-β1及Snail的阳性表达率分别为73.5%、65.5%、14.5%、53.1%、35.9%,正常胃黏膜组织中分别为100%、100%、0%、27.5%、2.5%,差异有统计学意义(P<0.05)。E-cadherin、β-catenin表达水平的降低及TGF-β1表达水平的升高与胃癌浸润程度有关(P<0.05)。E-cadherin与β-catenin的表达呈正相关,而与TGF-β1呈负相关(P<0.05)。N-cadherin与TGF-β1的表达呈正相关(P<0.05)。转移淋巴结中,E-cadherin和β-catenin的表达水平高于胃癌组织,而TGF-β1的表达低于胃癌组织(P<0.05)。结论TGF-β1和Snail的高表达以及E-cadherin、β-catenin、N-cadherin的低表达与胃癌的浸润和转移有密切关系;在胃癌的发展过程中,E-cadherin向N-cadherin转化及TGF-β1表达可能发挥重要作用。淋巴结转移灶中出现了间质上皮转化(MET)。
上皮间质转化标记蛋白;间质上皮转化;胃癌;淋巴结转移
胃癌在消化道肿瘤中发病率位于首位[1]。多种环境因素,如慢性幽门螺旋杆菌感染和饮食因素,均可促进胃癌的发生[2]。深入研究胃癌的发病和进展的分子机制将会为其治疗提供新的思路和方法。上皮细胞间质转化(epithelial-to-mesenchymal transition,EMT)是指上皮细胞向间质细胞转化的过程。在EMT过程中,细胞失去上皮极性并获得纤维细胞表型。这个过程包括紧密黏附连接蛋白(如E-cadherin、β-catenin)表达的减少或重新分布以及间质细胞蛋白(如N-cadherin、Vimentin、Snail)的表达上调,从而使细胞获得了较强的侵袭和转移能力[3]。EMT是多细胞生物发生过程中的基础过程,参与胚胎发育和器官塑型。EMT也存在于肿瘤的浸润和转移过程中,是肿瘤研究的热点之一[4~7]。本研究拟采用免疫组织化学方法检测EMT标志物E-cadherin,β-catenin,N-cadherin,Snail和转化生长因子β1(transforming growth factor-β1,,TGF-β1)在癌前病变、胃癌及转移淋巴结中的表达,并分析其相关性,旨在探讨EMT在胃癌发生和发展中的作用及意义。
1 材料与方法
1.1 材料
收集哈尔滨医科大学第一附属医院病理科2005年1月至2009年12月存档的胃癌组织标本145例,转移淋巴结42例,异常增生25例(取自胃癌的癌旁组织),肠化13例及正常胃黏膜40例(取自胃癌的断端)。所有患者术前均未接受化疗及放疗。临床参数,如年龄、性别、组织学类型、临床分期、淋巴结或远处转移,从病理资料库获得,组织学类型、Lauren、临床分期、分型参考2003版的世界卫生组织(WHO)的分级系统。
1.2 方法
采用免疫组织化学染色SP法。一抗E-cadherin(MAB-0589)、β-catenin(MAB-0259)、TGF-β1(1∶100,RAB-0238)购自福州迈新技术公司,N-cadherin(ZM-0094)购于北京中杉生物技术公司,Snail(1∶100,ab85936)购自Abcam公司。每次实验均设立阳性和阴性对照。试剂盒均购自福州迈新生物有限公司。染色操作严格按照试剂盒说明书进行。
1.3 免疫组化结果判断
每例切片选择10个高倍视野(×40)。E-cadherin和β-catenin阳性染色定位于细胞膜,按阳性细胞占全部癌细胞的比例计算百分比,阳性细胞比例<90%为阴性表达,≥90%为阳性表达。N-cadherin阳性染色定位于细胞膜和(或)细胞质,无阳性着色或阳性细胞比例<10%为阴性,阳性细胞比例≥10%为阳性。TGF-β1和Snail阳性染色分别定位于细胞质和细胞核,染色强度积分0分为阴性,1分为弱阳性,2分为强阳性;阳性细胞百分比1%~25%为1分,26%~50%为2分,51%~75%为3分,76%~100%为4分;按照阳性细胞×染色强度计算总分,<3为阴性,≥3为阳性。
1.4 统计学分析
采用SAS 9.1统计软件包对实验数据进行统计分析。多组数据阳性率的比较采用χ2检验、Spearman秩和相关分析,P<0.05为差异有统计学意义。
2 结果
2.1 EMT标志物在胃癌、肠化、异常增生及正常胃黏膜组织中的表达
在胃癌组织中,E-cadherin的阳性表达率(73.1%,106/145)低于正常胃黏膜组织(100%,40/40),差异有统计学意义(P=0.001 4);β-catenin的阳性表达率(65.5%,95/145)低于正常胃黏膜组织(100%,40/40),差异有统计学意义(P=0.001 6);N-cadherin的阳性表达率(14.5%,21/145)高于正常胃黏膜组织(0%,0/40),差异有统计学意义(P=0.011 5);TGF-β1的阳性表达率(53.1%,77/145)高于正常胃黏膜组织(27.5%,11/40),差异有统计学意义(P= 0.004 8);Snail异常细胞核表达(35.9%,52/145)高于正常胃黏膜组织(2.5%,1/40),差异有统计学意义(P=0.0013)。但是这5个标志物在肠化和异常增生组织中的表达与胃癌相比差异无统计学意义。见图1。
2.2 EMT标志物的表达与临床病理参数的关系
5种EMT标志物与临床病理因素的关系如表1所示:临床分期中,早期胃癌组E-cadherin和βcatenin的表达明显高于进展期胃癌组(P<0.05),而TGF-β1的表达则明显低于进展期胃癌组(P<0.05);无淋巴结转移组TGF-β1的表达显著低于有淋巴结转移组(P<0.05);β-catenin在未侵及浆膜层组的阳性表达明显高于侵及浆膜层组(P<0.05)。而它们与性别、年龄、大小、部位、分化程度、TNM分期、Lauren分型及远处转移均无关(P>0.05)。
2.3 胃癌组织中EMT标志物表达的相关性
在胃癌组织中,E-cadherin的表达与β-catenin的表达呈正相关(r=0.619 12,P=0.001 2),与TGF-β1呈显著负相关(r=-0.626 45,P=0.001 5);TGF-β1的表达与N-cadherin的表达呈显著正相关(r= 0.253 2,P=0.002 4)。
2.4 EMT标志物在转移淋巴结中的表达
如表2所示:与原发性肿瘤相比,在相应的转移淋巴结中,EMT上皮表型E-cadherin和β-catenin呈现高表达,间质表型TGF-β1表达减少(P<0.05)。提示了间质向上皮转化(mesenchymal-to-epithelial transition,MET)的发生。
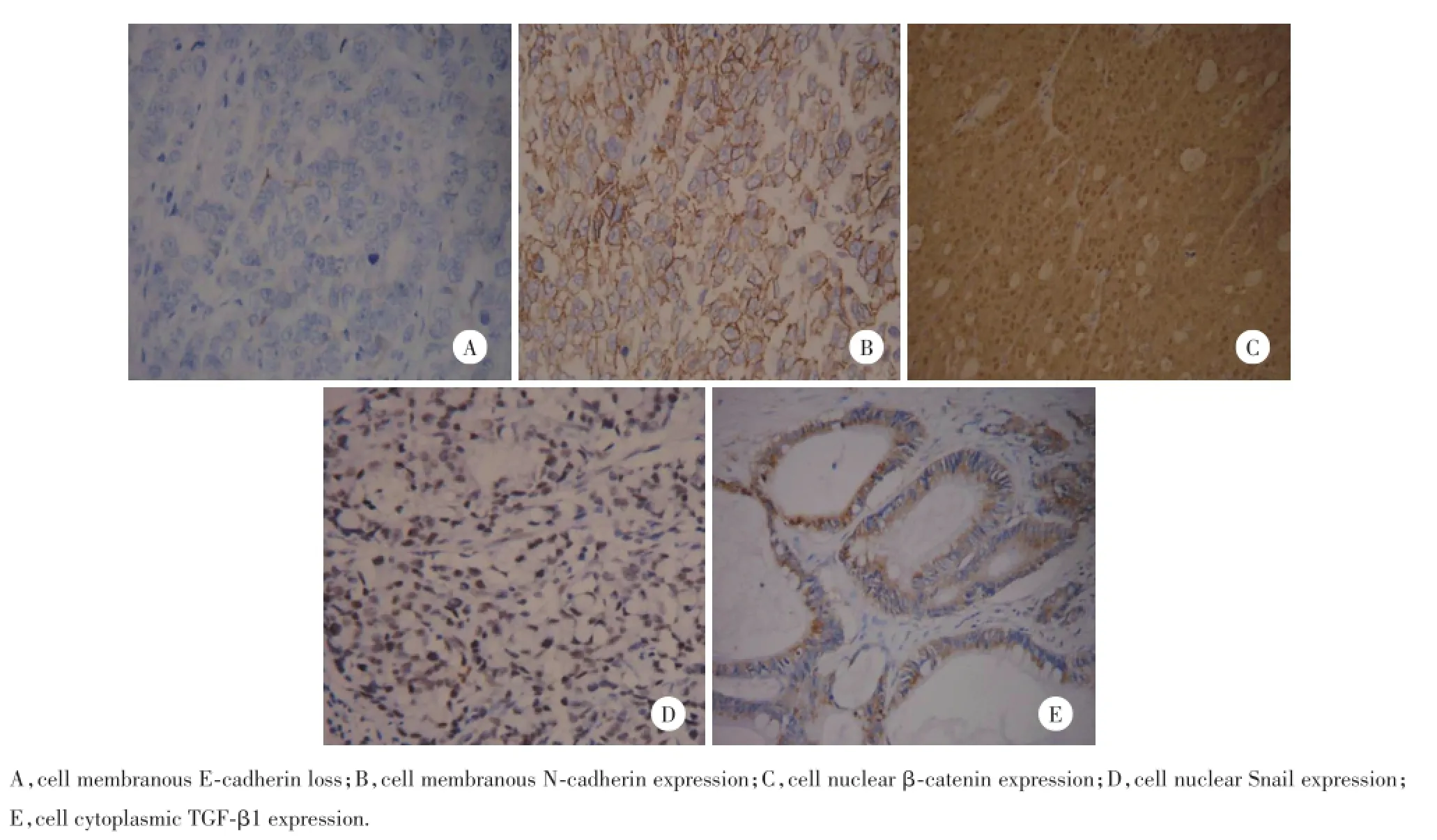
图1 免疫组化EMT标志物在胃癌中的表达Fig.1 Representative microphotographs of immunohistochemical expression of epithelial-to-mesenchymal transition markers in gastric cancer
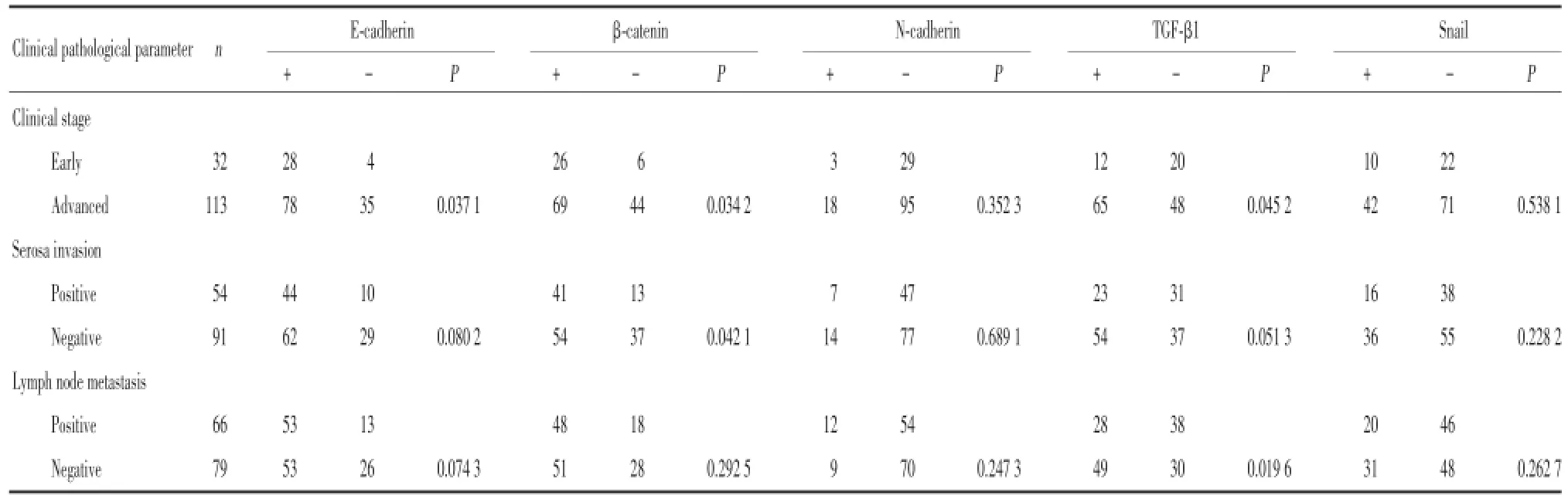
表1 临床病理参数和EMT标志物在胃癌中的关系Tab.1 Relationship of clinical pathological parameters and epithelial-to-mesenchymal transition markers in gastric cancer
3 讨论
EMT的主要特征是上皮细胞表型的缺失及间质特性的获得[8]。出现EMT的癌细胞的重要表型是细胞黏附分子表达减少。钙黏蛋白是细胞黏附分子家族之一,其中E-cadherin是影响肿瘤侵袭转移的EMT关键分子[9]。E-cadherin膜表达的缺失能降低细胞之间的黏附性,使细胞运动和迁移能力变强,这也是EMT发生的标志[10]。本研究结果显示:E-cadherin在胃癌中细胞膜缺失表达为26.9%,且在从正常向胃癌的过渡中表达呈逐渐减少趋势,提示在EMT中E-cadherin的低表达对胃癌的进展起着重要作用。β-catenin是一种多功能蛋白,与E-cadherin结合成复合体,使细胞间具有稳定的黏附性和细胞极性。研究表明,E-cadherin的表达缺失常伴随着βcatenin的膜表达减少,导致胞质中游离β-catenin增加,并最终聚集于细胞核中,启动相应的转录过程,从而促进了EMT的发生[11]。本研究结果显示:胃癌组织中E-cadherin和β-catenin的表达呈显著正相关,且β-catenin细胞膜表达减少而异位表达增加,且它们在进展期胃癌中的表达明显低于早期胃癌,其中,β-catenin与浆膜层侵及显著相关,提示了它们在胃癌的侵袭过程中起一定作用,促进了EMT的发生。
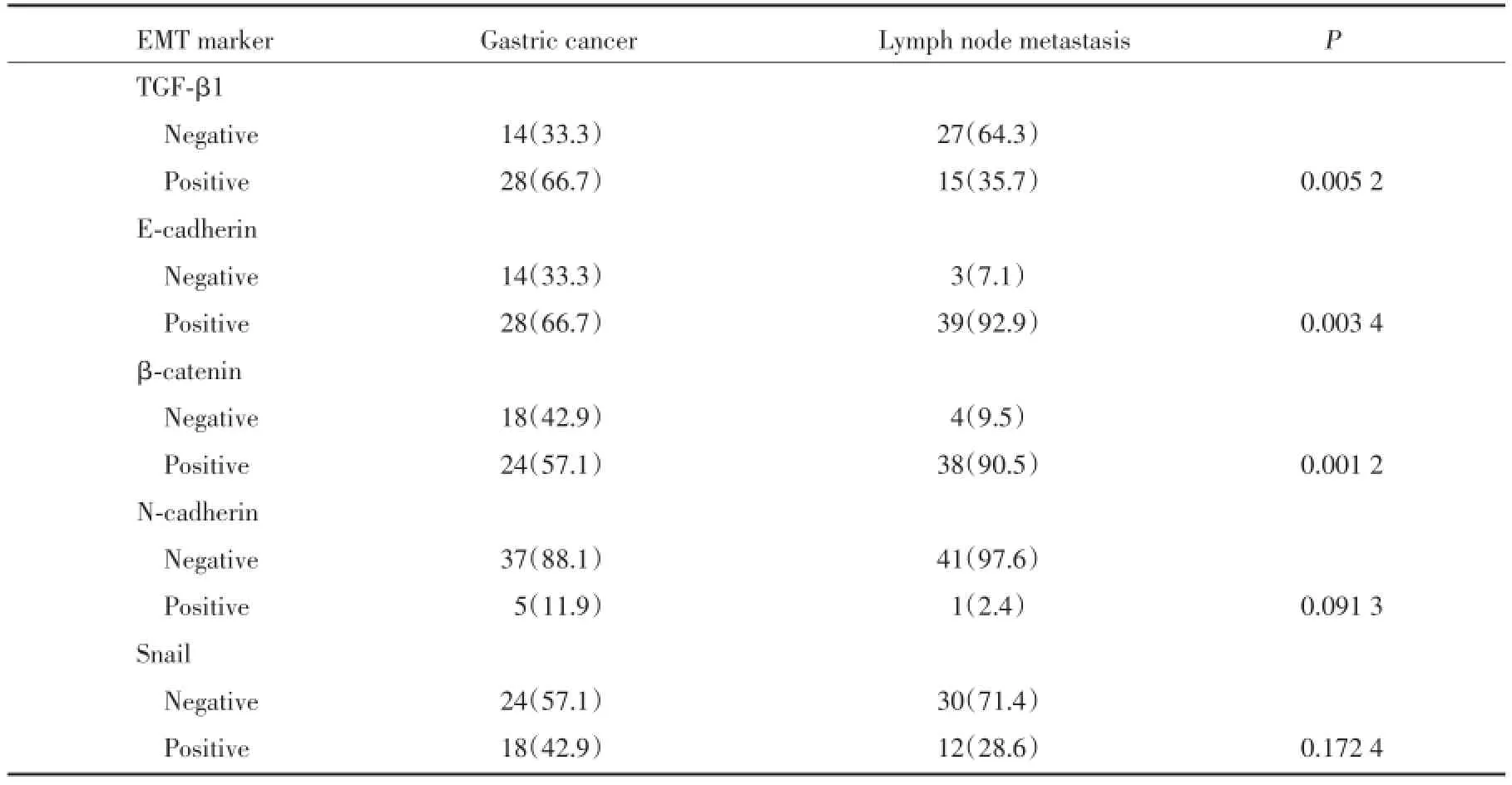
表2 EMT标记物在胃癌及转移淋巴结中的表达[n(%)]Tab.2 Expression of epithelial-to-mesenchymal transition markers in gastric cancer and lymph node metastases[n(%)]
癌细胞表达间叶分化标记是EMT更为直接的表型。Snail和TGF-β1是重要的转录调节因子,也是近年来EMT调控研究的热点,研究表明二者通过下调E-cadherin的表达参与EMT过程[12,13]。Snail是一种含有锌指结构的DNA结合蛋白,通过SIP1与E-cadherin竞争性结合启动子区E-box连接基序,抑制E-cadherin的表达,促进EMT发生[14]。而TGF-β1通过β-整合素信号转导途径发挥作用,参与调控Snail对EMT的促进[15~17]。本研究结果显示:E-cadherin与TGF-β1在胃癌中的表达呈显著负相关,而与Snail无相关性,与既往报道一致[18],可能因为在胃癌中TGF-β1通过调控其他转录因子表达诱导Smad依赖和非依赖途径下调E-cadherin的表达,而非Snail。N-cadherin是另一种重要的间叶分化标记,它在正常上皮组织中不表达,而在EMT过程中出现表达,并有促进肿瘤细胞运动和转移的作用[19]。本研究结果显示:N-cadherin在正常胃黏膜、肠化组织中无表达,而在胃癌组织中表达增加。文献报道,乳腺癌肿瘤细胞中N-cadherin可出现表达异位,并通过诱导肿瘤细胞向间质转化影响其侵袭和转移能力[20]。此外,TGF-β1在胰腺癌组织中通过促进N-cadherin的过表达参与肿瘤细胞上皮向间质转化的过程[21]。本研究结果显示TGF-β1在进展期胃癌中的表达明显高于早期胃癌,且与淋巴结转移呈明显负相关,同时TGF-β1与N-cadherin表达呈正相关。由此推测在胃癌进展过程中,TGF-β1可能抑制E-cadherin的表达,导致肿瘤细胞间黏附性减弱,并通过促进N-cadherin的过表达使肿瘤细胞获得间质特征,增强其转移及侵袭能力。
上皮肿瘤在进展的过程中暂时发生了向间质的转化,从而使肿瘤细胞获得了更多的侵袭和转移能力。而转移处扩散的间质表型肿瘤细胞又经历了EMT的逆转,即所谓的MET[22]。本研究结果显示:和原发性肿瘤相比,在转移的淋巴结中间质表型标志物表达呈较低水平,而上皮表型标志物则刚好相反,证实了细胞的可塑性,即细胞可以随着微环境的改变而做出适当的转化。
有研究表明,肺癌中EMT表型水平随着组织病理学的严重程度的增加而增高[6]。但是本研究并未发现EMT表型在正常胃黏膜向胃癌演进过程中逐渐增高的现象,而只是胃癌中EMT表型表达水平比正常胃黏膜高,推测EMT可能是促进胃黏膜恶性转化的一个重要致瘤因素。
综上所述,本研究结果提示EMT促进了胃癌的发生和转移。因此,EMT可能成为预防胃癌侵袭和转移的一个潜在靶点。
[1]Shah MA,Schwartz GK.Treatment of metastatic esophagus and gastric cancer[J].Semin Oncol,2004,31(4):574-587.
[2]Ebert MP,Schandl L,Malfertheiner P.Helicobacter pylori infection and molecular changes in gastric carcinogenesis[J].J Gastroenterol,2002,37(13):45-49.
[3]Wever OD,Pauwels P,Craene BD,et al.Molecular and pathological signatures of epithelial-mesenchymal transitions at the cancer invasion front[J].Histochem Cell Biol,2008,130(3):481-494.
[4]Boyer B,Valls AM,Edme N.Induction and regulation of epithelial mesenchymal transitions[J].Biochem Pharmacol,2000,60(8):1091-1099.
[5]Logullo AF,Nonogaki S,Pasini FS,et al.Concomitant expression of epithelial-mesenchymal transition biomarkers in breast ductal carcinoma:association with progression[J].Oncol Rep,2010,23(2):313-320.
[6]Prudkin L,Liu D,Ozburn NC,et al.Epithelial-to-mesenchymal transition in the development and progression of adenocarcinoma and squamous cell carcinoma of the lung[J].Mod Pathol,2009,22(5):668-678.
[7]Tomita K,Bokhoven A,Leenders GJ,et al.Cadherin switching in human prostate cancer progression[J].Cancer Res,2000,60(13):3650-3654.
[8]Jechlinger M,Grunert S,Tamir IH,et al.Expression profiling of epithelial plasticity in tumor progression[J].Oncogene,2003,22(46):7155-7169.
[9]Hirohashi S.Inactivation of the E-cadherin mediated cell adhesion system in human cancers[J].Am J Pathol,1998,153(2):333-339.
[10]Micalizzi DS,Farabaugh SM,Ford HL. Epithelial-mesenchymaltransition in cancer:parallels between normal development and tumorprogression[J]. J Mammary Gland Biol Neoplasia,2010,15(2):117-134.
[11]Bukholm IK,Nesland JM,Borresen-Dale AL.Reexpression of E-cadherin,α-catenin and β-catenin,but not of γ-catenin,in metastatic tissue from breast cancer patients[J].Pathology,2000,190(1):15-19.
[12]Alves C,Rosivatz E,Schott C,et al.Slug is overexpressed in gastric carcinomas and may act synergistically with SIP1 and Snail in the down-regulation of E-cadherin[J].J Pathol,2007,211(5):507-515.
[13]Rosivatz E,Becker K,Kremmer E,et al.Expression and nuclear localization of Snail,an E-cadherin repressor,inadenocarcinomas of the upper gastrointestinal tract[J].Virchows Arch,2006,448(3):277-287.
[14]Barrallo Gimeno A,Nieto MA.The Snail genes as inducers of cell movement and survival:implications in development and cancer[J].Development,2005,132(14):3151-3161.
[15]Rees JRE,Onwuegbusi BA,Save VE,et al.In vivo and in vitro evidence for transforming growth factor-mediated-β1 epithelial to mesenchymal transition in esophageal adenocarcinoma[J].Cancer Res,2006,66(19):9583-9590.
[16]Cho HJ,Baek KE,Saika S,et al.Snail is required for transforming growth factor-beta-induced epithelial-mesenchymal transition by activating PI3 kinase/Akt signal pathway[J].Biochem Biophys Res Commun,2007,353(2):337-343.
[17]Sun L,Diamond ME,Ottaviano AJ,et al.Transforming growth factorβ1 promotes matrix metallo proteinase-9-mediated oral cancer invasion through Snail expression[J].Mol Cancer Res,2008,6(1):10-20.
[18]祝迎锋,吴继锋,马伟,等.TGF-β1 Snail E-cadherin及N-cadherin在胃癌中的表达及意义[J].中国肿瘤临床,2007,34(24):1400-1404.
[19]Yanagimoto K,Sato Y,Shimoyama Y,et al.Co-expression of N-cadherin and alpha-fetoprotein in stomach cancer[J].Pathol Int,2001,51(8):612-618.
[20]Hazan RB,Phillips GR,Qiao RF,et al.Exogenous expression of N-cadherin in breast cancer cells induces cell migration,invasion,and metastasis[J].J Cell Biol,2000,148(4):779-790.
[21]Nakajima S,Doi R,Toyoda E,et al.N-cadherin expression and epithelial-mesenchymal transition in pancreatic carcinoma[J].Clin Cancer Res,2004,10(12):4125-4133.
[22]Hugo H,Ackland ML,Blick T,et al.Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression[J].J Cell Physiol,2007,213(2):374-383.
(编辑王又冬)
Expression and Significance of Epithelial-to-mesenchymal Transition in the Progression of GastricCarcinoma and Lymph Node Metastasis
CUIMiao-miao,SONGYue-jia,GU Yun-he,QIJi-ping
(Department of Pathology,The First Affiliated Hospital of Harbin Medical University,Harbin 150001,China)
Objective To investigate the association between expression of the epithelial-to-mesenchymal transition(EMT)biomarkers and the malignant progression of gastric cancer in primary tumors and metastases and their possible correlation with progression of gastric cancer(GC).MethodsThe EMT biomarkers including E-cadherin,β-catenin,N-cadherin,Snail and TGF-β1 were detected by immuno histochemical method for 145 cases of gastric cancer(GC),25 cases of abnormal hyperplasia,13 cases of intestinal metaplasia,42 cases of lymph node metastasis and 40 cases of normal gastric mucosa tissues.ResultsPositive rates of TGF-β1,Snail,E-cadherin,β-catenin and N-cadherin were 73.5%,65.5%,14.5%,53.1%and 35.9%,respectively,in gastric cancer tissues and 100%,100%,0%,27.5%and 2.5%,respectively,in normal gastric tissues,with a significant difference between the two groups(P<0.05).The decreased expression of E-cadherin and β-catenin and the increased expression ofTGF-β1 were related to the depth of invasion of gastric cancer(P<0.05).The expression of E-cadherin was correlated positively with the expression ofβ-catenin,butnegatively with the expression ofTGF-β1.Whereas,the expression of N-cadherin was correlated positively with the expression of TGF-β1(P<0.05).The expression of E-cadherin andβ-catenin in lymph node metastasis was significantly higher than that in gastric cancer tissues,while the expression of TGF-β1 was lower than in gastric cancer tissues(P<0.05).ConclusionThe increased expression of TGF-β1 and Snail and the decreased expression of E-cadherin,β-catenin,and N-cadherin are involved in the processes of invasion and metastasis of GC.The transformation of E-cad her in to N-cadherin and the expression of TGF-β1 may play an important role in the development of GC.In lymph node metastasis,the phenomenon of mesenchymal-to-epithelial transition(MET)occurs.
epithelial-to-mesenchymal transition markers;mesenchymal-to-epithelialtransition;gastric cancer;lymph node metastasis
R735.2
A
0258-4646(2015)05-0438-05
崔苗苗(1984-),女,医师,硕士研究生.
戚基萍,E-mail:qijiping2003@163.com
2015-01-12
网络出版时间:
