微囊藻毒素在太湖白鲢体内的累积规律及其影响因素
2014-09-21贾军梅罗维吕永龙
贾军梅,罗维,吕永龙
1.中国科学院生态环境研究中心城市与区域生态国家重点实验室,北京 100085 2.中国科学院大学,北京 100049
微囊藻毒素在太湖白鲢体内的累积规律及其影响因素
贾军梅1,2,罗维1,*,吕永龙1
1.中国科学院生态环境研究中心城市与区域生态国家重点实验室,北京 100085 2.中国科学院大学,北京 100049
太湖蓝藻水华及其次级代谢产物微囊藻毒素(MCs)的生物累积对生态系统和人体健康造成严重威胁,已成为最近环境科学研究的热点。本研究从太湖的不同区域(梅梁湖、西部沿岸区、南部沿岸区和湖心区)采集不同体重和体长的白鲢,利用固相萃取方法提取、高效液相色谱-质谱联用仪测定了白鲢不同器官中MCs的3种异构体MC-RR、MC-YR及MC-LR的含量,结合不同湖区的相关水质指标分析了MCs在白鲢体内的累积规律及其影响因素。研究结果表明:白鲢不同器官MCs的含量由高到低为:肠壁>肾脏>肝脏>肌肉>心脏,且肠壁累积的MCs显著高于肾脏、肝脏、肌肉和心脏。MC-RR含量是白鲢各器官累积MCs的异构体的主体,约占MCs的60%。梅梁湖鲢鱼的肌肉、肾脏和心脏中MCs均高于西部沿岸区、南部沿岸区和湖心区。生物指标(体重和体长)是影响白鲢肾脏内MCs和MC-RR含量以及肠壁内MCs含量重要因素。太湖水质指标总磷(TP)、藻细胞数量、湖泊营养指数及环节动物数量尤其是TP对白鲢肝脏累积MCs产生明显影响,TP、总氮(TN)、铵态氮(NH4-N)、内梅罗指数和环节动物数量尤其是NH4-N对肠壁累积MCs产生明显影响。
微囊藻毒素;白鲢;累积;水质指标;器官
蓝藻水华通常指有害的藻类水华,蓝藻大量滋生产生有害的微囊藻毒素(MCs),干扰水化学过程、减少溶解氧、破坏食物链,对饮用水、灌溉、娱乐和渔业造成严重影响[1-4],对生态系统和人体健康构成潜在威胁。国内外研究表明,在自然环境中MCs能在一系列水生动物(包括鱼、虾、蟹、蚌体)体内累积[5-12],不仅在它们的内脏中累积大量MCs,在可食用的肌肉等部位也有累积[13]。MCs在鱼体内累积的靶器官为肝脏,但有研究报道肾脏累积的MCs高于肝脏[7]。肌肉中累积MCs虽然低于内脏,但有研究显示长期食用会给人体健康带来一定的风险[6,8,12],已有研究表明,MCs可以通过水产品进入人体造成肝损伤[13]。尽管淡水鱼仅占世界鱼产量的很小部分,但是其在中国水产总量中却占40%~50%。因此,研究MCs在淡水鱼体内的累积,对于水生态系统和人体健康风险的评估尤为重要。MCs在鱼体内累积可能受生物因子和环境因子共同作用的影响,目前很少有研究同时探讨生物和环境因子对MCs累积的影响。尽管少量研究试图从鱼的食性来探讨MCs在鱼体内累积,但研究结论并不一致。有研究表明浮游食性鱼累积MCs较高,而另有研究则报道浮游食性鱼累积较低[14-15]。因此,从生物和环境的角度出发,探讨鱼体内MCs的累积规律及其影响因素十分必要。
太湖蓝藻水华及其MCs污染和危害是我国湖泊环境研究关注的焦点和热点。据统计,2004—2008年期间太湖具有明确时间、地点的蓝藻水华发生次数高达414次,蓝藻水华总面积为72 890 km2[16]。另一方面,太湖也是我国重要的淡水渔业基地,而白鲢是太湖优势鱼种之一。本研究试图从生物和水质角度出发,探讨MCs在白鲢体内不同器官的累积及其影响因素,以期为MCs的生物累积和水产品及其健康风险提供理论依据与参考。
1 材料与方法(Materials and methods)
1.1 研究区域
太湖是我国第三大淡水湖泊,面积约为2 338km2,平均水深为1.9m,水资源总量为195亿m3,对周边地区的渔业和供水发挥重要作用。太湖分为9个区,分别为梅梁湖(MLB)、竺山湖(ZSB)、五里湖(WLB)、贡湖(GHB)、西部沿岸区(WC)、湖心区(LC)、胥湖(EC)、南部沿岸区(SC)以及东太湖(ETH)(图1)。各湖区均有不同程度的蓝藻水华发生,一般竺山湖、梅梁湖、西部沿岸区最为严重,出现带状或油漆状蓝藻频次很多;湖心区和贡湖发生中等程度,且呈颗粒状的蓝藻水华;东部湖区蓝藻较少,程度较轻。
1.2 采样点布设及样品采集
基于以往的研究,选择受蓝藻污染最为严重的梅梁湖,相对较严重的西部沿岸区和南部沿岸区以及占太湖的面积比例较大且水产品量较大的湖心区作为研究对象(图1)。2011年9月,除梅梁湖只采集到1条白鲢外,在每个采样区域各采集3条白鲢(设3个重复),记录采样点、体重、体长以及采样的编号。现场进行解剖,每条鱼均取肌肉、肝脏、肾脏、肠壁以及心脏,并用清水洗涤避免交叉污染,然后放入采样管中封口。按顺序放入-20℃冷冻箱中,运回实验室进行分析。共采集10条代表性鲢鱼的各种器官样品共计50个。
1.3 微囊藻毒素提取和测定方法
固相萃取法具有简单、快速、高效和提取所用的有机溶剂用量少等特点,因而提取动物体内的MCs多采这一种方法[13,17-22]。根据Xie等[23]报道和使用的鱼体MCs的提取方法和步骤,首先将样品冷冻干燥,然后用研钵磨碎。每个器官称取约0.4 g的样品,加入10 mL混合溶液,混合液成分为V (丁醇)∶V (甲醇)∶V (水)=1∶4∶15,在摇床上振荡24 h,在18000r·min-1离心机上离心30 min,析出上清液,将加入混合液振荡到析出上清液这一过程重复3遍。将3次上清液合并,加超纯水稀释至甲醇体积分数
小于15%,稀释液过0.5 g C18固相萃取小柱(预先用50 mL甲醇和50 mL超纯水润洗),然后用100 mL 20%甲醇(体积分数)洗脱杂质,用100 mL 90%甲醇(体积分数)收集目标物,洗提液在旋转蒸发仪上蒸发近干。用5 mL纯甲醇分3次溶解,过10 mL纯甲醇润洗过的硅胶小柱,然后用20 mL 70%甲醇(体积分数)洗脱,洗提液用旋转蒸发仪蒸发近干,用1 mL纯甲醇分3次溶解,转入2 mL棕色样品瓶待测。
第三步建立方程(3),检验加入中介变量后文化与旅游产业融合的直接效应(γ1是否显著,是否与β1γ7同号)。
采用安捷伦6460 QQQ高效液相色谱质谱联用仪(HPLC/MS/MS)、配有ODS色谱柱(Cosmosil 5C18-AR, 4.6 mm×150 mm, Nacalai, Japan)测定MCs的各种异构体的含量[24]。流动相A(甲醇),流动相B(体积分数为0.1%甲酸水溶液),流速为0.5 mL·min-1,柱温为40℃,梯度洗脱程序为(tmin):t0= 80%;t3= 65%;t5= 65%;t7= 35%;t8= 35%;t8.5= 80%;t12= 80%。
目标成分在色谱柱分离后直接进入正离子ESI模式下的三重四级杆质谱检测器。氮气用作两级干燥和屏蔽气体以及碰撞气体。离子源参数设置如下:气体温度(gas temperature) 350 ℃,气体流速(gas flow )11 L·min-1,喷雾器气体压力(nebulizer gas pressure)50 psi,毛细管电压(capillary voltage)5 kV。样品检测在多反应检测模式(MultiReactionMonitor, MRM)进行,delta EMV设为(+) 400。破碎电压(100~250 V)和碰撞能量(20~100 eV)根据每种化合物成分不同而设置(表1)。
仪器控制、数据处理和分析都在Masshunter软件上进行。3种异构体的标准曲线R2>99%,通过与标准样品的测试曲线对比获得MCs浓度(标准样品MC-LR,MC-RR和MC-YR,来自AXXORA,EUROPE-Switzerland)。
1.4 回收率实验
平行称取6份0.4 g冷冻干燥的肝脏,按照1 μg·g-1的标准加入0.4μg MC-RR、MC-YR和MC-LR的标准品,按照1.3中的提取方法和分析方法进行提取和检测MC-RR、MC-YR和MC-LR的含量。MC-RR、MC-YR和MC-LR的回收率分别为48.2%、93.3%和86.2%,相应的相对标准差RSD分别为9.6%、0.2%和0.5%。

表1 多反应检测模式(MRM)的参数设置Table 1 Parametersfor multireactionmonitor (MRM)
注:MC-YR、MC-LR和MC-RR为微囊藻毒素MC的3种异构体。
Note: MC-YR,MC-LR and MC-RR are three isomers of microcystins.
1.5 太湖水质数据的获取
根据所采集的鲢鱼的体重和体长确定大部分鲢鱼的年龄在1年左右,因此选择采样期(2011年)全年太湖不同分区的水质指标作为鲢鱼暴露的水环境参数。本研究所使用的2011年太湖分区水质和生物指标的数据来源于太湖流域管理局的长期监测数据[25]。
1.6 统计分析方法
由于实验数据近似服从正态分布,因而可利用SPSS 17.0对不同区域的白鲢的不同器官累积MCs进行方差分析(ANOVA),对潜在影响MCs累积的生物和非生物因素进行Pearson相关分析。判断差异或相关性显著的标准为p <0.05,极显著的标准为p <0.01。
2 结果(Results)
2.1 太湖各湖区水质
由太湖分区水质状况可知,西部沿岸区的化学需氧量(COD)、总磷(TP)、总氮(TN)、铵态氮(NH4-N)、藻细胞数量、湖水污染指标内梅罗指数(表征湖泊污染综合指标,内梅罗指数越高,污染越严重)和湖泊营养指数(表征湖泊富营养化程度的指标)以及环节动物数量均最高;梅梁湖水体的COD、蓝藻数量、湖泊营养指数和环节动物数量也较高;湖心区水体的COD、TP、藻细胞数量、湖泊营养指数和环节动物数量均最低,而南部沿岸区水体的TN、NH4-N、内梅罗指数和原生动物均最低(表2)。
2.2 MCs在白鲢体内累积
由白鲢各器官累积的MCs及异构体(MC-RR、MC-LR和MC-YR)含量(表3)可知,鲢鱼不同器官累积的MCs由高到低的顺序为:肠壁>肾脏>肝脏>肌肉>心脏。鲢鱼肠壁内MCs含量显著高于肾脏、肝脏、肌肉和心脏(p <0.05),而肾脏、肝脏、肌肉和心脏的MCs含量不存在显著差异(p> 0.05)。鲢鱼肌肉累积的各异构体含量为:MC-RR > MC-YR > MC-LR,而肝脏、肾脏和肠壁的异构体含量则均为:MC-RR > MC-LR > MC-YR,但心脏中只检测出MC-RR。总体上白鲢不同器官中累积的MC-RR比较高,占MCs的60%。
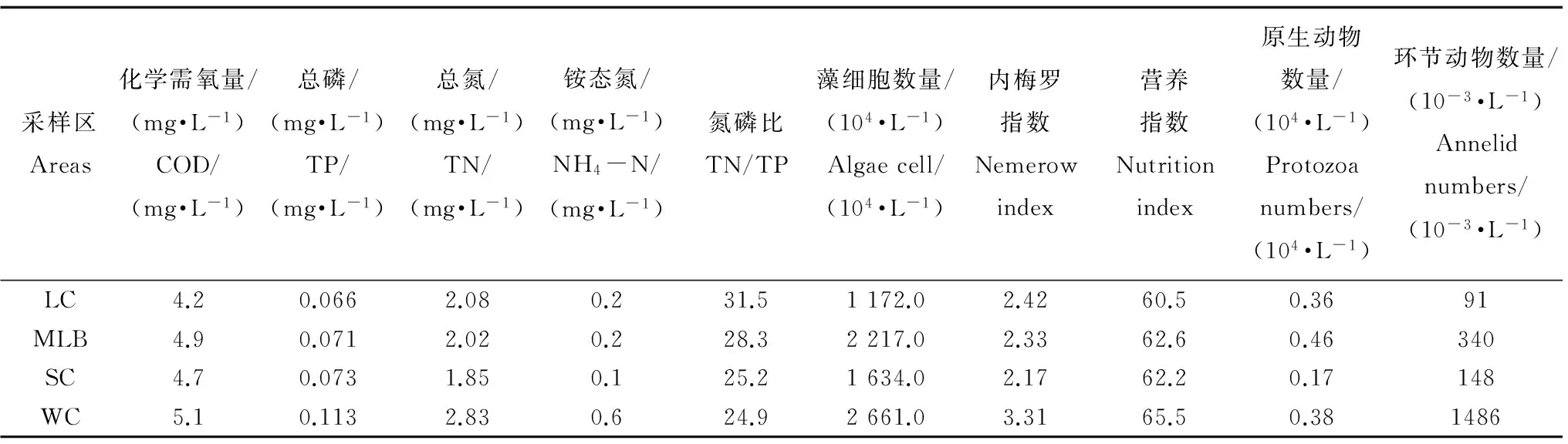
表2 2011年太湖采样区域水质指标Table 2 Water quality inde xin different sampling areas of Taihu Lake in 2011

表3 微囊藻毒素MC-RR、MC-YR、MC-LR在白鲢体内的累积(n =10)Table 3 Accumulation of microcystins (MC-RR, MC-YR, MC-LR) in silver carp(n =10)
注:*p <0.05;表中微囊藻毒素数据为所有样本的均值±标准偏差;n.d.表示未检出。
Note: *p <0.05; the concentration of microcystins were showed as mean value ± standard deviation; n.d., not detected.
鲢鱼体内不同器官累积的MCs数据表明(图2),梅梁湖的鲢鱼肌肉累积的MCs极显著高于南部沿岸区和西部沿岸区(p< 0.01),湖心区的鲢鱼肌肉中MCs同样显著高于南部沿岸区和西部沿岸区(p< 0.05)。不同区域白鲢肝脏中累积的MCs相差不大,仅西部沿岸区的鲢鱼肝脏中MCs显著高于湖心区(p< 0.05)。梅梁湖鲢鱼肾脏累积的MCs极显著地高于西部沿岸区、南部沿岸区和湖心区(p< 0.01),同时西部沿岸区的白鲢肾脏累积的MCs也极显著地高于南部沿岸区和湖心区(p< 0.01)。不同区域的鲢鱼肠壁中累积的MCs不存在显著的差异。梅梁湖鲢鱼心脏累积的MCs极显著地高于西部沿岸区、南部沿岸区和湖心区(p< 0.01),湖心区白鲢心脏中的MCs含量显著高于南部沿岸区(p< 0.05)。
2.3 影响MCs累积的因素
由白鲢不同器官内MCs及其异构体含量与白鲢的体重和体长的相关分析(表4)表明,肾脏中累积的MCs及MC-RR与体重、体长均呈显著正相关。肠壁中累积的MCs也与体重、体长显著相关。同一器官的异构体之间也呈显著的正相关,例如肌肉内的MC-LR与MC-RR呈显著正相关(P< 0.01)。此外,心脏中累积的MC-RR与肌肉和肾脏中累积的MCs及其异构体之间呈显著正相关。
MCs及其异构体在白鲢体内累积可能受太湖水质的影响。由相关分析(表5)表明,肝脏和肠壁中累积的MCs及异构体(MC-RR、MC-YR、MC-LR)与水质指标之间呈显著的相关关系。肠壁累积的MC-YR与TP、TN、NH4-N、内梅罗指数和环节动物数量呈显著相关、而与藻细胞数量并无显著相关。
3 讨论(Discussion)
本研究中白鲢肠壁累积的MCs最高,这与以往的研究结果一致,可能是由于肠壁能有效阻止MCs进入鱼体内,而本身能截留大量MCs的缘故[14,22-23]。白鲢肝脏累积的MCs较低,这与以往的研究不同[15,26-28],可能是由于本研究所采集的鱼样在累积-净化的动态过程中,净化处于优势[29],且胆汁在MCs净化过程中起到重要作用[30]。因此,肝脏中累积的MCs首先被净化排除体外,因而肝脏中MCs浓度较低。3种异构体中MC-RR所占比例最高,占MCs的60%,可能是由于鱼体首先净化毒性较高的MC-LR和MC-YR,然后净化毒性最小的MC-RR[31]。由于心脏累积MCs较少,因而心脏中只检测到MC-RR,其他2种异构体的含量均未检测到。对比不同区域采集的白鲢发现,梅梁湖鲢鱼的肌肉、肾脏和心脏中MCs均较高,可能是由于梅梁湖的蓝藻数量较大[25]、且相对于其他3个区域而言,梅梁湖比较封闭、蓝藻容易聚集所致。

图2 太湖不同区域白鲢的不同器官(M 肌肉,L 肝脏,K 肾脏,I 肠壁,H 心脏)中MCs的累积Fig. 2 Accumulation of MCs in different organs (M: muscle; L: liver; K: kidney; I: intestine wall; H: heart) of silver carp collected from different areas of Taihu Lake

表5 白鲢不同器官内MCs(MC-RR、MC-YR、MC-LR)及其异构体的含量与水质指标的相关关系
Table 5 Correlations between MCs (MC-RR, MC-YR, MC-LR) in
different organs of silver carp and water quality of Taihu Lake

Pearson相关Pearsoncorrelation化学需氧量COD/(mg·L-1)总磷TP/(mg·L-1)总氮TN/(mg·L-1)铵态氮NH4-N/(mg·L-1)氮磷比TN/TP藻细胞数量Algaecell(104·L-1)内梅罗指数Nemerowindex营养指数Nutritionindex原生动物数量Protozoanumbers/(104·L-1)环节动物数量Annelidnumbers/(10-3·L-1)MCs(肌肉Muscle)-0.31-0.64-0.45-0.500.74-0.23-0.48-0.530.65-0.52MC-RR(肌肉Muscle)0.610.910.750.79-0.810.610.770.84-0.280.83MC-YR(肌肉Muscle)-0.36-0.68-0.48-0.540.77-0.29-0.51-0.580.62-0.56MC-LR(肌肉Muscle)0.048-0.48-0.38-0.440.420.075-0.41-0.260.66-0.35MCs(肝脏Liver)0.940.890.800.78-0.680.99*0.790.98*0.370.92MC-RR(肝脏Liver)0.790.17-0.030-0.061-0.550.68-0.0430.480.210.20MC-YR(肝脏Liver)0.940.730.660.63-0.570.99*0.650.880.520.79MC-LR(肝脏Liver)0.790.99**0.930.93-0.670.860.930.97*0.210.99**MCs(肾脏Kidney)0.46-0.0440.006-0.0590.0890.51-0.0260.190.780.082MC-RR(肾脏Kidney)0.570.0560.0680.006-0.0460.610.0380.310.730.17MC-YR(肾脏Kidney)0.29-0.23-0.16-0.220.240.33-0.19-0.0020.75-0.10MC-LR(肾脏Kidney)0.12-0.21-0.031-0.100.470.24-0.066-0.0730.90-0.063MCs(肠壁Intestinewall)-0.350.350.430.470.012-0.260.450.045-0.370.28MC-RR(肠壁Intestinewall)-0.180.400.360.42-0.28-0.180.390.16-0.620.29MC-YR(肠壁Intestinewall)0.610.98*0.99*0.99**-0.500.730.99*0.870.240.98*MC-LR(肠壁Intestinewall)-0.85-0.33-0.041-0.0340.81-0.69-0.041-0.600.10-0.30MC-RR(心脏Heart)0.093-0.41-0.30-0.360.410.14-0.33-0.200.72-0.28
注:*p <0.05,**p< 0.01;MCs(MC-RR、MC-YR、MC-LR)单位为ng·g-1, dw。
Note:*p <0.05, **p< 0.01; the unit of MCs (MC-RR, MC-YR, MC-LR) is ng·g-1, dw.
由白鲢体内累积MCs和体重及体长的相关关系可知,体重和体长是影响白鲢肾脏累积MCs和MC-LR、肠壁累积MCs的重要因素。另外,同一器官中MCs的异构体之间也呈显著的正相关,例如肌肉内的MC-LR与MC-RR呈显著正相关(p< 0.01),由此说明同一器官累积MCs的各异构体容易相互影响。此外,心脏中累积的MC-RR与肌肉和肾脏中累积的MCs及其异构体之间呈显著正相关,由此解释了白鲢肌肉、肾脏和心脏累积MCs在同一个区域均为最高的原因。
由白鲢各器官累积MCs和水质指标的相关关系可知,肝脏累积MCs及异构体(MC-RR、MC-YR、MC-LR)容易受到TP、藻细胞数量、湖泊营养指数及环节动物数量的影响,可能是由于TP、湖泊营养指数及环节动物数量会影响藻细胞数量,进而间接影响肝脏中MCs及异构体的累积。肠壁累积的MC-YR与TP、TN、NH4-N、内梅罗指数和环节动物呈显著相关,而与藻细胞数量并无显著相关,其原因有待进一步研究。
[1] Wiegand C, Pflugmacher S. Ecotoxi cological effects of selected cyanobacterial secondary metabolites a short review [J]. Toxicology and Applied Pharmacology, 2005, 203(3): 201-218
[2] Otten T G, Xu H, Qin B, et al. Spatiotemporal patterns and ecophysiology of toxigenic microcystis bloomsin Lake Taihu, China: Implications for water quality management [J]. Environmental Science & Technology, 2012, 46(6): 3480-3488
[3] Blaha L, Babica P, Marsalek B. Toxins produced in cyanobacterial water blooms -Toxicity and risks [J]. Interdisciplinary Toxicology, 2009, 2(2): 36-41
[4] Paskerová H, Hilscherová K, Bláha L. Oxidative stress and detoxification biomarker responses in aquatic freshwater vertebrates exposed to microcystins and cyanobacterial biomass [J]. Environmental Science and Pollution Research, 2012, 19(6): 2024-2037
[5] Mekebri A, Blondina G J, Crane D B. Method validation of microcystins in water and tissue by enhanced liquid chromatography tandem mass spectrometry [J]. Journal of Chromatography A, 2009, 1216(15): 3147-3155
[6] Magalhães V F, Marinho M M, Domingos P, et al. Microcystins (cyanobacteria hepatotoxins) bioaccumulation in fish and crustaceans from Sepetiba Bay (Brasil, RJ) [J]. Toxicon, 2003, 42(3): 289-295
[7] Mohamed ZA, Carmichael W W, Hussein A A. Estimation of microcystins in the freshwater fish Oreochromis niloticus in an Egyptian fish farm containing a Microcystis bloom[J]. Environmental Toxicology, 2003, 18(2): 137-141
[8] Chen J, Xie P. Tissue distributions and seasonal dynamics of the hepatotoxic microcystins-LR and -RR in two freshwater shrimps, Palaemon modestus and Macrobrachium nipponensis , from a large shallow, eutrophic lake of the subtropical China [J]. Toxicon, 2005, 45(5):615-625
[9] Zurawell R W, Kotak B G, Prepas E E. Influence of lake trophic status on the occurrence of microcystin-LR in the tissue of pulmonate snails [J]. Freshwater Biology, 1999, 42(4): 707-718
[10] Zhang J Q, Wang Z, Song Z Y, et al. Bioaccumulation of microcystins in two freshwater gastropods from a cyanobacteria-bloom plateau lake, Lake Dianchi [J]. Environmental Pollution, 2012, 164: 227-234
[11] Williams D E, Dawe S C, Kent M L, et al. Bioaccumulation and clearance of microcystins from salt water, mussels, Mytilu sedulis, and in vivo evidence for covalently bound microcystins in mussel tissues [J]. Toxicon, 1997, 35(11): 1617-1625
[12] Chen J, Xie P. Seasonal dynamics of the hepatotoxic microcystins in various organs of four freshwater bivalves from the large eutrophic Lake Taihu of subtropical China and the risk to human consumption [J]. Environmental Toxicology, 2005, 20(6): 572-584
[13] Chen J, Xie P, Li L, et al. First identification of the hepatotoxic microcystinsin the serum of a chronically exposed human population together with indication of hepatocellular damage [J]. Toxicological Sciences, 2009, 108(1): 81-89
[14] Zhang D W, Xie P, Liu Y Q, et al. Transfer, distribution and bioaccumulation of microcystins in the aquatic food web in Lake Taihu, China, with potential risks to human health [J]. Science of the Total Environment, 2009, 407(7): 2191-2199
[15] Xie L Q, Xie P, Guo L G, et al. Organ distribution and bioaccumulation of microcystins in freshwater fish at different trophic levels from the eutrophic Lake Chaohu, China [J]. Environmental Toxicology, 2005, 20(3): 293-300
[16] 刘聚涛, 杨永生, 高俊峰, 等. 太湖蓝藻水华分级及其时空变化[J]. 长江流域资源与环境, 2011(2): 156-160
Liu J T, Yang Y S, Gao J F, et al. Characteristics of cuanobacteria bloom grading and its temporal and spatial variation in Taihu Lake [J]. Resources and Environment in the Yangtze Basin, 2011(2): 156-160 (in Chinese)
[17] Zhang D, Xie P, Liu Y, et al. Bioaccumulation of the hepatotoxic microcystins in various organs of a freshwater snail from a subtropical Chinese lake, Taihu Lake, with dense toxic microcystis blooms [J]. Environmental Toxicology and Chemistry, 2007, 26(1): 171-176
[18] Chen J, Xie P. Accumulation of hepatotoxic microcystins in freshwater mussels, aquatic insect larvae and oligochaetes in a large, shallow eutrophic lake (Lake Chaohu) of subtropical China [J]. Fresenius Environmental Bulletin, 2008, 17(7A): 849-854
[19] Zhang D, Xie P, Chen J, et al. Determination of microcystin-LR and its metabolites in snail (Bellamyaaeruginosa), shrimp (Macrobrachium nipponensis) and silver carp (Hypophthalmichthys molitrix) from Lake Taihu, China [J]. Chemosphere, 2009, 76(7): 974-981
[20] Wang Q, Xie P, Chen J, et al. Distribution of microcystins in various organs (heart, liver, intestine, gonad, brain, kidney and lung) of Wistar rat via intravenous injection [J]. Toxicon, 2008, 52(6): 721-772
[21] Zhang D, Xie P, Chen J. Effects of temperature on the stability of microcystins in muscle of fish and its consequences for food safety [J]. Bulletin of Environmental Contamination and Toxicology, 2010, 84(2): 202-207
[22] Chen J, Xie P, Zhang D, et al. In situ studies on the distribution patterns and dynamics of microcystins in a biomanipulation fish -Bighead carp (Aristichthysnobilis ) [J]. Environmental Pollution, 2007, 147(1): 150-157
[23] Xie LQ, Xie P, Ozawa K, et al. Dynamics of microcystins-LR and -RR in the phytoplanktivorous silver carp in a sub-chronic toxicity experiment [J]. Environmental Pollution, 2004, 127(3): 431-439
[24] 李建中. Agilent1260U HPLC/6460QQQ用于微囊藻毒素的检测[J]. 环境化学, 2011, 30(3): 731-733
Li J Z. Agilent 1260 U HPLC/6460QQQ used for the detection of microcystins [J]. Environmental Chemistry, 2011, 30(3): 731-733 (in Chinese)
[25] 水利部太湖流域管理局. 太湖健康状况报告2011[R]. 上海: 水利部太湖流域管理局, 2012
[26] Malbrouck C, Trausch G, Devos P, et al. Hepatic accumulation and effects of microcystin-LR on juvenile goldfish Carassius auratus L. [J]. Comparative Biochemistry and Physiology C-Toxicology & Pharmacology, 2003; 135(1): 39-48
[27] Li X Y, Chung I K, Kim J I, et al. Subchronic oral toxicity of microcystin in common carp (Cyprinusc arpio L.) exposed to microcystisunder laboratory conditions [J]. Toxicon, 2004, 44(8): 821-827
[28] Kankaanpää H T, Holliday J, Schröder H, et al. Cyanobacteria and prawn farming in northern New South Wales, Australia-Acase study on cyanobacteria diversity and hepatotoxin bioaccumulation [J]. Toxicology and Applied Pharmacology, 2005, 203(3): 243-256
[29] Ibelings B W, Chorus I. Accumulation of cyanobacterial toxins in freshwater "seafood" and its consequences for public health: A review [J]. Environmental Pollution, 2007, 150(1): 177-192
[30] Tencalla F, Dietrich D. Biochemical characterization of microcystin toxicity in rainbow trout (Oncorhynchus mykiss) [J]. Toxicon, 1997, 35(4): 583-595
[31] Gupta N, Pant S C, Vijayaraghavan R, et al. Comparative toxicity evaluation of cyanobacterial cyclic peptide toxin microcystin variants (LR, RR, YR) in mice [J]. Toxicology, 2003, 188(2-3): 285-296
◆
AccumulationofMicrocystinsinSilverCarpfromTaihuLakeanditsInfluencingFactors
JiaJunmei1,2, Luo Wei1,*, LuYonglong1
1. State Key Laboratory of Urban and Regional Ecology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China 2. University of Chinese Academy of Sciences, Beijing 100049, China
31 May 2013accepted26 July 2013
Cyanobacterial blooms took place in Taihu Lake every year and were widely concerned. Microcystins (MCs), the metabolites of cyanobacteria, could have potential adverse effects on ecosystems and human health. In order to examine accumulations of MCs in fish from different parts of Taihu Lake and its influencing factors, silver carp was sampled and MCs (MC-LR, MC-YR and MC-RR) were analyzed by high performance liquid chromatography tandem mass spectrometric. The results showed that MCs in different organs of silver carp was of such order: intestine wall > kidney > liver > muscle >heart, and MCs concentration in intestine wall was significantly higher than those in kidney, liver, muscle and heart. MC-RR was the main isomer of MCs which accounted for 60% of MCs. Concentrations of MCs in muscle, kidney and heart of silver carp from the Meiliang Bay were higher than those from West Coast, South Coast and Lake Center of Taihu Lake. Based onthe significant relationships between body length, weight and MCs concentrations in organs of silver carp, it wasindicated that body length and weight were important factors that influenced accumulation of MCs and MC-RR in kidney and that of MCs in intestine wall.The water quality index of Taihu Lake including TP, algae cell number, nutrition index and number of annelid, especially TP, played important roles in MCs accumulation in liver of silver carp whilethe water quality index including TP, TN, NH4-N, Nemerow index and number of annelid, especially NH4-N also played important roles in MCs accumulation in intestine wall of silver carp.
microcystins; silver carp; accumulation; water quality index; organs
国家重点基础研究计划973计划课题(2008CB418106);国家自然科学基金资助项目(41271502;C031001);科技部科技基础性工作专项课题(2013FY111100)
贾军梅(1988-),女,硕士,研究方向为区域生态风险,E-mail:jiadao_mei@126.com;
*通讯作者(Corresponding author),E-mail:luow@rcees.ac.cn
10.7524/AJE.1673-5897.20130531001
贾军梅,罗维,吕永龙. 微囊藻毒素在太湖白鲢体内累积及其影响因素[J]. 生态毒理学报, 2014, 9(2): 382-390
Jia J M, Luo W, Lv Y L. Accumulation of microcystins in silver carp from the Taihu Lake and its influencing factors [J]. Asian Journal of Ecotoxicology, 2014, 9(2): 382-390 (in Chinese)
2013-05-31录用日期2013-07-26
1673-5897(2014)2-382-09
X171.5
A
罗维(1972—),男,博士,副研究员,主要研究方向为城市与区域污染过程及生态风险评价,景观生态毒理,有机废物堆肥与资源化利用及生态风险。
