Modified arteriaIization of orthotopic Iiver transpIantation in a mouse modeI
2010-07-05
Chongqing, China
Modified arteriaIization of orthotopic Iiver transpIantation in a mouse modeI
De-Rong Huang, Zhong-Jun Wu and Yu Zhu
Chongqing, China
BACKGROUND: With the establishment of genetically modified and gene knock-out models, the mouse has become an important animal model for liver transplantation. We examined hepatic rearterialization after liver transplantation in a mouse model.
METHODS: Orthotopic liver transplantation was performed in 70 mice and sham-operation was performed in a control group of 40 mice. Based on the "two-cuff" method, a continuous suture approach was applied to the suprahepatic inferior vena cava and a cuff approach to the portal vein and the infrahepatic inferior vena cava. A biliary stent was inserted into the bile duct. The hepatic artery was reconstructed with end-to-side anastomosis. The survival rate of recipients was monitored at 24 hours, one week, and one month after the operation. Liver function and morphology were evaluated one month postoperatively.
RESULTS: Postoperative survival rates were 94.3% at 24 hours, 91.4% at one week, and 85.7% at one month. No significant difference was seen between the experimental and control groups in liver function. The hepatic tissue preserved normal structure.
CONCLUSION: Owing to its high survival rate and stability, this surgical approach is ideal for establishing an orthotopic liver transplantation mouse model with hepatic artery reconstruction.
(Hepatobiliary Pancreat Dis Int 2010; 9: 264-268)
liver transplantation; model, animal; hepatic rearterialization
Introduction
The hepatic arterial system supplies most of the blood to the bile duct in a transplanted liver and anastomotic stoma.[1]An adequate blood supply is essential to the success of the transplant. Poor blood supply by the hepatic artery in the early stage after liver transplantation is an independent risk factor for an ischemic-type biliary lesion and lethal biliary complications. Insufficient blood supply dramatically increases the liver re-transplantation rate and mortality rate after surgery.[2,3]The formation of hepatic artery thrombosis without collateral compensation following liver transplantation results in a mortality rate of 50%[4]and is a significant cause of graft loss.[5]In addition, hepatic rearterialization following liver transplantation in mice reduces hepatic tissue injury, protects the bile duct, and improves the long-term survival rate.[6-8]Most of the transplants performed do not restore adequate blood flow and have mixed results. This study aimed to explore the outcomes after orthotopic liver transplantation using hepatic arterial reconstruction in mice.
Methods
Animals and equipment
One hundred and eighty healthy C57 inbred male mice (purchased from the Animal Center of Chongqing University of Medical Sciences, China) of clean stage, aged 8-12 weeks and weighing 23-28 g, were randomly assigned to an experimental group (n=140) or a control group (n=40). The 140 mice in the experimental group were classified as either donors or recipients. The weight of a recipient was equal to or slightly more than (no more than 5 g) that of the donor. All mice were fasted for 12 hours before operation, without water deprivation. The equipment consisted of a double binocular microscope (Shanghai Medical Instrument Factory, China), a set of microsurgical instruments, venous cannulas for the inferior vena cava and portal vein (made by stretching 7F and 5F interventional catheters), biliarystents made by stretching disposable epidural catheters (length ≤4 mm, inner diameter 0.28 mm, outer diameter 0.61 mm), 10-0 and 11-0 suture needles with thread, self-made retractor, hemostatic forceps, tissue scissors, miniature aorta clamp, and routine surgical equipment for this procedure as previously reported.
Experimental group
Donor liver procurement
Ether inhalation was used to anesthetize the mice. A transverse abdominal incision was made to the xiphisternum to expose the liver. The perihepatic ligament, the portal vein (PV), the infrahepatic inferior vena cava (IHVC), the pyloric vein, and the right suprarenal vein were blunt dissected. After this, the duodenum adjoining the common bile duct was beveled and a biliary stent was inserted and secured. The abdominal aorta above the coeliac trunk was blunt dissected and a No. 1 thread was placed on the opposite portion of the abdominal aorta. Then, 0.4 ml of physiological saline containing 100 U heparin sodium was injected into the IHVC, and a preset thread was used to ligate the coeliac trunk in order to block the abdominal aorta and quickly puncture its lower section. Then, a syringe pump continuously pumped 2-3 ml 4 ℃ lactated Ringer's solution at 10-30 ml/h, and the diaphragm was quickly sheared off and the supra-phrenic inferior vena cava was cut off, so that the perfusate could flow out. Physiological saline (4 ℃) was continuously showered on the liver to keep it at a low temperature. After that, the cystic duct was ligated and the gallbladder was removed along the gallbladder bed and close to the left renal vein, while the inferior vena cava above the left renal vein was excised. After the left renal vein and left suprarenal vein were ligated and separated, the inferior vena cava cuff was installed using the "two-cuff method".[9]The splenic artery and left gastric artery were subsequently ligated and blunt dissected along the coeliac trunk, and then isolated from the abdominal aorta. The superior mesenteric artery, right renal artery, left renal artery and lumbar arteries were separated and ligated successively, and the abdominal aorta was transversely cut and excised 2 cm inferior to the left renal artery. When the perfused liver uniformly turned yellowish gray, the portal vein was separated above the splenic vein, and the portal vein cuff was installed. Afterwards, the common bile duct was transected inferior to the biliary stent, and the suprahepatic vena cava (SHVC) was excised near its attachment to the diaphragm. The donor liver was removed and placed in 4 ℃ lactated Ringer's solution and stored in a refrigerator at 4 ℃.
Recipient operation
Before the operation, the abdominal cavity of the recipient was injected with 1-1.5 mg ketamine combined with ether for anesthesia. The liver was separated in a manner similar to that of the donor. The PV and IHVC were blunt dissected at the level of the right renal vein and pyloric vein, respectively, and the common bile duct and hepatic artery proper were ligated at the bifurcation of left and right hepatic ducts. A No. 1 thread was present at the posterior side of the SHVC. Hemostatic forceps were used to ensure no blood entered the PV and IHVC. During the anhepatic phase, 0.2-0.3 ml of physiological saline was injected into the bifurcation of the portal vein to wash out blood from the liver, then the preset thread was tightened. Following this, an arterial clamp was placed in close proximity to the superior portion of the diaphragm to block the IHVC. The SHVC and IHVC were transected near the liver, and the portal vein was excised at the bifurcation. Finally, the recipient liver was removed and the donor liver was placed orthotopically.
From the left corner, the SHVC was sutured by a continuously evaginating method, and bubbles in the vein were completely removed before closing. The portal fissure was exposed and the portal vein was anastomosed by the cuff method. The hemostatic forceps that were placed on the PV and SHVC were loosened at the end of the anhepatic phase. The quickly reperfused liver turned red and the IHVC was anastomosed in the same way. A segment of the abdominal aorta was separated below the left renal artery and was blocked at the proximal and distal end of the artery clamp. An opening was made on this section of the recipient's abdominal aorta in order to anastomose the abdominal aorta of the donor by "end-to-side anastomosis". When the artery clamps at the proximal and distal ends were loosened, the hepatic artery was quickly filled with blood. The donor biliary stent was placed into the recipient common bile duct and secured. The greater omentum was covered without active hemorrhage, and the blood in the gastrointestinal tract cycled well. Then, the abdominal cavity was washed with metronidazole and sutured with No. 1 thread (Fig. 1).
Intra-operative and post-operative observations
During the operation, the duration of surgery for the donor and recipient was recorded, as well as the duration of the anhepatic phase. After the operation, reflection, diet, sleep, defecation and irritability, were observed. The mortalities of the mice at 24 hours, one week, and one month were recorded. The dead mice were dissected to explore their causes of death.
Postoperative treatments
After operation, the mice were kept warm for 3 hours by incandescent light. Penicillin (800 000 units) was given via intramuscular injection but immunosuppressant agents were not used. After the operation, the mice were fed separately, without food and water deprivation. One month after the operation, 10 mice were sacrificed for the measurement of serum alanine aminotransferase (ALT) and aspartic acid aminotransferase (AST). Hepatic tissue was collected and preserved in paraformaldehyde. Paraffin sections were stained with hematoxylin and eosin for pathohistological examination.
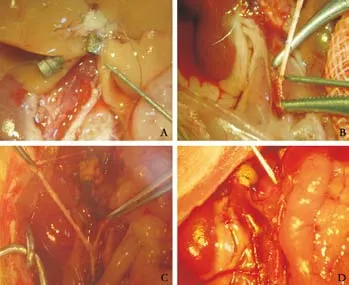
Fig. 1. A: Hepatic arteries of a donor mouse liver (original magnification ×10); B: Anastomosis of the recipient hepatic artery (original magnification ×20); C: After anastomosis of the hepatic artery, prior to blood flow (original magnification ×10); D: After reconstruction of hepatic arteries, blood filled the hepatic artery (original magnification ×10).
Control group
The 40 mice in the control group underwent a sham-operation by which the abdomen was opened and sutured without further treatment. The mice were fed separately and sacrificed one month later for testing of ALT and AST.
Statistical analysis
Data were expressed as mean±SD. They were analyzed using SPSS software version 13.0 (SPSS Inc., Chicago, IL, USA). Student'sttest was used to compare the ALT and AST data of the experimental and control groups. APvalue less than 0.05 was considered statistically significant.
Results
Orthotopic liver transplantation was performed in 70 mice. The warm ischemia time was less than 30 seconds and the average cold reserve time was 40 minutes. The average duration of the donor surgery was 45±2 minutes and that of the recipient surgery was 55±3 minutes. The mean anhepatic phase was 15±2 minutes and the time for the anastomosis of the hepatic artery was 6±1 minutes. The survival rate of mice was 94.3% (66/70) at 24 hours, 91.4% (64/70) at one week, and 85.7% (60/70) at one month. Of the 4 mice that died at 24 hours, 2 died from anastomotic leakage of the SHVC, 1 from portal vein cuff failure, and 1 from anastomotic leakage of the hepatic artery. Of the 2 mice that died in the period from 24 hours to one week, 1 died from thrombosis of the hepatic artery and 1 from abscessation of the abdomen. Of the 4 mice that died in the period from one week to one month, 2 died from a biliary fistula, 1 from infection, and 1 from an unidentified cause. These results were confirmed by autopsy.
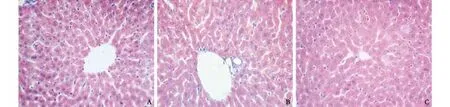
Fig. 2. A: One month after the surgery, the hepatic tissue was well-structured and the liver cells were normal in size (HE, original magnification ×400); B: No ductule proliferation was found in the portal area and no cholestasis was present (HE, original magnification ×400); C: Hepatic tissue section one month after sham-operation (HE, original magnification ×400).
Liver function results
One month after surgery, the results of the liver function tests were 39.2±2.09 U/L for ALT and 75.6± 3.24 U/L for AST in the experimental group. The values in the control group were 37.6±2.06 U/L for ALT, and 72.4±1.65 U/L for AST. The difference between the experimental and control groups was not statistically significant.
Morphology of hepatic grafts
One month after surgery, the hepatic tissue was wellstructured, the hepatic lobule demonstrated structural integrity, and the liver cells were of normal size and were equally stained with occasional small fibroblastic proliferation. A few lymphocytes infiltrated the portal area, and ductule proliferation was not visible, without cholestasis (Fig. 2).
Discussion
Stent, cuff and suture approaches have been adopted in the arterialization of orthotopic liver transplantation in rat models.[10,11]However, it should be noted that anastomosis by stent or cuff is not suitable for the thin hepatic artery in mouse. The suture method is very difficult and requires a skilled operator. The technical difficulties and precautions in mouse are discussed below.
根据《EPO专利审查指南》的上述规定可知,单纯的机器学习算法以及计算模型本身仍然被排除在保护客体之外;一旦将人工智能相关的算法和模型应用于特定的技术领域以解决技术问题,则有望通过可专利性的审查。EPO的最新指南修改表达了对人工智能技术的特别关注,也通过增加专门章节的形式作了细化规定。从实践来看,EPO关于人工智能领域专利保护客体的审查标准和判断原则与我国现阶段的专利审查实践并无不同。
In this study, the coeliac trunk with the hepatic artery was removed, along with the entire abdominal aorta below the coeliac trunk. Then, the donor abdominal aorta and recipient abdominal aorta were end-to-side anastomosed, thus increasing the length of the donor vessel, avoiding the end-to-end anastomosis of thin hepatic arteries, and reducing the difficulty of the procedure. This procedure also increased the diameter of anastomosed vessels and reduced the chances of surgical complications. The incidence of hepatic artery thrombosis can be reduced by the introduction of large caliber artery microsurgical techniques under a high resolution microscope or loupe, thus reducing postoperative biliary complications.[12]This method was used by Tian et al.[13]In this procedure, the donor superior mesenteric artery, the coeliac trunk, and the abdominal aorta medial to these structures were taken out as a whole. Then an anastomosis was made between the donor superior mesenteric artery and the recipient abdominal aorta. This method was used in our study, however, as the donor vessels were quite short and the anastomosis between the recipient left renal artery and the abdominal aorta was tensioned by the hepatic artery, reducing the hepatic arterial flow.
When suturing, the distance between stitches should not be too wide. The stent could be implanted into the abdominal aorta and further inserted into the recipient abdominal aorta, thus preventing injury to the side of the vessel wall and vascular stenosis after anastomosis. After suturing, the stent could be removed from the other end of the donor abdominal aorta. The air in this region of the abdominal aorta was expelled before ligation.
Slight anastomotic hemorrhage is not uncommon when the blood refills the vessels. In this case, a cotton swab should be used to stop bleeding by maintaining pressure on the vessel until the bleeding subsides. It is important that the hepatic artery blood flow be evaluated after refilling with blood in order to prevent torsion of the vessel, which influences hepatic arterial flow.
Finally, variations in the hepatic artery should be assessed. Variations frequently occur in the hepatic vessels and may complicate hepatic artery reconstruction. The integrity of the donor hepatic artery contributes to good recovery of liver function and lowers rate of postoperative biliary complications.[14]In this study, the variations in the hepatic artery were mainly seen in the superior mesenteric artery and left gastric artery. Hence, the whole hepatic artery should be taken out. Rarely the abdominal aorta demonstrates ectopic changes. In this case it is better not to use the donor liver, since if the hepatic artery is too thin the chance of successful reconstruction is low. On very rare occasions, the hepatic artery enters the liver from the ramus communicans of the esophagus and liver. In such a case it is better to abandon the donor liver.
In summary, the orthotopic liver transplantation model in mice using hepatic artery reconstruction is a difficult operation that requires a highly skilled operator to perform the operation under a microscope. The microoperation procedure in our hepatic artery reconstruction was successfully performed to ensure the stability of the model. When Qian et al[15]performed non-arterialized orthotopic mouse liver transplantation, three negative characteristics of the portal vein were discovered through the pathological examination of hepatic tissues. They found moderate fibrosis, ductule proliferation, and mild lymphocytic infiltration. However, Steger et al[16]did not find similar characteristics when using an arterialized orthotopic liver transplantation model in mice, and the ductule proliferation correlated with the hepatic artery reconstruction. Most of researchers have not included hepatic rearterialization because its omission is unrelated to the typical sideeffects seen after the procedure.[17-20]Although we have highlighted the importance of rearterialization in the mouse model, further studies are still needed.
Funding: This study was supported by a grant from the National Natural Science Foundation of China (No. 30772055).
Ethical approval: Not needed.
Contributors: HDR proposed the study, wrote the first draft, and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. HDR is the guarantor.
Competing interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Xu X, Zheng SS. Variations and reconstruction of the hepatic artery in liver transplantation. Hepatobiliary Pancreat Dis Int 2006;5:170-172.
2 Nishida S, Nakamura N, Kadono J, Komokata T, Sakata R, Madariaga JR, et al. Intrahepatic biliary strictures after liver transplantation. J Hepatobiliary Pancreat Surg 2006;13:511-516.
3 Vivarelli M, Cucchetti A, La Barba G, Bellusci R, De Vivo A, Nardo B, et al. Ischemic arterial complications after liver transplantation in the adult: multivariate analysis of risk factors. Arch Surg 2004;139:1069-1074.
4 Shen XD, Gao F, Ke B, Zhai Y, Lassman CR, Tsuchihashi S, et al. Inflammatory responses in a new mouse model of prolonged hepatic cold ischemia followed by arterialized orthotopic liver transplantation. Liver Transpl 2005;11:1273-1281.
5 Zheng SS, Yu ZY, Liang TB, Wang WL, Shen Y, Zhang M, et al. Prevention and treatment of hepatic artery thrombosis after liver transplantation. Hepatobiliary Pancreat Dis Int 2004;3:21-25.
6 Gao W, Lemasters JJ, Thurman RG. Development of a new method for hepatic rearterialization in rat orthotopic liver transplantation. Reduction of liver injury and improvement of surgical outcome by arterialization. Transplantation 1993;56: 19-24.
7 Steffen R, Ferguson DM, Krom RA. A new method for orthotopic rat liver transplantation with arterial cuff anastomosis to the recipient common hepatic artery. Transplantation 1989;48:166-168.
8 Engemann R, Ulrichs K, Thiede A, Müller-Ruchholtz W, Hamelmann H. Value of a physiological liver transplant model in rats. Induction of specific graft tolerance in a fully allogeneic strain combination. Transplantation 1982;33:566-568.
9 Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation 1979;28:47-50.
10 Zhang LD, Wang SG, Li XW, Xiong Y, Zhang YJ, Dong JH. The influence of cold preservation on the microcirculation of intrahepatic bile duct after liver transplantation. Zhonghua Wai Ke Za Zhi 2007;45:339-343.
11 Ma Y, Wang GD, Guo ZY, Guo ZG, He XS, Chen GH. Surgical techniques of arterialized orthotopic liver transplantation in rats. Chin Med J (Engl) 2007;120:1914-1917.
12 Yan S, Zhang QY, Yu YS, He JJ, Wang WL, Zhang M, et al. Microsurgical reconstruction of hepatic artery in living donor liver transplantation: experiences and lessons. Hepatobiliary Pancreat Dis Int 2009;8:575-580.
13 Tian Y, Rüdiger HA, Jochum W, Clavien PA. Comparison of arterialized and nonarterialized orthotopic liver transplantation in mice: prowess or relevant model? Transplantation 2002;74: 1242-1246.
14 Li GM, Zhu JY, Huang L, Wang D, Gao J, Leng XS. The analysis for the variation of hepatic arteries of the donor livers. Zhonghua Wai Ke Za Zhi 2005;43:447-449.
15 Qian SG, Fung JJ, Demetris AV, Ildstad ST, Starzl TE. Orthotopic liver transplantation in the mouse. Transplantation 1991;52:562-564.
16 Steger U, Sawitzki B, Gassel AM, Gassel HJ, Wood KJ. Impact of hepatic rearterialization on reperfusion injury and outcome after mouse liver transplantation. Transplantation 2003;76:327-332.
17 Sriwatanawongsa V, Davies HS, Calne RY. The essential roles of parenchymal tissues and passenger leukocytes in the tolerance induced by liver grafting in rats. Nat Med 1995;1:428-432.
18 Morimoto Y, Kamiike W, Nishida T, Hatanaka N, Shimizu S, Huang TP, et al. Improvement of rat liver graft function by insulin administration to donor. Gastroenterology 1996;111: 1071-1080.
19 Kamada N, Sumimoto R, Kaneda K. The value of hepatic artery reconstruction as a technique in rat liver transplantation. Surgery 1992;111:195-200.
20 Ramos E, Closa D, Hotter G, Roselló-Catafau J, Gelpi E, Fernandez-Cruz L. The impact of arterialization on prostanoid generation after liver transplantation in the rat. Transplantation 1994;58:140-144.
Received August 11, 2009
Accepted after revision January 26, 2010
Author Affiliations: Department of Hepatobiliary Surgery, First Affiliated Hospital, Chongqing Medical University, Chongqing 400016, China (Huang DR, Wu ZJ and Zhu Y)
Zhong-Jun, Wu, MD, Department of Hepatobiliary Surgery, First Affiliated Hospital, Chongqing Medical University, Chongqing 400016, China (Tel: 86-23-89011015; Email: zjingjing1011@163.com)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
猜你喜欢
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Pancreas transplantation in the mouse
- Adult-to-adult living donor liver transplantation for malignant metastatic melanoma to the liver
- Relationship between the expression of IP-10 and IP-10 mRNA in peripheral blood and HBV DNA level in patients with cirrhosis
- Integrity of the pancreatic duct-acinar system in the pathogenesis of acute pancreatitis
- T29C genotype polymorphism of estrogen receptor alpha is associated with initial response to interferon-alpha therapy in chronic hepatitis B patients
- An effective model for predicting acute kidney injury after liver transplantation
