Pancreas transplantation in the mouse
2010-07-05
Hangzhou, China
Pancreas transplantation in the mouse
Xiang-Yan Liu, Liang Xue, Xiang Zheng, Sheng Yan and Shu-Sen Zheng
Hangzhou, China
BACKGROUND: Pancreas transplantation is the only established treatment to achieve long-term normoglycemia and insulin independence in patients with insulin-dependent diabetes mellitus. However, many complications both during and post-transplantation have limited the progress of pancreas transplantation. Mice are the widely used laboratory animals that have been used to establish pancreas transplant models. The pathogenesis and the treatment of pancreas allograft rejection have been studied during the last twenty years. This review introduces four different mouse pancreas transplantation models established by different centers.
DATA SOURCES: We reviewed the three mostly reported mouse pancreas transplantation models in the literature (PubMed), and compared them with a novel mouse model established at our center.
RESULTS: In this review, four different models of mouse pancreas transplantation were compared in terms of surgical technique, immediate success rate, advantages and disadvantages.
CONCLUSIONS: The mouse model is a useful tool to study pancreas transplantation-related diseases and their treatment. The findings from this model help to improve human pancreas transplantation in the future.
(Hepatobiliary Pancreat Dis Int 2010; 9: 254-258)
pancreas transplantation; model, animal; mouse
Introduction
The first human pancreas transplant[1]was performed by a team headed by Dr. Kelly and Dr. Lillehei, Minnesota State University of America, in 1966. Almost half a century later this technique remains the only established treatment to achieve long-term normoglycemia and insulin independence in patients with insulin-dependent diabetes mellitus.[2-4]
However, progress in pancreas transplantation has been limited by a number of challenges including fatal surgical complications, allogeneic immunological barriers as well as autoimmune recurrence and various other nonsurgical problems. Therefore, the establishment of an ideal model for pancreas transplantation is imperative for investigating the molecular basis of pathophysiological and immunological phenomena encountered. These include post-transplant pancreatitis of the graft, ischemiareperfusion injury (IRI), transplant immunology of the pancreas, and regeneration of the pancreas post-surgery.
Mouse models have been used for decades in many areas. They represent an ideal experimental model because of their clear genetic background, short breeding cycle and low cost of colony maintenance. In addition, a greater number of immunological research methods can be applied to mouse models compared to any other animal models.[5]The extensive range of murine transgenic, knock-in and knock-out models currently available to researchers provides a rich resource for a comprehensive study design. A wider variety of agents such as monoclonal antibodies and fusion proteins can be used in research with mice other than with any other animals. Recently, Mood and colleagues[6]published a comprehensive review about pancreas transplantation in the rat. Compared to murine heart, small bowel, kidney and liver transplantation models, the establishment of a pancreatic transplant model in the mouse is much more challenging on a technical and organ-specific level. Thus, developing a practical mouse model of pancreas transplantation would be a great challenge to the international scientific community.
Segmental pancreas transplantation in mice
Purcell et al[7,8]described the segmental pancreas transplantation model in mice in 1992. In this segmental approach the donor segment of the pancreas supplied by the splenic artery was first procured along with the portal vein and the aorta cuff attached to the celiac trunk. This was followed by cauterization of the pancreatic duct of the graft. In the recipient operation the aortic cuff of the graft was anastomosed to the recipient aorta in an end-to-side fashion, while the donor portal vein was anastomosed to the recipient inferior vena cava. Segmental pancreas transplantation has already been successfully employed to determine whether immunosuppressive antibody treatment in pancreatic transplant recipients prolongs survival by delaying or stopping autoimmune disease recurrence and graft rejection.[9-11]
However, the model of segmental pancreas transplantation has critical shortcomings such as the occlusion of the pancreatic duct. Several studies[12,13]and ours have revealed evident inflammation and exocrine atrophy in the graft, which definitely affect the endocrine function.[14]Thus, the long-term preservation of graft function could be hindered by these chronic pathological processes in the graft parenchyma. This may also explain the reason why the model has been suspended since 1999.
Pancreaticoduodenal transplantation in mice
The first combined pancreaticoduodenal transplantation in the mouse was reported by Tori et al[15]from Osaka University Medical School of Japan in 1999. These authors modified Lee's pancreaticoduodenal transplantation in the rat.[16]They harvested the donor pancreas together with a two-centimeter strip of the duodenum including the ampulla of Vater. The donor aortic patch was then anastomosed to the celiac trunk, and the cranial mesenteric artery to the recipient aorta, the donor portal vein to the recipient inferior vena cava, and the graft duodenum to the recipient's duodenum. Using this model, they achieved an immediate success rate of 46%, with a total operation time of 80 minutes. Successful outcomes resulted in normalization of blood glucose levels from the first post-transplantation day.
In this pancreaticoduodenal transplantation model, the graft's exocrine secretion was drained to the recipient's gut, and the endocrine part to the systemic circulation. The model was physiological and relevant to clinical pancreas transplantation if there was a higher survival rate. However, limitations of this transplantation include complicated surgical procedures, long surgical time, and various complications during post-transplantation, such as shock (31%), arterial thrombosis (7%), venous stenosis, ileus and others.[15]
Although this transplantation has been resulted in good long-term pancreatic function, the failure rate is still over 50%, which may explain the scarcity of published studies using this model.
Murine pancreas transplantation in the neck
Recently, Maglione[17]and his team have developed a novel technique for heterotopic vascularized pancreas transplantation in the diabetic mouse. They used a notouch technique for the isolation of the donor pancreas, and implanted the donor pancreas in the recipient's cervical region by connecting the portal vein to the external jugular vein cuff and the donor aortic segment over the carotid cuff.
Using this technique, the immediate success rate was increased to >90%, and the pancreas ischemia time was reduced to 33±6 minutes. Thromboembolic complications were eliminated by technical modifications, and all recipients' blood glucose levels returned to normal within one day. On post-operation days 10 and 30, all grafts in the 16-hour cold ischemia group and non-ischemia group showed almost normal islet cell and acinar architecture. However, on post-operation day 100 all grafts showed interstitial fibrosis along with atrophy and inflammation. In contrast to the control group, graft functional capillary density values were significantly reduced in the prolonged ischemic group, paralleled by macroscopically marked edema and areas of subcapsular hemorrhage.[17]Hematoxylin and eosin (HE) staining in both groups showed increases in interstitial edema, hemorrhage, necrosis, and inflammation according to Schmidt's method.[18]
However, ligation of the exocrine drainage of the graft leads to some long-term complications such as acinar cell atrophy and interstitial fibrosis after 3-4 weeks. This defect precludes the utility of this model in transplant immunology research. In addition, it has been suggested that pancreatic duct occlusion plays a role in graft edema and recipient hyperamylasemia after transplantation.[13]Therefore, although this model may be acceptable for ischemia-reperfusion studies, consequences of pancreatic duct ligation must be considered. In our experience, the occlusion of the pancreatic duct profoundly increased the severity of parenchymal edema when compared with the parent pancreatic duct model. Since graft edema is one of the parameters for histological assessment of graft impairment by IRI, the grade of graft injury might have surpassed acceptable limits.
Modified segmental pancreas transplantation with an opening pancreatic duct
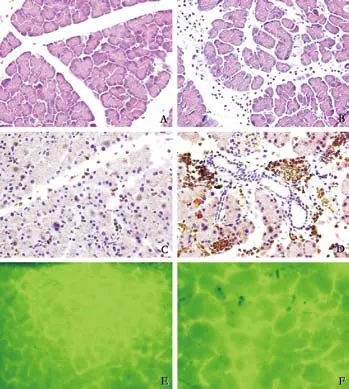
Fig. 1. A: HE staining of non-ischemic graft, (original magnification ×200); B: HE staining of 16-hour prolonged cold ischemia graft (original magnification ×200); C: myeloperoxidase staining of non-ischemic graft (original magnification ×200); D: myeloperoxidase staining of 16-hour prolonged cold ischemia graft (original magnification ×200); E: Microcirculation changes of non-ischemic graft at 2 hours post-transplantation (original magnification ×200); F: Microcirculation changes of 16-hour prolonged cold ischemia graft at 2 hours post-transplantation (original magnification ×200).
We have modified Maglione's technique of the heterotopic vascularized segmental pancreas transplantation in the neck. Anatomically the murine pancreas has three separate lobes, namely the gastric, duodenal and splenic lobes. The exocrine drainage of the gastric and splenic lobes is conducted by a common pancreatic duct, opening to the mid of the bile duct instead of the ampulla of Vater. Fortunately, the demarcation of vascularized parts is distal to the duodenal ampulla after ligation of the superior mesenteric artery. Therefore, a single aortic patch with the celiac trunk is sufficient to provide the blood supply for the gastric and splenic lobes when the duodenal part is abandoned. Importantly, the duodenal ampulla is preserved for an enlargement in the end of the common bile duct enabling stent implantation for drainage of pancreatic juice.
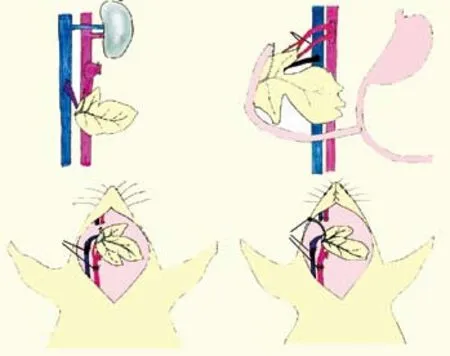
Fig. 2. Sketch of the four different models. A: Purcell's model; B: Tori's model; C: Maglione's model; D: our model. Duo: duodenum; Ao: aorta; IVC: inferior vena cava; PV: portal vein; CeA: celiac artery; EJV: external jugular vein; CA: carotid artery; PD: pancreatic duct; SMA: superior mesenteric artery.
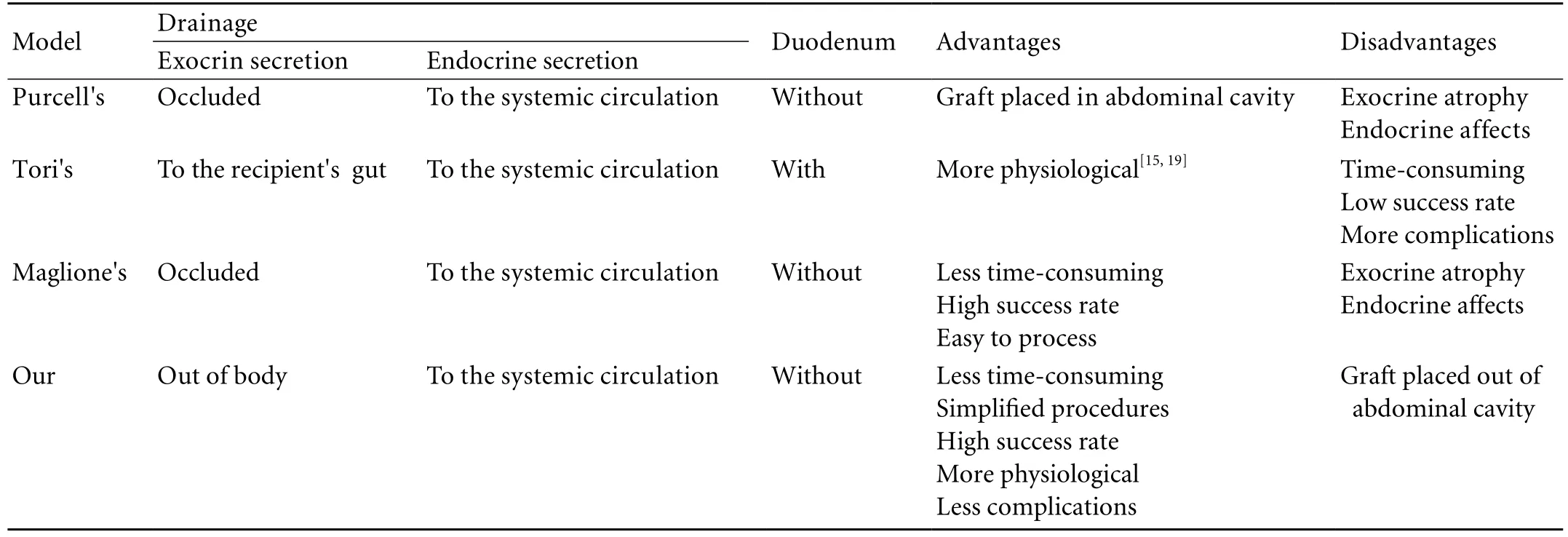
Table. Comparison of the four different models
We have also used the no-touch technique to harvest the donor pancreas, ligate and cut the head of the pancreas, and keep the pancreatic duct open. Usually we position the graft in the recipient's cervical region. The vascularization procedure is similar to that used in Maglione's model; the pancreatic duct is fixed to the skin using a suitable cuff, thus providing unobstructed drainage of the graft's exocrine secretion. When the graft is implanted in the abdomen, the celiac trunk with an aortic patch is used for anastomosis with the subrenal aorta, and the venous reconstruction is performed as described in Mottram's model. Because we use a patch with a single artery (celiac trunk, without superior mesenteric artery) and less pancreatic tissue, the incidence of thrombosis, hypovolemic shock and fatal pancreatitis is reduced in our model.
Using this model in pancreas transplantation studies, we achieved an immediate high success rate of more than 90%, and reduced the total warm ischemia time to only 30 minutes. All grafts were harvested at 6 hours post-transplantation for histological studies. Fig. 1 shows the results of our model. HE staining (Fig. 1A, B) and myeloperoxidase staining (Fig. 1C, D) showed that grafts exposed to 16-hour cold ischemia got more severe inflammation and acinus edema than those without cold ischemia.
In another experiment using the same model, grafts both in the 16-hour prolonged cold ischemia group and the non-ischemia group were harvested at 2 hours posttransplantation. The immunofluorescence technique was used to study the microcirculation changes. Graft microcirculation in the prolonged cold ischemia group was more significantly reduced than in the non-ischemia group (Fig. 1E, F).
Compared the four different models, our model has several merits (Table). First, the donor pancreatic duct is not ligated but its drainage is necessary during the experimental procedure, thereby avoiding posttransplantation complications of acinar cell atrophy and interstitial fibrosis.[12,13]We believe that this produces more physiological conditions than the original model. Second, in the donor operation, the donor hepatic artery, superior mesenteric artery, and their supply branches are ligated or cut to the pancreatic head. The splenic artery is left for its supply to the pancreatic body and tail. This modification not only simplifies the surgical procedure and efficiently streamlines the vascularization, but also streamlines the vascularization. In addition blood flow turbulence is controlled to achieve long-term hemodynamic stability and reduce the risk of thrombosis, thereby ensuring adequate blood supply to the graft (Fig. 2).
Although human organ transplantation has made great progress during the past half century, some transplantation-related diseases, IRI in particular, remain one of the biggest obstacles, especially in pancreas transplantation.[20]IRI can induce graft pancreatitis, several post-transplantation complications and finally graft failure.[21]Many centers have developed practical models to study the pancreas transplantationrelated diseases, IRI-related mechanisms and methods of treatment. In this review we have addressed the mouse models of pancreatic transplantation and some problems to be solved in the future.
Funding: This study was supported by grants from the National Key Technology R&D Program of China (No. 2008BAI60B06), the National Natural Science Foundation of China (No. 30700769), and Science and Technology Bureau of Zhejiang Province (No. 2008C14028).
Ethical approval: Not needed.
Contributors: LXY wrote the main body of the article under the supervision of ZSS. XL, ZX and YS provided advice on medical aspects. ZSS is the guarantor.
Competing interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery 1967;61:827-837.
2 Odorico JS, Sollinger HW. Technical and immunosuppressive advances in transplantation for insulin-dependent diabetes mellitus. World J Surg 2002;26:194-211.
3 Sutherland DE, Gruessner A, Hering BJ. Beta-cell replacement therapy (pancreas and islet transplantation) for treatment of diabetes mellitus: an integrated approach. Endocrinol Metab Clin North Am 2004;33:135-148, x.
4 White SA, Shaw JA, Sutherland DE. Pancreas transplantation. Lancet 2009;373:1808-1817.
5 Niimi M. The technique for heterotopic cardiac transplantation in mice: experience of 3000 operations by one surgeon. J Heart Lung Transplant 2001;20:1123-1128.
6 Mood ZA, Mehrabi A, Schmied BM, Müller SA, Engelmann G, Schemmer P, et al. Review of various techniques of pancreas transplantation in rat model. J Surg Res 2008;145:205-213.
7 Purcell LJ, Mottram PL, Green MK, Mandel TE. Transplantation of the segmental pancreas in STZ-treated diabetic mice. Transplant Proc 1992;24:236-237.
8 Purcell LJ, Mottram PL, Mandel TE. The transplantation of segmental pancreas isografts in nonobese diabetic mice. Transplant Proc 1992;24:2299.
9 Purcell LJ, Mottram PL, Mandel TE. Immunosuppressive antibody treatment prolongs graft survival in two murine models of segmental pancreas transplantation. Immunol Cell Biol 1993;71:349-352.
10 Purcell LJ, Mottram PL. Prevention of both rejection and recurrence of autoimmune disease in the NOD/Lt mouse following segmental pancreas transplantation. Transplant Proc 1995;27:2166-2167.
11 Mottram PL, Murray-Segal LJ, Han W, Maguire J, Stein-Oakley A, Mandel TE. Long-term survival of segmental pancreas isografts in NOD/Lt mice treated with anti-CD4 and anti-CD8 monoclonal antibodies. Diabetes 1998;47:1399-1405.
12 Brynger H, Mjörnstedt L, Olausson M. Heterotopic grafting of pancreas to the neck in the rat--an experimental model. Transplant Proc 1980;12:148-149.
13 Panozzo MP, Basso D, Plebani M, Valente ML, Rasia E, Balint L. Effects of pancreaticobiliary duct obstruction on the exocrine and endocrine rat pancreas. Pancreas 1995;11:408-414.
14 Ohshio G, Okada N, Manabe T, Imamura M. Pancreatic exocrine secretion in short-term pancreatic duct obstruction induced acute pancreatitis in rats: an in vivo and in vitro study. Digestion 1994;55:200-207.
15 Tori M, Ito T, Matsuda H, Shirakura R, Nozawa M. Model of mouse pancreaticoduodenal transplantation. Microsurgery 1999;19:61-65.
16 Lee S, Tung KS, Koopmans H, Chandler JG, Orloff MJ. Pancreaticoduodenal transplantation in the rat. Transplantation 1972;13:421-425.
17 Maglione M, Hermann M, Hengster P, Schneeberger S, Mark W, Obrist P, et al. A novel technique for heterotopic vascularized pancreas transplantation in mice to assess ischemia reperfusion injury and graft pancreatitis. Surgery 2007;141:682-689.
18 Schmidt J, Rattner DW, Lewandrowski K, Compton CC, Mandavilli U, Knoefel WT, et al. A better model of acute pancreatitis for evaluating therapy. Ann Surg 1992;215:44-56.
19 Spadella CT, Mercadante MC, Machado JL, Schellini SA, de Macedo AR. Microsurgical pancreatoduodenal transplantation in rats. Technique and results following 12 years of investigation. Arq Gastroenterol 1996;33:158-166.
20 Fernández-Cruz L, Sabater L, Gilabert R, Ricart MJ, Saenz A, Astudillo E. Native and graft pancreatitis following combined pancreas-renal transplantation. Br J Surg 1993;80:1429-1432.
21 Drognitz O, Obermaier R, von Dobschuetz E, Pisarski P, Neeff H. Pancreas transplantation and ischemia-reperfusion injury: current considerations. Pancreas 2009;38:226-227.
Received December 31, 2009
Accepted after revision April 13, 2010
Author Affiliations: Division of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Liu XY, Xue L, Zheng X, Yan S and Zheng SS)
Shu-Sen Zheng, MD, PhD, FACS, Division of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Tel: 86-571-87236601; Fax: 86-571-87236601; Email: shusenzheng@zju.edu.cn)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Gallbladder cancer with tumor thrombus in the superior vena cava
- Budd-Chiari syndrome secondary to caval recurrence of renal cell carcinoma
- A prospective study on radiofrequency ablation locally advanced pancreatic cancer
- Liver graft vascular variant with 3 extra-hepatic arteries
- An effective model for predicting acute kidney injury after liver transplantation
- Integrity of the pancreatic duct-acinar system in the pathogenesis of acute pancreatitis
