Relationship between the expression of IP-10 and IP-10 mRNA in peripheral blood and HBV DNA level in patients with cirrhosis
2010-07-05
Huainan, China
Relationship between the expression of IP-10 and IP-10 mRNA in peripheral blood and HBV DNA level in patients with cirrhosis
Jian Wang, Ping-Ping Wang, Gui-Ju Xiang and Xiao-Bin Hu
Huainan, China
BACKGROUND: Post-hepatitic cirrhosis is regarded as common and severe form of liver damage. Interferon γ-inducible protein 10 (IP-10), a member of the non-ELR (glutamic-leucinearginine) motif CXC chemokine family, has recently been shown to recruit and activate specific subsets of leukocytes to sites of inflammation or an immune response during the development of hepatic cirrhosis. However, the effects of IP-10 and IP-10 mRNA on inflammatory infiltration at local sites and in the peripheral blood of patients with post-hepatitic cirrhosis as well as their relationship with viral load are still poorly defined. This study aimed to detect the relationship between the expression of IP-10 in serum, IP-10 mRNA in peripheral blood mononuclear cells (PBMCs), and the levels of HBV DNA in the serum of patients, and to explore their role in the pathogenesis of cirrhosis.
METHODS: Typical patients with cirrhosis after HBV infection were selected, and their serum IP-10 concentrations were evaluated with ELISA, the content of IP-10 mRNA in PBMCs was measured by real-time PCR, and the load of HBV DNA in serum and PBMCs was assessed by semi-quantitative analysis of gel imaging.
RESULTS: The levels of IP-10 in serum and IP-10 mRNA in PBMCs of patients with cirrhosis were 299.9±77.2 pg/ml and 0.7500±0.1495, respectively. They were higher than those of controls (P<0.05) and also increased in the HBV DNA(+) groups (P<0.05,P<0.01) to 343.0±80.3 pg/ml and 0.8465±0.1528, respectively. The levels of IP-10 in serum and IP-10 mRNA in PBMCs were clearly correlated with the load of HBV DNA (P<0.01).
CONCLUSIONS: The levels of IP-10 and IP-10 mRNA in the peripheral blood of patients with cirrhosis increase are closely correlated with the load of HBV DNA in serum, and play a key role in the progression of post-hepatitic cirrhosis.
(Hepatobiliary Pancreat Dis Int 2010; 9: 280-286)
hepatic cirrhosis; interferon γ-inducible protein 10; mRNA; HBV DNA; peripheral blood mononuclear cells
Introduction
Hepatic cirrhosis, including cirrhotic compensation and decompensation, is generally stratified by the Child-Pugh classification,[1]and assessed by albumin, bilirubin, prothrombin time, ascites, and encephalopathy.[2]It has been confirmed that biochemical and anatomical dysfunction in hepatic cirrhosis results from both reduced liver cell number and portal vascular derangement due to destructive, sclerosing, and inflammatory injury of the liver, in which various chemokines and cytokines, inflammatory cells, and hepatocytes orchestrate the inflammatory response, while hepatic stellate cells, myofibroblasts, and matrix molecules participate in the process of fibrogenesis.[3,4]
Chemokines, a type of small heparin-binding protein and a new sensitive marker of inflammatory activity, have strong chemoattractant effects and are involved in a variety of immune inflammatory reactions, such as attracting activated T lymphocytes, neutrophils, monocytes, and natural killer cells,[5]via the G proteincoupled receptor pathway. They direct circulating leukocytes to the sites of inflammatory injury and contribute to wound repair. There are four groups of chemokines based on the relative positions of the first two of the four conserved half-cystine residues (CXC, CC, C, and CX3C families), of which the largest subgroups are the CXC and CC families. The CXC family can befurther subdivided into CXC chemokines containing the ELR (glutamic-leucine-arginine) motif (the 3 amino acid residues that immediately precede the first half-cysteine), which is important in neutrophil chemotaxis, and the non-ELR CXC chemokines, such as interferon γ-inducible protein 10 (IP-10), a monokine induced by interferon-γ (Mig), interferon-inducible T cell alpha chemoattractants (I-TAC).[6]Up to now, more than 800 sequences of this G protein-coupled receptor superfamily are available,[7]and these have been classified into a number of protein families including chemokine receptors.
Interferons are a family of proteins first identified by their ability to induce cellular resistance to infection by many viruses. IP-10, an important non-ELR-CXC chemokine, was found to be a gene induced by IFN-γ in a human myeloid cell line, human mononuclear cells, fibroblasts, and endothelial cells.[8]The importance of IP-10 expression during hepatic cirrhosis has recently been emphasized. In order to identify molecules involved in the IFN-γ response and characterize their modulation, Luster et al[9]isolated genes that were induced after recombinant IFN-γ treatment of U937 cells, a histiocytic lymphoma cell line with monocytic characteristics, and then confirmed that the IFN-γ-inducible protein might be a member of a family of proteins involved in the inflammatory process. However, kinetic studies sequentially reported that pIFN-γ-31 mRNA expression from challenged U937 cells showed that it is a cycloheximide-insensitive primary response gene, which is rapidly elevated within 30 minutes and long lasting up to 34 hours post-stimulation. Interestingly, induction of pIFN-γ-31 mRNA is not restricted to U937 but also occurs in IFN-γ-stimulated normal human spleen, peripheral blood monocytes, HUVECs, and primary human foreskin fibroblasts, indicating its potentially ubiquitous role. Besides IFN-γ, other stimuli typically elevated during infection or inflammation, such as type I IFNs, tumor necrosis factor (TNF)-α, double-stranded RNA (dsRNA), lipopolysaccharide (LPS), and certain viruses also induce IP-10 expression.[10]
Previous reports have confirmed that chemokines and chemokine receptors form a complicated network and play a critical role during the migration of leukocytes into areas of inflammation in cirrhosis. However, the relationship between the expression of IP-10 and IP-10 mRNA in peripheral blood and the level of HBV DNA in cirrhosis had not been fully identified.
Methods
Clinical data
Thirty-nine patients with hepatic cirrhosis were selected from the Second Miner's Hospital of Huainan, Anhui, China from January 2007 to May 2008. The clinical diagnosis of these patients (27 men and 12 women) aged from 31.2 to 62.5 years (average 44.5) was based on the modified diagnostic criteria affirmed at the Chinese Viral Hepatitis Conference, 2005. All patients met the diagnostic criteria of liver cirrhosis: 1) positive course of HBsAg >12 months; 2) clear clinical manifestations of chronic liver disease such as hepatosplenomegaly, liver palms, spider angioma, level of serum aspartate transaminase (AST) greater than that of alanine transaminase (ALT); 3) absence of laboratory evidence of co-infection with HAV, HCV, HDV, HEV, and HIV and autoimmune diseases; 4) absence of hepatic encephalopathy, ascites, alimentary tract hemorrhage, and other severe complications; and 5) B-ultrasonic examination clearly indicating cirrhosis of the liver. Twenty-five healthy volunteers (16 men and 9 women), whose ages ranged from 20 to 43 years (average 32), served as normal controls. All participants provided informed consent.
Reagents and instruments
The diagnostic reagents of HBV DNA with PCR were from Shanghai Zhongya Gene Institute. The ELISA test kit for the quantitative measurement of IP-10 was purchased from Sigma, and Trizol reagent from Invitrogen Co., USA. Ficoll-Hypaque (1.077±0.001) was from the Second Reagent Factory of Shanghai, and LightCycler FastStart DNA Master SYBR Green Ⅰ reagent from Roche Co., Germany. The semiautomatic biochemistry analyzer was from Eppendorf® Co., Germany, the nucleic acid/protein gel image analysis system (Hema GSM-301 Version 3.40) from Hema Co., China, and the iCyclez® real-time quantitative PCR instrument was from Bio-Rad Co., USA. The auto tissue processor (ZMN-9802) and auto tissue embedding machine were from Lihua Electron Co., Ltd., Changzhou. The microtome (Leica 2128) was from Leica Co., Ltd., Germany.
Samples
Five ml peripheral venous blood was collected before breakfast from patients with hepatic cirrhosis, and stored in two heparin anticoagulant tubes and another two sterile Eppendorf test tubes. The former were used for measurement of IP-10 mRNA in peripheral blood mononuclear cells (PBMCs), and the latter were used for measurement of IP-10 and HBV DNA in serum. HBV markers were detected in fresh serum as soon as possible.
Detection of HBV DNA
Both HBV DNA load in serum and PBMCs weredetected by routine PCR and then semi-quantitative analysis was made with the nucleic acid gel image analysis system. HBV DNA in PBMCs was detected after the heparinized blood was mixed with an equal volume of Hank's solution without Ca2+and Mg2+. PBMCs were separated and purified in lymphocyte separation medium, and then diluted to 1-2×106/ml. The total RNA in PBMCs was isolated with trizol and then the RNA transcribed into cDNA by random primers. Both positive and negative controls were set up separately at the same time for comparison in each test. The primers for PCR were selected from the HBV core conserved region, and their sequences and specific amplified fragment lengths are shown in the Table. PCR amplification of HBV DNA was carried out with 1.25 units of Taq DNA polymerase in 25 μl reaction mixture containing 10 pmol of specific primers, 2.5 mmol MgCl2, 1×PCR buffer (500 mmol KCl, 100 mmol Tris, 20 mg/ml gelatin, pH 8.3), and 2 μl of standard reference materials or sample HBV DNA. The sample optical-grade PCR tubes with 25 μl reactions were preheated at 94 ℃ for 5 minutes, followed by 35 cycles of heating at 94 ℃ for 50 seconds, 55 ℃ for 40 seconds and 72 ℃ for 90 seconds. The PCR tubes were then heated at 72 ℃ for 4 minutes and cooled to 4 ℃ until electrophoresis. The reaction products (25 μl) were mixed with 6 μl gel loading buffer and separated on 2% agarose gels with ethidium bromide (5 μg/ml) for 15 minutes. After electrophoresis, the positive result was that the sample and positive control had identical fluorescence bands, and then semiquantitative analysis was made with the nucleic acid gel image analysis system.
Measurement of IP-10 in serum
Ninety-six-well polystyrene plates were coated with specific anti-IP-10 McAb diluted in phosphate buffer as capture antibody. Then, 100 μl of standard, control, or test sample per well was added and incubated for 2 hours at 37 ℃. One hundred microliters of the specific anti-IP-10 McAb labelled with horseradish peroxidase was put into each well of 96-well polystyrene plates, warmed to room temperature, and mixed gently. The bound horseradish peroxidase was visualized withtetramethylbenzidine and H2O2diluted in sodium acetate buffer at pH 6.0. The color reaction was stopped by addition of 1.2 mol H2SO4in less than 10 minutes, and absorbance was measured at 450 nm. IP-10 marker concentrations were calculated from standard curves using standard samples in the kits. The control groups of IP-10 markers were made in each test with two blank reaction pores i.e., two negative reaction pores and two positive reaction pores, respectively. Every titer was measured twice with an ELISA analyzer and the final average OD of the titer was calculated. The positive threshold was ≥2.1, which was the ratio of the average OD of a sample to the average titer OD of the negative control. The minimum detectable dose of human IP-10 was typically less than 19 pg/ml and its detection range was from 78 to 5000 pg/ml.
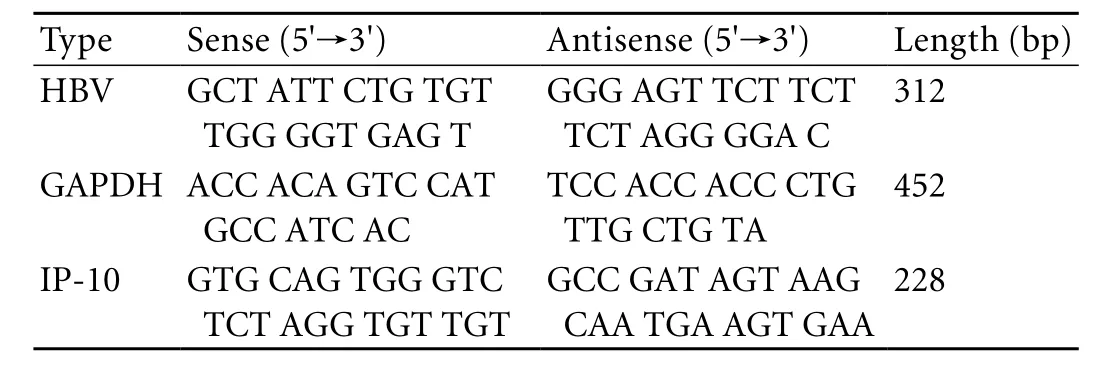
Table. Primer sequences of HBV, IP-10 and their PCR products
Detection of IP-10 mRNA from PBMCs
Total RNA of PBMCs was isolated with Trizol reagent and then reverse-transcribed to cDNA by oligo (dT)18 primers. Positive and negative controls were set up at the same time for comparison in each test. PCR amplification of cDNA was carried out with 1.25 units of Taq DNA polymerase in 25 μl reaction mixture containing 10 pmol of specific primers, 2.5 mmol MgCl2, 1.25 U Ex-Taq, 0.2 mmol dNTPs, 1×PCR buffer (500 mmol KCl, 100 mmol Tris, 20 mg/ml gelatin, pH 8.3), 2×SYBRTM GreenⅠ and 2 μl of a standard or cDNA. The cDNA template was diluted in the proportion of 1∶5. The primer sequences were designed according to those used by Shields et al.[11]The sequences for IP-10 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and their specific amplified products are also shown in the Table. The cycling conditions for GAPDH and IP-10 were the same: preheated for 5 minutes at 94 ℃, followed by 35 cycles of heating at 95 ℃ for 50 seconds, 55 ℃ for 40 seconds, 72 ℃ for 90 seconds, and a final elongation for 5 minutes at 72 ℃, then cooling to 4 ℃until electrophoresis.
Plotting the standard curve
To ensure the amplification efficiency and sensitivity, the PCR product of GAPDH was considered as the standard. Subsequently, the product was diluted to seven graded concentrations (106, 105, 104, 103, 102, 10, and 0 copies/μl), which were used to plot the standard curve.
Statistical analysis
The ratio of l g cDNA/l g GAPDH was regarded as the extreme level of IP-10 mRNA and the data were expressed as mean±SD. Differences between the groupswere assessed by Student'sttest and the relationship was considered statistically significant when thePvalue was less than 0.05.
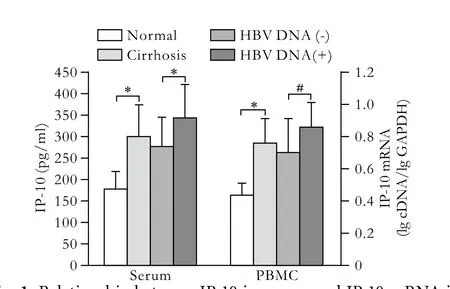
Fig. 1. Relationship between IP-10 in serum and IP-10 mRNA in PBMCs with HBV DNA in patients with cirrhosis. *:P<0.05, #:P<0.01, vs. the corresponding group.
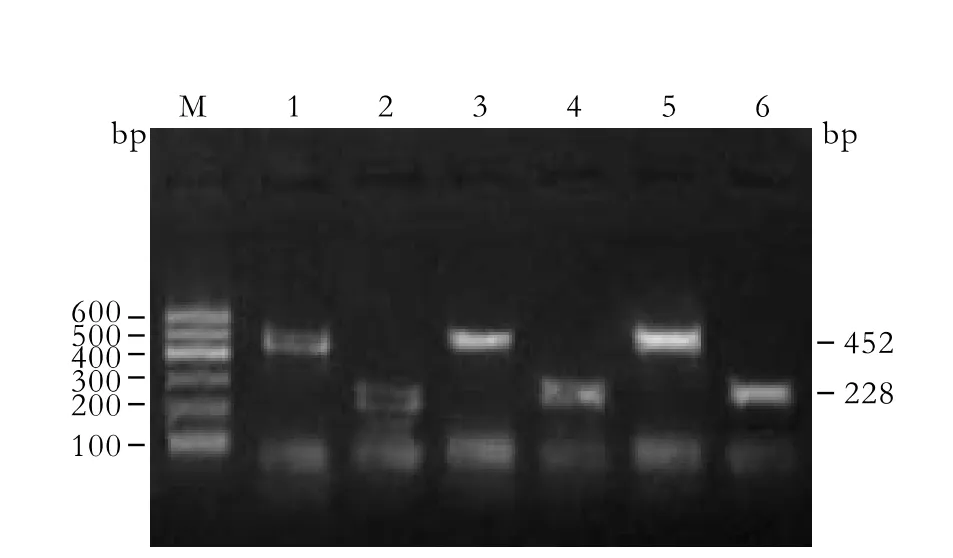
Fig. 2. Relationship of IP-10 mRNA in PBMCs with HBV DNA expression in patients with cirrhosis. M: marker; 1, 3, 5: GAPDH 452bp; 2, 4, 6: IP-10 228bp; 1, 2: Normal; 3, 4: HBV DNA(-) patients; 5, 6: HBV DNA(+) patients.
Results
The chemokine concentration was all normally very lowin vitroorin vivo, but increased markedly within a short time after stimulation by a foreign antigen, such as various viruses, bacteria. Chemokines combined with cell receptors in organs to exert biological effects against local inflammation. The levels of IP-10 in serum and IP-10 mRNA in PBMCs were 299.9±77.2 pg/ml and 0.7500±0.1495 all clearly identified and turned out to be higher in hepatic cirrhosis than those in normal controls (t'=7.3604,P<0.05;t'=9.9867,P<0.05; Figs. 1, 2). Notably, the expression levels of IP-10 and its mRNA in the HBV DNA(+) group were 343.0±80.3 pg/ml and 0.8465±0.1528, and were significantly increased in the HBV DNA(+) group than those in the HBV DNA(-) group (t=2.6439,P=0.0135;t=2.7911,P=0.0095; Fig. 1).
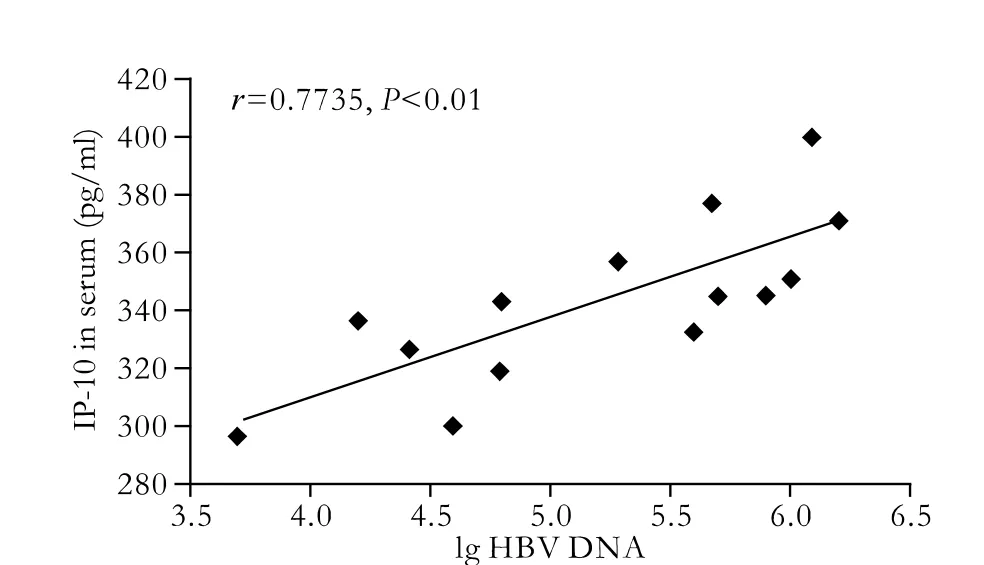
Fig. 3. Correlation between IP-10 in serum and HBV DNA load in patients with cirrhosis.
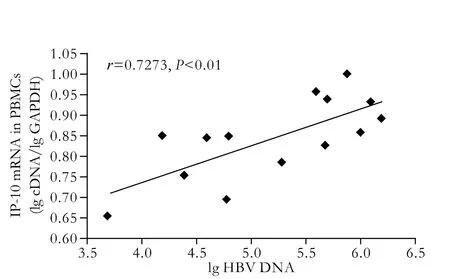
Fig. 4. Correlation between IP-10 mRNA in PBMCs and HBV DNA load in patients with cirrhosis.
In our previous studies,[12]the expression level of IP-10 and its mRNA were closely correlated, as were IP-10 and ALT in serum, and IP-10 and HBV DNA in serum. Few relationships between IP-10 and ALT in serum were identified in normal controls. In this paper, the level of IP-10 in serum was correlated with duplication of HBV DNA (r=0.7735,P<0.01, Fig. 3). Interestingly, the expression of IP-10 mRNA was also associated with the load of HBV DNA (r=0.7273,P<0.01, Fig. 4).
Discussion
In our study, significantly higher levels of IP-10 were found in patients with hepatic cirrhosis than in control subjects. Further studies also showed that the levels of IP-10 in serum were associated with HBV DNA load. The higher the level of IP-10, the greater the load of HBV DNA. The data imply that HBV-specific cytotoxic T cells recruit antigen-nonspecific lymphomononuclear and polymorphonuclear inflammatory cells which could contribute to liver disease. This finding is consistent with that of Chuang et al[13]who measured plasma levels of chemokines IP-10 and Mig, and also studied the expression of CXCR3 chemokine receptors in 105 subjects. Interestingly, they showed that thelevels of plasma IP-10 and Mig in primary biliary cirrhosis increase significantly compared to controls and appear to increase with disease progression. By immunohistochemistry, IP-10 and Mig expressions are evident in the portal areas in primary biliary cirrhosis. Further, the frequency of CXCR3-expressing cells in peripheral blood is also significantly higher in primary biliary cirrhosis, and CXCR3-positive cells occur in the portal areas of diseased livers, primarily on CD4+cells. Similar studies[14,15]also showed that IP-10 played a key role in the local inflammatory reaction and contributed to the hepatic damage featured in chronic HBV infection. High levels of IP-10 in serum could be induced by disordered cellular immune function, portal hypertension, alteration of gastrointestinal flora, ischemia and hypoxia of the intestinal mucosa, and other pathogenic factors such as IL-1, TNF-α, and LPS invading the peripheral blood through the damaged mucosae. Recently, Deng et al[16]demonstrated that among progressing carriers, patients with severe hepatitis B had significantly higher serum CXCL10 levels than those with liver cirrhosis or hepatocellular carcinoma. Strong CXCL10 staining occurs in the cytoplasm of hepatocytes but not in other cell types, and the most intense staining is near areas with lymphoid infiltration. Their reports also showed that the -1596T-201A haplotype was associated with higher CXCL10 transcription in IFN-γ-stimulated peripheral blood mononuclear cells, thus providingin vivofunctional evidence of association of this risk variant. Augmented CXCL10 molecules in hepatocytes chemoattract activate T lymphocytes and natural killer cells in the liver parenchyma; this leads to chronic liver inflammation, which is correlated with severe hepatitis and cirrhosis.
In this study, the higher expression of IP-10 mRNA and protein secretion in patients with hepatic cirrhosis played a decisive role in the recruitment and accumulation of monocytes and lymphocytes within the liver tissue through the interaction with its receptor CXCR3 expressed in target cells, thus contributing to the hepatic necroinflammatory damage in cirrhosis. The high expression of IP-10 mRNA was detectable by real-time PCR in PBMCs from patients with hepatic cirrhosis, and was paralelled by IP-10 levels in serum. This indicated that the transcriptional level of mRNA in PBMCs of patients with hepatic cirrhosis was so strong that more IP-10 could be secreted into serum and high IP-10 levels could induce various inflammatory injuries. Zeremski et al[17]also reported that chemokines might promote hepatic inflammation in chronic hepatitis C through the recruitment of lymphocytes to the liver parenchyma. Multivariable analysis showed that intrahepatic CXCL10 mRNA expression levels were significantly associated with lobular necroinflammatory grade. In the lobular region, CXCL10-expressing and CXCL9-expressing hepatocytes predominate in areas with necroinflammation. Most intrahepatic lymphocytes express the CXCR3 receptor, and the number of CXCR3(+) lymphocytes increases in patients with advanced necroinflammation.
Recent studies have reported that the level of HBV DNA in serum is an important index to evaluate viral replication in the host and the clinical therapeutic effect.[18]In our study, the levels of IP-10 and IP-10 mRNA were all markedly higher in the group with viral replication than in the group without replication. The IP-10 plasma level was correlated with the HBV DNA plasma level in serum and in PBMCs (r=0.7735,r=0.7273,P<0.01). The high load of HBV DNA might be the main reason for inducing IP-10 in patients with hepatic cirrhosis, and both factors contribute to local tissue damage. Interestingly, a different expression pattern has been defined, in that IP-10 expression is mainly restricted to the sinusoidal endothelium. In addition, the intrahepatic expression level of these chemokines are correlated positively with the histological marker of hepatic cirrhosis.[13]Both IP-10 and Mig are synthesized predominantly by macrophages on exposure to IFN-γ, and are involved in the selective recruitment of lymphocytes, especially of Th1 cells via CXCR3. CXCR3 is highly expressed on activated T cells, and yet is undetectable on resting T cells. It is clear that HBV DNA load is a marker of replication in patients with hepatic cirrhosis, and HBV DNA is positive in patients whose hepatocytes have been infected. After HBV invades hepatocytes, immune complex and lipopolysaccharide released by concurrent infection with Gram-negative bacteria have a positive effect on Kupffer cells, monocytes, and other activated immune cells so that various cytokines and chemokines, such as TNF-α, IL-6, and IP-10, increase gradually in peripheral blood. The high concentrations of IP-10 could further attract increasing numbers of activated T lymphocytes, neutrophils, monocytes, and natural killer cells to sites of inflammatory injury, and then persist, where chronic inflammatory and hepatic fibrosis would emerge step by step. Large inflammatory cytokines, for example TNF-α and IL-1, might be attracted and most immunocytes would change from nonactivated to activated.
The above results are similar to those reported by Harvey,[19]who found that IP-10 as a histological marker of inflammation and fibrosis could recruit activated T lymphocytes in the liver during chronic HCV infection, and the most intense immunoreactivity was evident in the areas of lobular inflammation. IP-10 recruits Tlymphocyte subsets expressing the CXCR3 receptor, including activated T lymphocytes of the T-helper type 1 phenotype, a proportion of which are likely to be antigenspecific CD4 cells. In addition, the likely effector cells of antiviral immunity, CD8 cytotoxic T cells and NK cells also express CXCR3 and are responsive to IP-10 by chemotaxis. The IP-10 receptor is expressed on a significantly higher proportion of T lymphocytes in the liver compared with blood. CD8 T lymphocytes, which predominate in the liver lobule, are almost uniformly CXCR3-positive.[20]The expression of IP-10 mRNA correlates with lobular necroinflammatory activity but not with inflammation or fibrosis in the portal tracts. We have demonstrated here that the IP-10 level in serum was highly correlated with the level of HBV DNA in serum. This further supports the idea that IP-10 attracts monocytes and lymphocytes to liver tissues through interaction with its receptor CXCR3 expressed on target cells, so that many hepatocytes are destroyed and release quantities of ALT into the blood, which can not only achieve an effective antiviral response but also lead to tissue damage, thus contributing to progressive liver injury.
In summary, IP-10, an important predictor of liver disease progression, can be not only induced by HBV within hepatocytes but also recruits multiple inflammatory cells into the lobule, and is involved in inflammatory infiltration and hepatic fibrosis.[21]High levels of IP-10 in the serum of patients with hepatic cirrhosis is closely associated with the load or duplication of HBV DNA. The regulation of chemokines and their receptors may be regarded as a new and potential therapeutic target to decrease liver inflammation and to increase specific T lymphocytes and other non-specific immunocyte migration to the infected liver.[22,23]In the future, chemokines may be used to monitor the natural course and progression of HBV-associated liver disease, and also may be regarded as new potential therapeutic targets.[24]
Funding: This study was supported by grants from the Natural Science Foundation of Anhui Province (090413138) and the Natural Science Foundation of the Department of Education of Anhui Province (KJ2007A019, KJ2009A032, KJ2010A086).
Ethical approval: The studies on human participants were approved by the Ethics Committee of the Hospital and adhered to the International Ethical Sandards.
Contributors: WJ proposed the study. WPP wrote the first draft. XGJ and HBX analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. WJ is the guarantor.
Competing interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol 2005;42:S100-107.
2 Chu CM, Liaw YF. Incidence and risk factors of progression to cirrhosis in inactive carriers of hepatitis B virus. Am J Gastroenterol 2009;104:1693-1699.
3 Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology 2008;134:1655-1669.
4 Xu J, Lin Y, Wang YP, Chen YX, Shi B, Lu J, et al. Hepatitis B virus DNA in patients with hepatitis B-related liver cirrhosis with or without hepatocellular carcinomas: a matched casecontrol study. J Dig Dis 2009;10:138-144.
5 Ono SJ, Nakamura T, Miyazaki D, Ohbayashi M, Dawson M, Toda M. Chemokines: roles in leukocyte development, trafficking, and effector function. J Allergy Clin Immunol 2003;111:1185-1200.
6 Korniejewska A, Watson M, Ward S. Analysis of CXCR3 and atypical variant expression and signalling in human T lymphocytes. Methods Mol Biol 2010;616:125-147.
7 Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, et al. Insights into G protein structure, function, and regulation. Endocr Rev 2003;24:765-781.
8 Neville LF, Mathiak G, Bagasra O. The immunobiology of interferon-gamma inducible protein 10 kD (IP-10): a novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev 1997;8:207-219.
9 Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature 1985;315:672-676.
10 Zeremski M, Petrovic LM, Talal AH. The role of chemokines as inflammatory mediators in chronic hepatitis C virus infection. J Viral Hepat 2007;14:675-687.
11 Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol 1999;163:6236-6243.
12 Wang J, Zhao JH, Wang PP, Xiang GJ. Expression of CXC chemokine IP-10 in patients with chronic hepatitis B. Hepatobiliary Pancreat Dis Int 2008;7:45-50.
13 Chuang YH, Lian ZX, Cheng CM, Lan RY, Yang GX, Moritoki Y, et al. Increased levels of chemokine receptor CXCR3 and chemokines IP-10 and MIG in patients with primary biliary cirrhosis and their first degree relatives. J Autoimmun 2005; 25:126-132.
14 Zhao JH, Wang J, Jiang SQ, Xiang GJ. Expression of chemokine monokine induced by interferon-gama in patients with chronic hepatitis B. Nan Fang Yi Ke Da Xue Xue Bao 2006;26:1589-1592.
15 Wang J, Wang PP, Xiang GJ. Antibody against interferon-α 2b in serum of the patients with chronic hepatitis C and its clinical significance: a clinical trial. Hepatitis Monthly 2009;9:103-109.
16 Deng G, Zhou G, Zhang R, Zhai Y, Zhao W, Yan Z, et al. Regulatory polymorphisms in the promoter of CXCL10 gene and disease progression in male hepatitis B virus carriers. Gastroenterology 2008;134:716-726.
17 Zeremski M, Petrovic LM, Chiriboga L, Brown QB, Yee HT, Kinkhabwala M, et al. Intrahepatic levels of CXCR3-associated chemokines correlate with liver inflammation and fibrosis in chronic hepatitis C. Hepatology 2008;48:1440-1450.
18 Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol 2008;6:1315-1341.
19 Harvey CE, Post JJ, Palladinetti P, Freeman AJ, Ffrench RA, Kumar RK, et al. Expression of the chemokine IP-10 (CXCL10) by hepatocytes in chronic hepatitis C virus infection correlates with histological severity and lobular inflammation. J Leukoc Biol 2003;74:360-369.
20 Maini MK, Boni C, Lee CK, Larrubia JR, Reignat S, Ogg GS, et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med 2000;191:1269-1280.
21 Reiberger T, Aberle JH, Kundi M, Kohrgruber N, Rieger A, Gangl A, et al. IP-10 correlates with hepatitis C viral load, hepatic inflammation and fibrosis and predicts hepatitis C virus relapse or non-response in HIV-HCV coinfection. Antivir Ther 2008;13:969-976.
22 Larrubia JR, Benito-Martínez S, Calvino M, Sanz-de-Villalobos E, Parra-Cid T. Role of chemokines and their receptors in viral persistence and liver damage during chronic hepatitis C virus infection. World J Gastroenterol 2008;14:7149-7159.
23 Zhao X, Town JR, Li F, Zhang X, Cockcroft DW, Gordon JR. ELR-CXC chemokine receptor antagonism targets inflammatory responses at multiple levels. J Immunol 2009; 182:3213-3222.
24 Yates CC, Whaley D, Y-Chen A, Kulesekaran P, Hebda PA, Wells A. ELR-negative CXC chemokine CXCL11 (IP-9/I-TAC) facilitates dermal and epidermal maturation during wound repair. Am J Pathol 2008;173:643-652.
Received July 22, 2009
Accepted after revision February 5, 2010
Patience is the basis of all intellect and wisdom.
— Plato
Author Affiliations: Department of Aetiology and Immunology, Medical College, Anhui University of Science and Technology, Huainan 232001, China (Wang J and Wang PP); Department of Infectious Diseases, Xinhua Hospital of Huainan (the Second Miner's Hospital of Huainan), Huainan 232052, China (Xiang GJ and Hu XB)
Jian Wang, MD, Department of Aetiology and Immunology, Medical College, Anhui University of Science and Technology, Huainan 232001, China (Tel: 86-554-6659942; Email: wangjian8237@sina. com)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Pancreas transplantation in the mouse
- Adult-to-adult living donor liver transplantation for malignant metastatic melanoma to the liver
- Modified arteriaIization of orthotopic Iiver transpIantation in a mouse modeI
- Integrity of the pancreatic duct-acinar system in the pathogenesis of acute pancreatitis
- T29C genotype polymorphism of estrogen receptor alpha is associated with initial response to interferon-alpha therapy in chronic hepatitis B patients
- An effective model for predicting acute kidney injury after liver transplantation
