An effective model for predicting acute kidney injury after liver transplantation
2010-07-05
Hangzhou, China
An effective model for predicting acute kidney injury after liver transplantation
Xiao Xu, Qi Ling, Qiang Wei, Jian Wu, Feng Gao, Zeng-Lei He, Lin Zhou and Shu-Sen Zheng
Hangzhou, China
BACKGROUND: Acute kidney injury (AKI) is a common complication in the early period after liver transplantation (LT), posing an enormous obstacle to treatment efficiency and patient survival. However, the exact influencing factors of AKI are still unclear and a predictive model is desperately required in the clinic.
METHODS: Data of 102 consecutive LTs were reviewed. A model for predicting AKI was established and further validated in a prospective study of 44 patients receiving LT.
RESULTS: The incidence of AKI was 32.4%. AKI patients showed a significantly lower survival rate than non-AKI patients. Multivariate analysis demonstrated the independent influencing factors of AKI were preoperative serum creatinine >1.2 mg/dl, intraoperative urine output ≤60 ml/h, intraoperative hypotension status, and intraoperative use of noradrenaline. A model was then established and showed a sensitivity of 75.0%, a specificity of 93.8%, and an accuracy of 88.6% in predicting AKI.
CONCLUSIONS: High preoperative serum creatinine, low intraoperative urine output, and intraoperative hypotension contribute to the development of AKI, and intraoperative use of noradrenaline serves as a protective factor. The predictive model could potentially facilitate early prediction and surveillance of AKI.
(Hepatobiliary Pancreat Dis Int 2010; 9: 259-263)
acute kidney injury; liver transplantation; risk factors; complications; prognosis
Introduction
Acute kidney injury (AKI) is the loss of renal function over a period of hours to days and reflects the entire spectrum of acute renal failure.[1]It is one of the most common complications after liver transplantation (LT), especially in the early postoperative period, and is associated with a high mortality.[2-8]It is defined as serum creatinine >1.5 mg/dl with an increase of 50% above the baseline level or/and the presence of need for renal replacement therapy in the first week post-LT.[2,9]The development of AKI has been associated with markedly increased costs and consumption of health care resources in the general hospital population and the LT population.[5,10]
Unfortunately, it seems that the incidence of AKI, which varies from 17% to 95%[2-7]has not shown a significant decline with the remarkable improvement of surgical technique, the invention of drugs with low nephrotoxicity and the development of perioperative management during the past decade, and AKI remains an unsolved problem.
The difficulties in early intervention contribute significantly to the poor prognosis of AKI. Serum creatinine, which is the most important index of renal function, is strongly correlated with AKI.[2-5]However, it is apparent that preoperative renal function alone may be insufficient to elucidate the underlying mechanisms of AKI and to predict AKI effectively. There also have been many studies concerning the multifactorial etiology of AKI and a variety of preoperative, intraoperative and postoperative variables are suggested.[5,6]
One of the main indications for LT in Asia is HBV-related liver cirrhosis. According to an early report on a relatively small cohort, recipients with HBV-related liver cirrhosis might be more susceptible to AKI than others.[7]However, much less is known about the incidence and the exact factors influencing AKI in the mainland of China, where the majority of recipients receive LT for HBV-related end-stage liver diseases.
The present study consisted of a retrospectivereview and a prospective identification in two cohorts of patients with end-stage liver disease who received LT in our center. The aim of the study was to evaluate the current status of AKI in the first week post-LT and its impact on patient survival, to identify the factors critical to the presence of AKI, and to establish a valid predictive model. The ultimate goal of the present study was to identify patients at great risk for developing AKI so that effective rescue or protective strategies can be promptly undertaken.
Methods
Retrospective cohort
The records of all patients (88 males and 14 females) with benign end-stage liver disease who had undergone cadaveric related LT at the First Affiliated Hospital, Zhejiang University School of Medicine, from January 2004 until September 2005 were retrospectively analyzed. Their main indication for LT was HBV-related cirrhosis. All of the patients received a triple immunosuppression regimen consisting of a calcineurin inhibitor, prednisolone and mycophenolate mofetil. Patient characteristics are shown in Table 1. Informed consent was given by all donors and recipients before transplantation. Each organ donation or transplant complied with the guidelines of the Ethics Committee of the hospital, the Organ Transplant Committee of Zhejiang Province, and theDeclaration of Helsinki.
All patients underwent orthotopic LT without venovenous bypass. To maintain an intraoperative mean arterial pressure above 60 mmHg, some vasoactive drugs were used. In some patients, continuous infusion of noradrenalin was initiated at 1 μg/min and subsequently raised to a maximum of 50 μg/min according to the clinical response during the anhepatic phase or extended to the neohepatic phase. In general, adrenalin was used when noradrenalin or high-dose dopamine failed to work. Intraoperative hypotension status was defined as a systolic blood pressure <90 mmHg for >15 minutes.
AKI was determined by a serum creatinine >1.5 mg/dl with an increase of 50% above the baseline level or/and the presence of need for renal replacement therapy in the first week post-LT.[11]According to the definition of AKI, the patients were divided into an AKI group and a non-AKI group. All patients were routinely followed up closely at the outpatient clinic.
Validation cohort
To further evaluate the clinical predictive ability of the model established in a previous retrospective study,we prospectively investigated another cohort of 44 adult patients with benign end-stage liver disease who had undergone LT from November 2005 to July 2006 at our center. Different from the retrospective cohort, the validation cohort were treated with the piggyback technique and tacrolimus. Other perioperative strategies were the same as in the retrospective study. The main parameters of the two cohorts are compared in Table 1.
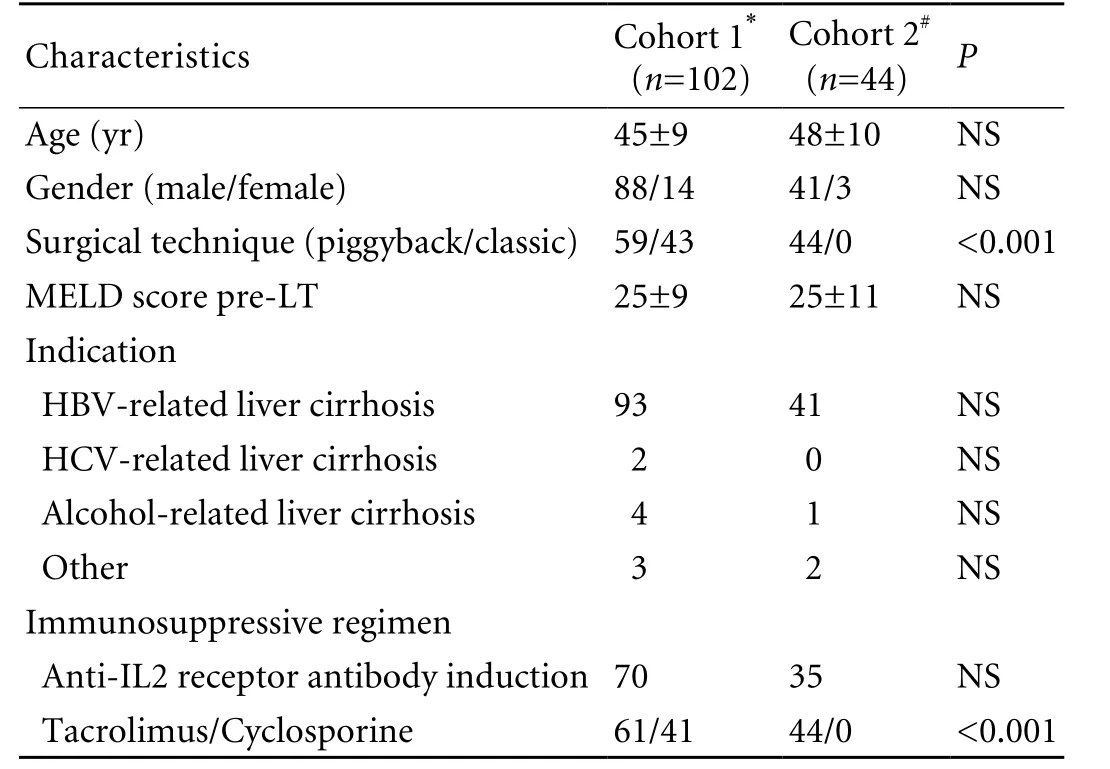
Table 1. Patient characteristics
Statistical analysis
The data were analyzed statistically using SPSS, version 11.0 (SPSS Inc, Chicago, IL). Descriptive variables were expressed as mean±SD or median and range. The Kaplan-Meier method and the log-rank test were used to assess the impact of AKI on the survival. Appropriate cutoff levels for all potential influencing factors were selected for their clinical significance. All the variables were detected by univariate analysis and aPvalue of less than 0.05 was considered statistically significant. Variables with statistical significance were taken for a forward stepwise multivariate logistic regression analysis. The area under the receiver operating characteristic (ROC) curve was generated to assess the model's discrimination, and the method of Hosmer and Lemeshow was used to assess its goodness of fit.
Results
Prevalence of AKI and patient survival
The mean postoperative follow-up for the retrospective cohort was 304±214 days (range 7-686 days). Duringthe first week post-LT, 33 patients (32.4%) developed AKI including 10 (9.8%) requiring renal replacement therapy. The median time for appearance of AKI was 2 days post-LT. The median duration of AKI was 17 days (range 7-481 days). The difference in the 1-year overall survival rates between patients in the AKI and non-AKI groups was statistically significant (70.1% vs. 92.3%,P=0.001, Fig. 1).
Clinical features pre-LT
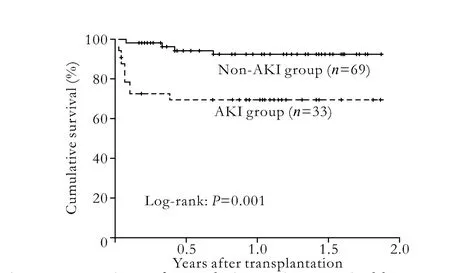
Fig. 1. Comparison of cumulative patient survival between the AKI group (broken line) and the non-AKI group (solid line); the log-rank test,P=0.001.
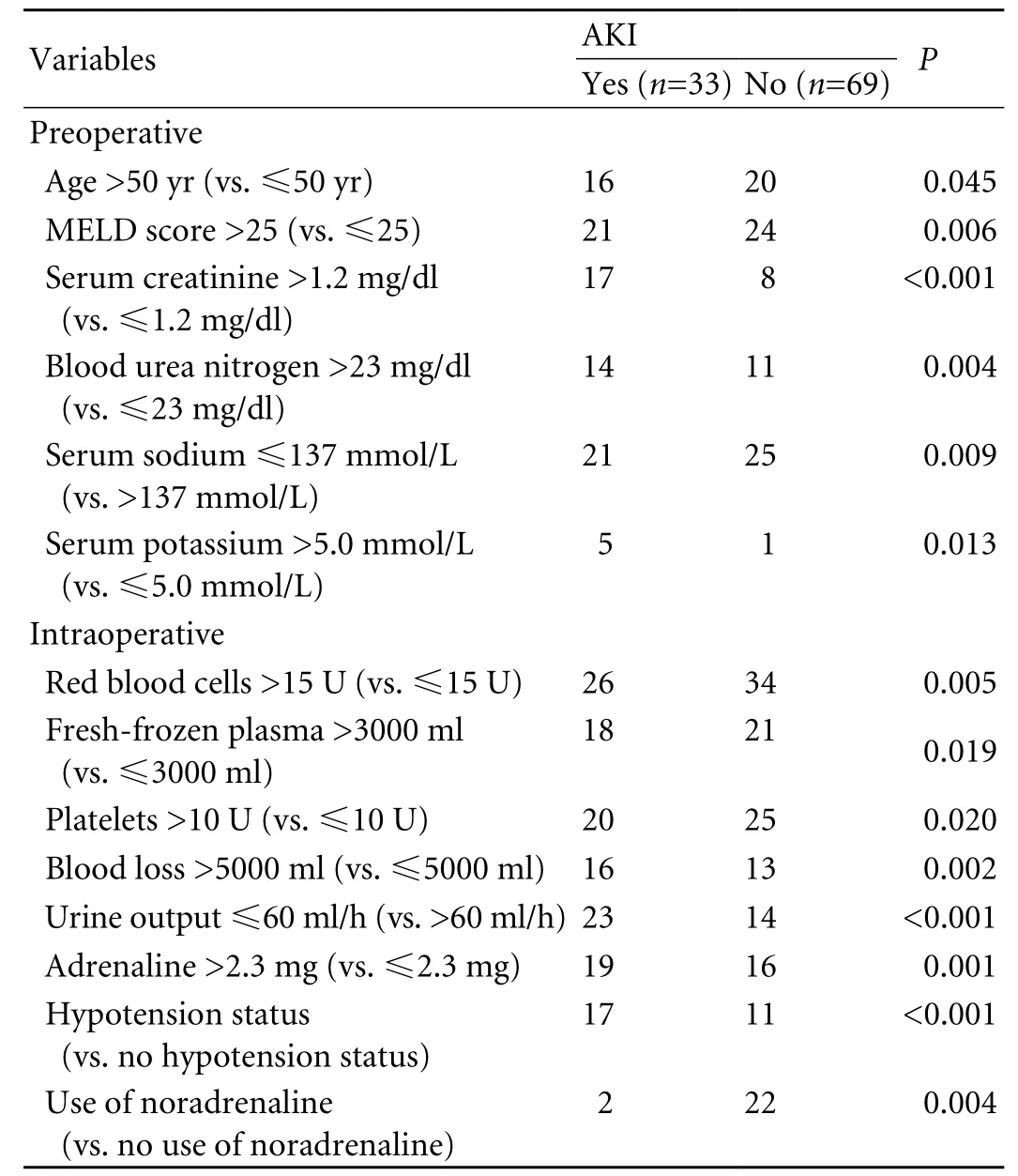
Table 2. Univariate analysis of influencing factors for AKI
The pre-LT clinical features of the AKI and non-AKI groups showed differences in age, MELD score, serum creatinine, blood urea nitrogen, serum sodium, and serum potassium (allP<0.01; Table 2).
Risk factors of AKI
In our cohort, norepinephrine was used intraoperatively as a protective drug. Statistically significant variables associated with AKI were revealed by the univariate analysis. Fourteen parameters were significantly associated with a high risk of AKI (Table 2). Of the 25 patients with preoperative serum creatinine >1.2 mg/dl, 10 including 2 who needed renal replacement therapy developed hepatorenal syndrome as defined by the International Ascites Club and the other 15 had a slight elevation of serum creatinine (<2 mg/dl) without intrinsic kidney disease.
Predictive model for AKI
Preoperative model
Six preoperative parameters were subject to multivariate logistic regression analysis. Serum creatinine >1.2 mg/dl (odds ratio (OR)=8.603,P<0.001) and serum sodium ≤137 mmol/L (OR=3.349,P=0.015) were independent risk factors of AKI. The preoperative model for the prediction of AKI was established as: -1.961+2.152× (serum creatinine >1.2 mg/dl)+1.209×(serum sodium≤137 mmol/L). For predicting patients at risk for AKI, the area under the ROC curve was 0.765 (Fig. 2).
Postoperative model
The results of multivariate logistic regression analysis are shown in Table 3. Of all 14 potential influencing factors analyzed, preoperative serum creatinine >1.2 mg/ dl, intraoperative hypotension status, intraoperative urine output ≤60 ml/h and intraoperative use of noradrenaline proved to have the best fit in our predictive model: risk score=[-2.128+1.109×(preoperative serum creatinine>1.2 mg/dl)+2.243×(intraoperative urine output ≤60ml/h)+1.542×(intraoperative hypotension status)-2.463× (intraoperative use of noradrenaline)]. Probability of AKI=EXP (risk score)/[1+EXP (risk score)].
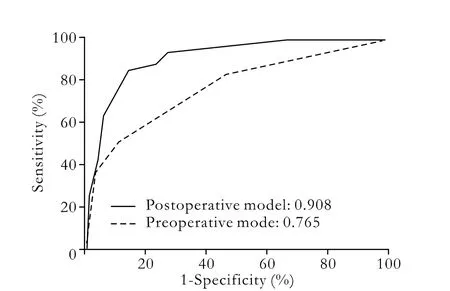
Fig. 2. Area under ROC curve of the preoperative model (broken line, 0.765) and postoperative model (solid line, 0.908).
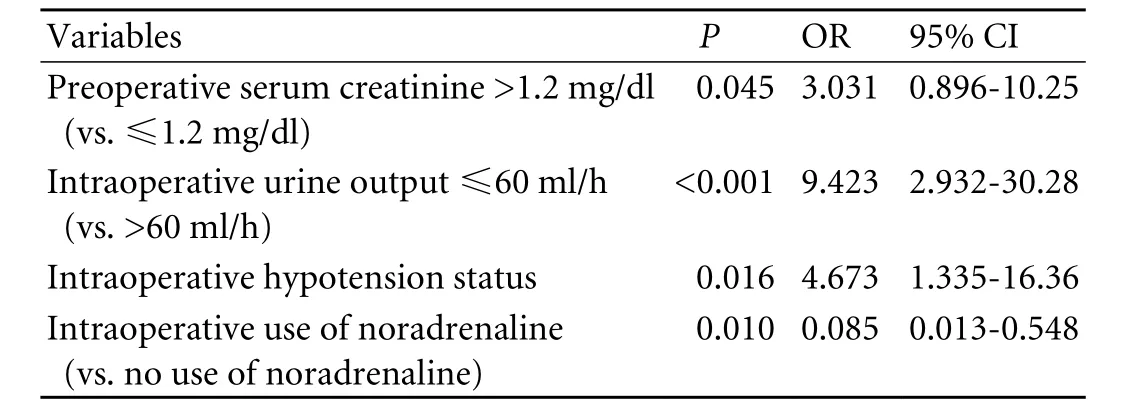
Table 3. Multivariate analysis of influencing factors for AKI
The model discriminated well (area under ROC curve: 0.908, Fig. 2) and fitted excellently (P=0.971 to reject model fit). Considering both sensitivity and specificity, we selected the risk score as a cutoff of -0.2 to predict AKI. This implied that patients with a risk score≥-0.2 (at high risk) were more likely to develop AKI than those with a risk score <-0.2 (at low risk).
The consequent prospective evaluation using the established model showed good predictive results. According to the predictive rule, 9 of 11 patients at high risk developed AKI, and 30 of 33 patients at low risk did not develop AKI. This model obtained a sensitivity of 75.0% (9/12), a specificity of 93.8% (30/32) and an accuracy of 88.6% (39/44).
Discussion
In this study, we examined renal function in the early period post-LT in patients most of whom had hepatitis B related liver disease. Our retrospective study confirmed the previous studies that AKI has a relatively high incidence (32.4%) and has a marked negative impact on the short-term and long-term survival of patients. Thus it would be better if there were an effective model for early prediction of AKI.
A preoperative model could identify patients going into LT who are at risk for AKI. However, we found it was difficult to predict the development of AKI based on the preoperative data alone, as the predictive ability of the preoperative model was far from desirable (area under ROC curve <0.8). The etiology of AKI is multifactorial and the potential risk factors include preoperative, intraoperative and postoperative factors.[2-7]Our finding that most AKI cases appeared within 3 days post-LT suggested that it might be mainly associated with the preoperative and intraoperative conditions. This study demonstrated that patients at high risk could be clearly discriminated from those at low risk for developing AKI by a predictive model established on the basis of one preoperative variable (high serum creatinine level) and three intraoperative variables (low urine output, hypotension status and nonuse of noradrenalin). Using this model, we can rapidly and simply assess the natural course of renal function post-LT and take prompt strategies for the prevention and treatment of AKI.
It has frequently been mentioned in Western reports that preoperative impairment of renal function plays a crucial role in the occurrence of AKI.[2-5]Serum creatinine has traditionally been considered as a simple and cheap marker for assessing renal function in clinical practice. Nevertheless, patients with end-stage liver disease always present high levels of serum creatinine.[3,12]Even a mild elevation of preoperative serum creatinine level (1.0-1.5 mg/dl) may forebode poor renal function post-LT.[13]To better evaluate preoperative renal function, we tried different serum creatinine levels between 1.0 and 1.5 mg/dl for analysis and finally selected >1.2 mg/dl as the best cutoff value for the threshold of preoperative renal dysfunction.
Intraoperative injury to the kidneys could be induced by unstable hemodynamics (massive blood loss, hypotension status, low urine output, and large requirement for blood products), surgical technique or nephrotoxic drugs.[14-16]Our data indicated that massive blood loss, major blood product transfusion, hypotension status, low urine output and moderate or even high doses of vasopressors were significantly related to AKI. To some extent, intraoperative massive blood loss is inevitable in patients with refractory disturbance of coagulation and severe varices.[17]Large amounts of blood loss result in unstable hemodynamics and inadequate vital organ perfusion, reflected by hypotension and low urine output. Fluid transfusion and vasopressors are definitely required under these conditions, and some drugs might aggravate poor kidney perfusion. Of particular interest, noradrenaline, an available and common agent in clinic use, was found to be significantly associated with a reduced incidence of AKI, which has not been reported. Recently, both clinical and animal studies have indicated that noradrenaline functions are effective not only in elevating arterial blood pressure, but also in improving renal blood flow and urine output in vasodilated conditions such as septic shock.[18-20]Recipients with cirrhosis present characteristic hemodynamic changes, including low arterial blood pressure and increased cardiac output before and during operation, which might be similar to septic shock.[20,21]Further studies such as a randomized and double-blind clinical trial are desperately required to certify the potential renal protective function of noradrenalin in LT.
The actual impact on the development of AKI of other potential influencing factors such as age, serum sodium and serum potassium is still not well defined. Although the univariate analysis did not find that the immunosuppressive regimen is a risk factor of AKI, in our experience the application of calcineurin inhibitors should be postponed if the calculated risk score is above -0.2 according to this predictive model. Veno-venous bypass has been abandoned since 2001 at our center because our previous study did not find any positive effect from this technique on renal function.[22]
In conclusion, AKI remains a common complication after LT with a poor prognosis among patients with HBV-related liver diseases. In China, the preoperative serum creatinine, intraoperative urine flow, intraoperative hypotension status and intraoperative use of noradrenalin are strongly associated with AKI. The predictive model established on the basis of these four independent influencing factors may be a reliable and effective tool to identify patients at high risk for developing AKI, and thus prompt salvaging interventions are required. Further well-designed studies are needed to clearly describe the strengths and limitations of this predictive model.
Funding: This study was supported by a grant from the Projects of Ministry of Public Health (No. 20082006).
Ethical approval: Not needed.
Contributors: XX proposed the study and wrote the first draft. All authors contributed to the design and interpretation of the study and to further drafts. ZSS is the guarantor.
Competing interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31.
2 Niemann CU, Walia A, Waldman J, Davio M, Roberts JP, Hirose R, et al. Acute kidney injury during liver transplantation as determined by neutrophil gelatinase-associated lipocalin. Liver Transpl 2009;15:1852-1860.
3 Lima EQ, Zanetta DM, Castro I, Massarollo PC, Mies S, Machado MM, et al. Risk factors for development of acute renal failure after liver transplantation. Ren Fail 2003;25:553-560.
4 Lebrón Gallardo M, Herrera Gutierrez ME, Seller Pérez G, Curiel Balsera E, Fernández Ortega JF, Quesada García G. Risk factors for renal dysfunction in the postoperative course of liver transplant. Liver Transpl 2004;10:1379-1385.
5 Smith JO, Shiffman ML, Behnke M, Stravitz RT, Luketic VA, Sanyal AJ, et al. Incidence of prolonged length of stay after orthotopic liver transplantation and its influence on outcomes. Liver Transpl 2009;15:273-279.
6 Pawarode A, Fine DM, Thuluvath PJ. Independent risk factors and natural history of renal dysfunction in liver transplant recipients. Liver Transpl 2003;9:741-747.
7 Chuang FR, Lin CC, Wang PH, Cheng YF, Hsu KT, Chen YS, et al. Acute renal failure after cadaveric related liver transplantation. Transplant Proc 2004;36:2328-2330.
8 Peeters P, Van Laecke S, Vanholder R. Acute kidney injury in solid organ transplant recipients. Acta Clin Belg Suppl 2007: 389-392.
9 Rimola A, Gavaler JS, Schade RR, el-Lankany S, Starzl TE, Van Thiel DH. Effects of renal impairment on liver transplantation. Gastroenterology 1987;93:148-156.
10 Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005;16:3365-3370.
11 Barri YM, Sanchez EQ, Jennings LW, Melton LB, Hays S, Levy MF, et al. Acute kidney injury following liver transplantation: definition and outcome. Liver Transpl 2009;15:475-483.
12 Sharma P, Schaubel DE, Guidinger MK, Merion RM. Effect of pretransplant serum creatinine on the survival benefit of liver transplantation. Liver Transpl 2009;15:1808-1813.
13 Bilbao I, Charco R, Balsells J, Lazaro JL, Hidalgo E, Llopart L, et al. Risk factors for acute renal failure requiring dialysis after liver transplantation. Clin Transplant 1998;12:123-129.
14 Sural S, Sharma RK, Singhal M, Sharma AP, Kher V, Arora P, et al. Etiology, prognosis, and outcome of post-operative acute renal failure. Ren Fail 2000;22:87-97.
15 Cabezuelo JB, Ramirez P, Acosta F, Torres D, Sansano T, Pons JA, et al. Does the standard vs piggyback surgical technique affect the development of early acute renal failure after orthotopic liver transplantation? Transplant Proc 2003;35:1913-1914.
16 Park Y, Hirose R, Dang K, Xu F, Behrends M, Tan V, et al. Increased severity of renal ischemia-reperfusion injury with venous clamping compared to arterial clamping in a rat model. Surgery 2008;143:243-251.
17 Findlay JY, Rettke SR. Poor prediction of blood transfusion requirements in adult liver transplantations from preoperative variables. J Clin Anesth 2000;12:319-323.
18 Di Giantomasso D, Morimatsu H, May CN, Bellomo R. Intrarenal blood flow distribution in hyperdynamic septic shock: Effect of norepinephrine. Crit Care Med 2003;31:2509-2513.
19 Martin C, Viviand X, Leone M, Thirion X. Effect of norepinephrine on the outcome of septic shock. Crit Care Med 2000;28:2758-2765.
20 Albanèse J, Leone M, Delmas A, Martin C. Terlipressin or norepinephrine in hyperdynamic septic shock: a prospective, randomized study. Crit Care Med 2005;33:1897-1902.
21 Møller S, Bendtsen F, Henriksen JH. Splanchnic and systemic hemodynamic derangement in decompensated cirrhosis. Can J Gastroenterol 2001;15:94-106.
22 Zheng SS, Liang TB, Wang WL, Huang DS, Shen Y, Zhang M, et al. Clinical experience in liver transplantation from an organ transplantation center in China. Hepatobiliary Pancreat Dis Int 2002;1:487-491.
Received May 14, 2009
Accepted after revision November 6, 2009
Author Affiliations: Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Xu X, Ling Q, Wei Q, Wu J, Gao F, He ZL, Zhou L and Zheng SS)
Shu-Sen Zheng, MD, PhD, FACS, Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Tel: 86-571-87236567; Fax: 86-571-87236567; Email: zyzss@zju.edu.cn)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Gallbladder cancer with tumor thrombus in the superior vena cava
- Budd-Chiari syndrome secondary to caval recurrence of renal cell carcinoma
- A prospective study on radiofrequency ablation locally advanced pancreatic cancer
- Liver graft vascular variant with 3 extra-hepatic arteries
- Pancreas transplantation in the mouse
- Integrity of the pancreatic duct-acinar system in the pathogenesis of acute pancreatitis
