Integrity of the pancreatic duct-acinar system in the pathogenesis of acute pancreatitis
2010-07-05
Chengdu, China
Integrity of the pancreatic duct-acinar system in the pathogenesis of acute pancreatitis
Guo-Jun Wang, Yuan Li, Zong-Guang Zhou, Cun Wang, and Wen-Jian Meng
Chengdu, China
BACKGROUND: Acute pancreatitis is an acute inflammatory process of the pancreas that frequently involves peripancreatic tissues and at times remote organ systems. For a long time, the etiology and pathogenesis of acute pancreatitis has been intensively investigated worldwide, but the pathogenetic theories are controversial. The integrity of the pancreatic ductacinar system might play an important role in the pathogenesis of this disease.
DATA SOURCES: Web of Science and PubMed databases were searched for published studies (between January 1966 and June 2009) to identify relevant articles using the keywords "acinar hyperstimulation", "pathogenesis", "acute pancreatitis", "pancreatic duct-acinar system", and "pancreatic duct pressure". Most of the relevant articles were reviewed.
RESULTS: From critical reading of the relevant articles, we found that the underlying mechanisms involved in the pathogenesis of acute pancreatitis are still under debate and ill-understood. On the basis of the relevant studies, we propose a hypothesis for the pathogenesis of acute pancreatitis, in which the integrity of the pancreatic duct-acinar system plays an essential role in the onset and progression of various forms of the disease.
CONCLUSIONS: In our hypothesis, pancreatic duct obstruction and hyperstimulation of the exocrine pancreas are preconditions for the onset of acute pancreatitis; under the common conditions of pancreatic duct obstruction and acinar hyperstimulation, acute pancreatitis arises and develops. This may be an important common pathophysiological mechanism causing various forms of acute pancreatitis.
(Hepatobiliary Pancreat Dis Int 2010; 9: 242-247)
pancreatic duct-acinar system; pathogenesis; acute pancreatitis; acinar hyperstimulation; pancreatic duct pressure
Introduction
Acute pancreatitis is an acute inflammatory process of the pancreas that frequently involves peripancreatic tissues and at times remote organ systems. The severity of the disease varies widely from mild forms only affecting the pancreas to severe disease with multisystem organ failure and death.[1,2]Although the pathogenesis of acute pancreatitis has been intensively investigated for a long time, the exact mechanism still eludes us.[3-5]Many theories about its pathogenesis have been proposed, but they are still under debate.[6-8]Presently, most investigators accept that pancreatic duct obstruction and acinar hyperstimulation play important roles in the early stages, and pancreatic duct pressure might have a key effect during the onset and progression.[9-11]But the triggering and development mechanisms have still not been satisfactorily elucidated.[12]Considering the close association between pancreatic duct pressure and the incidence of acute pancreatitis, the integrity of the pancreatic duct-acinar system should logically be essential to the pathogenesis.
Long-term clinical confusion about acute pancreatitis
Acute pancreatitis has been studied for many years, but its pathogenesis remains an enigma.[13]The classic finding by Opie in 1901 of ampullary obstruction by a gallstone during the autopsy of a patient who succumbed to acute hemorrhagic pancreatitis is a historical landmark in the field.[14]Acosta demonstrated that passage of a stone through the ampulla can initiate acute pancreatitis.[15]Furthermore, Lee et al emphasized that biliary sludge is sufficient to causeacute pancreatitis.[16]These studies laid an essential foundation for the common channel theory of the pathogenesis of acute pancreatitis which is still accepted by many investigators worldwide. But the common channel theory is not sufficient to satisfactorily explain the exact triggering and development mechanisms of acute pancreatitis. The mechanism whereby such a stone might precipitate acute pancreatitis is still a subject of many studies and continues to be an issue of considerable controversy.[17]Many theories attempt to explain the pathogenesis of acute pancreatitis. The very existence of multiple theories for one disease shows that little is understood about its pathogenesis. Many clinical phenomena still confuse most researchers.
Though distal common bile duct stone impaction has long been thought to be a major cause of acute pancreatitis, many patients with similar conditions do not develop the disease.[18]The mechanism whereby gallstone passage through the choledochoduodenal junction initiates acute pancreatitis is not yet known. Although the incidence of pancreatic disease increases as a function of the prevalence of alcohol abuse in a population, only a minority of subjects who abuse alcohol develop pancreatitis.[19]Also, for the most part, alcohol feeding does not lead to pancreatitis in animals.[20]Though acute pancreatitis is the most common and serious complication of diagnostic and therapeutic ERCP, many clinical studies report that many acute pancreatitis patients benefit from therapeutic ERCP.[21-27]Some case reports implicate certain drugs in the onset of this disease, but no persuasive direct evidence is available.[28]Whether pancreas divisum is related to pancreatitis is also a highly controversial issue.[29-31]There are many other debatable issues about acute pancreatitis. To settle all these confusions depends on the eventual elucidation of its pathogenesis.
Roles of duct obstruction and acinar hyperstimulation
To date, there have been many reports on animal models of acute pancreatitis.[32-35]They mainly include noninvasive models such as those induced by caerulein, diet, or L-arginine, and invasive models including closed duodenal loop, antegrade pancreatic duct perfusion, biliopancreatic duct injection, and duct ligation.[36-42]In non-invasive models, strikingly similar phenomena occur during the early stages. Although these observations might be relevant to the genesis of those experimental models of pancreatitis, it is not clear that these observations can be extrapolated to the situation in clinical pancreatitis, because the clinical disease obviously does not result from ethionine ingestion and is unlikely to be the result of supramaximal secretagogue stimulation or L-arginine.[43,44]Rather, gallstone pancreatitis, which is the most common form of acute pancreatitis, appears to be triggered by passage of a stone into or through the terminal bile duct. It has been suggested that such a stone might obstruct the pancreatic duct, but the mechanism whereby this could result in pancreatitis has not been clarified.[45,46]In invasive models, to be of clinical significance, inducing conditions should be possible in the human body. Thus, the duct ligation-induced pancreatitis model seems to be ideal for investigating the pathogenesis of acute pancreatitis.
Presently, many investigators accept that pancreatic duct obstruction and acinar hyperstimulation play essential roles in the pathogenesis of various forms of acute pancreatitis.[47,48]The role of refluxed bile in the etiology of gallstone pancreatitis remains contested. Several studies show that bile reflux is not necessary in the pathogenesis of acute pancreatitis.[49,50]Although several studies claim that acute pancreatitis can be successfully induced without pancreatic duct obstruction, in clinical conditions, pancreatic juice outflow blockage seems an indispensable factor in the pathogenesis.[51]There are also many duct ligationinduced animal models of acute pancreatitis. In rats (and rabbits), pancreatic duct obstruction leads to a relatively mild edematous pancreatitis. It is interesting that in the opossum, ligation of the pancreatic duct alone, or bile and pancreatic ducts separately, or the common biliopancreatic duct, each causes a haemorrhagic pancreatitis.[52]These studies show that duct obstruction plays a very important role in the pathogenesis of acute pancreatitis (Fig. 1).
Lampel and Kern first observed that high dosesof caerulein infused into the rat evoke a reversible, nonlethal edematous pancreatitis.[53]Since then, caerulein hyperstimulation has become a very popular model of acute pancreatitis.[54-56]Since supramaximal secretagogue stimulation is not observed or claimed to be involved in clinical pancreatitis, the relevance of this model to the etiology of the human disease remains to be established. Nevertheless, there is no question that intensive study of experimental pancreatitis produced by caerulein hyperstimulation has contributed a wealth of data on the events underlying the onset and progression of pancreatitis. It does underline the possibility that acinar hyperstimulation is a very important factor in triggering acute pancreatitis.
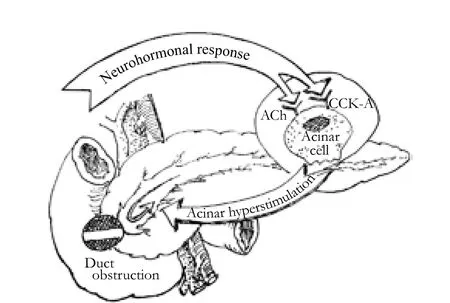
Fig. 1. Possible triggering and development mechanism of acute pancreatitis.
Hypothesis of duct pressure and the integrity of the pancreatic duct-acinar system
Recently, many reports on animal models show that pancreatic duct obstruction and acinar hyperstimulation synergistically exert effects on the onset and progression of acute pancreatitis, which is similar to clinical conditions.[57]Logically, pancreatic duct pressure should be an indispensable factor underlying the genesis of the disease. But the exact mechanism still awaits a satisfactory explanation.[58]We hypothesize that there is a keystone in the pathogenesis of acute pancreatitis in which the integrity of the pancreatic duct-acinar system plays an essential role.
In our hypothesis, pancreatic duct obstruction and acinar hyperstimulation of the exocrine pancreas are preconditions for the genesis of acute pancreatitis. Various causative factors exert their effects through pancreatic duct orifice obstruction which blocks the outflow of pancreatic juice, and this is an initiating event. But duct obstruction alone is not sufficient to cause acute pancreatitis. In the presence of pancreatic duct obstruction, pancreatic acinar hyperstimulation inevitably causes an increase in pancreatic intraductal pressure which might break through the integrity of the pancreatic duct-acinar system. Pancreatic duct pressure is therefore a critical factor in the onset and progression.
Under the circumstance of pancreatic duct obstruction, when the pancreatic duct pressure is not sufficiently high, the integrity of the pancreatic duct-acinar system is not destroyed, and only a small amount of activated enzyme extravasates into the pancreatic interstitium through clefts between the acinar cells, which is rapidly cleared up by protective mechanisms such as capillaries or lymphatics. Then, interstitial pancreatitis arises, which is generally symptomless. If pancreatic duct obstruction continues, the final consequences are acinar cell apoptosis and pancreatic atrophy.
Combined with pancreatic duct obstruction, pancreatic acinar hyperstimulation causes increasing pancreatic duct pressure. When the pressure is high enough, it may eventually destroy the integrity of the pancreatic duct-acinar system, which is a very important protective barrier. Rupture of the pancreatic ductacinar system is the triggering event in the pathogenesis of acute pancreatitis. Under this circumstance, a large amount of activated enzyme refluxes into the pancreatic interstitium and protective mechanisms to prevent trypsinogen activation or reduce trypsin activity are overwhelmed, so acute pancreatitis arises and develops to exhibit clinical features.
Once the acute pancreatitis is induced, if the pancreatic duct obstruction is transient, the duct pressure decreases, the vicious cycle of activated enzyme reflux and pancreatic tissue autodigestion discontinues, and the acute pancreatitis ceases to develop. The consequence of this situation is normally an edematous pancreatitis which often clinically corresponds to mild acute pancreatitis. On the other hand, if the pancreatic duct obstruction is persistent and the pancreatic duct pressure is higher than pancreatic interstitial pressure, the vicious cycle of activated enzyme reflux and pancreatic tissue autodigestion continues, and the acute pancreatitis keeps on developing. The consequence of this situation is normally a necrotic pancreatitis which often clinically corresponds to severe acute pancreatitis (Fig. 2).
Evaluation of the proposed hypothesis
For many years, the pathogenesis of acute pancreatitis has been intensively investigated worldwide, and many theories have been proposed attempting to explain the pathogenetic mechanisms. But these theories are still under debate or controversial.[59]Gallstone pancreatitis is the most common form, but the mechanism whereby an obstructive factor precipitates acute pancreatitis has been a subject of many studies and continues to be controversial. Many hypotheses have been advanced, but there have been numerous objections to each one, not the least of which is that none of them clearly explains how activated digestive enzymes gain access to the gland parenchyma.
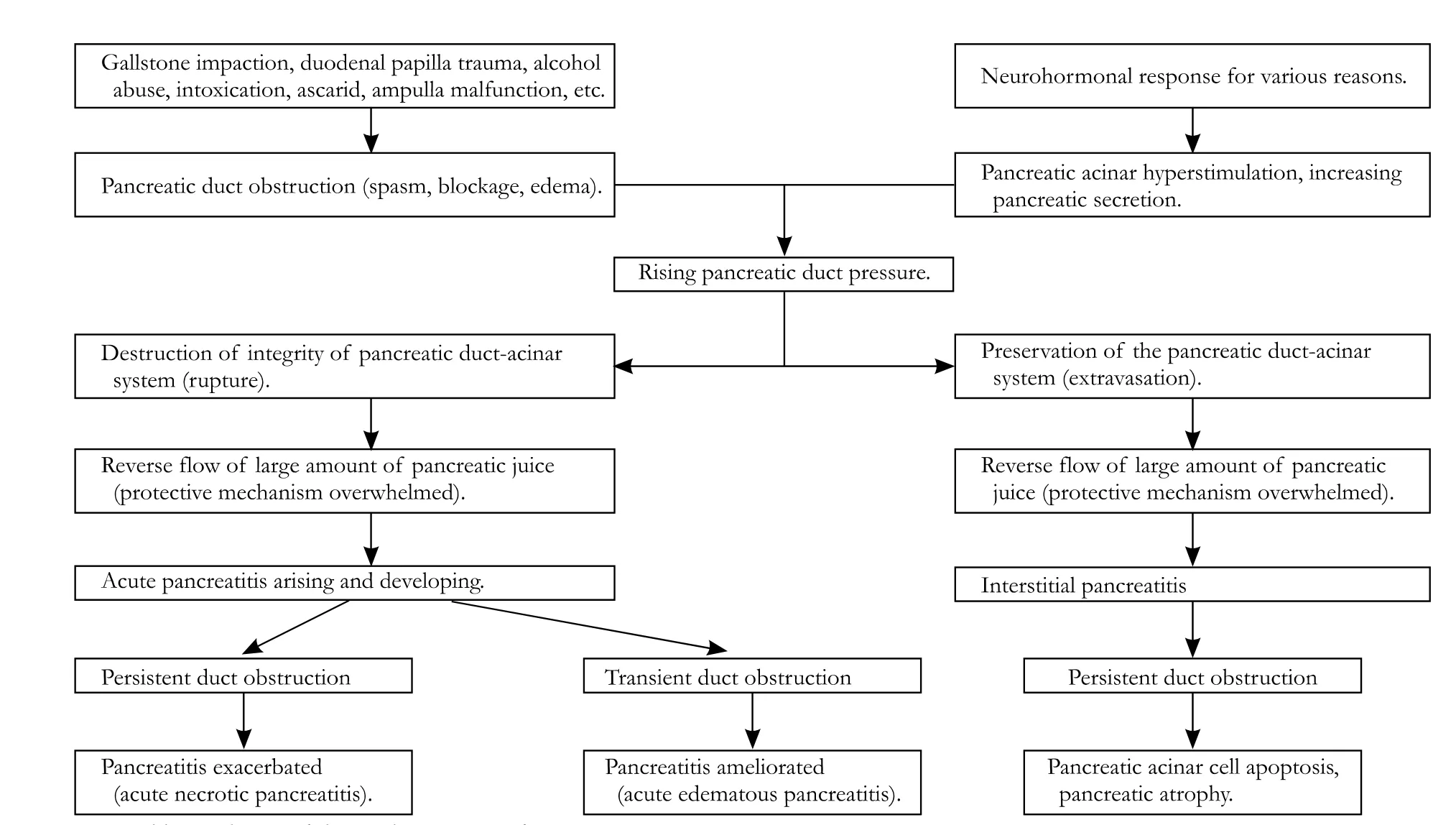
Fig. 2. Proposed hypothesis of the pathogenesis of acute pancreatitis.
This new theoretical framework offers an ideal explanation for the pathogenesis of various forms of acute pancreatitis, and may clearly elucidate many seemingly paradoxical clinical phenomena and experimental results. In various forms of the disease, pancreatic obstruction is frequently implicated in its genesis. But in the setting of pancreatic obstruction, some people only manifest pancreatic duct dilation and pancreatic atrophy without obvious clinical symptoms, some manifest mild acute pancreatitis which mainly involves pancreatic edematous inflammation, and others manifest severe acute pancreatitis which mainly involves pancreatic necrotic inflammation. In rat models of acute pancreatitis, pancreatic duct ligation alone only results in acinar cell apoptosis and atrophy, but in a setting of continued pancreatic secretion, edematous, even necrotic acute pancreatitis is the result. These phenomena can be satisfactorily explained by the proposed theoretical framework. Furthermore, there is no definite contradictory evidence against this framework.
This hypothesis may describe an important common pathophysiological mechanism causing various forms of acute pancreatitis. The hypothesis draws on fresh evidence to present a new paradigm that reexamines the role of the integrity of the pancreatic duct-acinar system in pathogenesis. If our hypothesis is confirmed, the traditional therapeutic strategies may be improved; elimination of pancreatic duct obstruction and inhibition of hyperstimulation of the exocrine pancreas should be basic therapeutic strategies; and the decompression of pancreatic duct pressure should rationally be advocated as an essential approach in the treatment, which may greatly improve the outcome of various forms of acute pancreatitis.[60,61]
Conclusions
The genesis of acute pancreatitis has been intensively investigated for a long time, but a satisfactory pathogenetic theory still eludes us. We hypothesize that the integrity of the pancreatic duct-acinar system is essential to the pathogenesis of various forms of acute pancreatitis. Destruction of the integrity of the pancreatic duct-acinar system caused by increased pancreatic duct pressure is the triggering event. Once the integrity of this system is destroyed under the common effects of duct obstruction and acinar hyperstimulation, acute pancreatitis arises and develops. This might be an important common pathophysiological mechanism underlying various forms of acute pancreatitis.
Funding: This work was supported by a grant from the National Natural Science Foundation of China (No. 30830100).
Ethical approval: Not needed.
Contributors: WGJ wrote the main body of the article under the supervision of ZZG. LY, WC, and MWJ provided advice on medical aspects. ZZG is the guarantor.
Competing interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Chooklin S. Pathogenic aspects of pulmonary complications in acute pancreatitis patients. Hepatobiliary Pancreat Dis Int2009;8:186-192.
2 Fétaud V, Frossard JL, Farina A, Pastor CM, Bühler L, Dumonceau JM, et al. Proteomic profiling in an animal model of acute pancreatitis. Proteomics 2008;8:3621-3631.
3 Samuel I. Bile and pancreatic juice exclusion activates acinar stress kinases and exacerbates gallstone pancreatitis. Surgery 2008;143:434-440.
4 Perides G, Laukkarinen JM, Vassileva G, Steer ML. Biliary acute pancreatitis in mice is mediated by the G-protein-coupled cell surface bile acid receptor Gpbar1. Gastroenterology 2010; 138:715-725.
5 Arendt T, Nizze H, Monig H, Kloehn S, Stüber E, Folsch UR. Biliary pancreatic reflux-induced acute pancreatitis--myth or possibility? Eur J Gastroenterol Hepatol 1999;11:329-335.
6 Gabryelewicz A. Etiology and pathogenesis of acute pancreatitis--current view. Rocz Akad Med Bialymst 1995;40: 218-226.
7 Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology 2007;133:1056.e1-1056. e25.
8 Arendt T. The pathogenesis of acute biliary pancreatitis: a controversial issue. Part I. The concept of biliopancreatic reflux. Gastroenterol J 1989;49:50-53.
9 Yamasaki M, Takeyama Y, Shinkai M, Ohyanagi H. Pancreatic and bile duct obstruction exacerbates rat caerulein-induced pancreatitis: a new experimental model of acute hemorrhagic pancreatitis. J Gastroenterol 2006;41:352-360.
10 Yeung YP, Lo SF, Yip AW. Role of ERCP in the management of predicted mild acute biliary pancreatitis. Asian J Surg 2003; 26:197-201.
11 Baillie J. Predicting and preventing post-ERCP pancreatitis. Curr Gastroenterol Rep 2002;4:112-119.
12 Talukdar R, Vege SS. Recent developments in acute pancreatitis. Clin Gastroenterol Hepatol 2009;7:S3-S9.
13 Chen JW, Thomas A, Woods CM, Schloithe AC, Toouli J, Saccone GT. Sphincter of Oddi dysfunction produces acute pancreatitis in the possum. Gut 2000;47:539-545.
14 Bruennler T, Langgartner J, Lang S, Wrede CE, Klebl F, Zierhut S, et al. Outcome of patients with acute, necrotizing pancreatitis requiring drainage-does drainage size matter? World J Gastroenterol 2008;14:725-730.
15 Acosta JM, Ledesma CL. Gallstone migration as a cause of acute pancreatitis. N Engl J Med 1974;290:484-487.
16 Lee SP, Nicholls JF, Park HZ. Biliary sludge as a cause of acute pancreatitis. N Engl J Med 1992;326:589-593.
17 Saluja A, Saluja M, Villa A, Leli U, Rutledge P, Meldolesi J, et al. Pancreatic duct obstruction in rabbits causes digestive zymogen and lysosomal enzyme colocalization. J Clin Invest 1989;84:1260-1266.
18 Zhou ZG, Zheng YC, Shu Y, Hu WM, Tian BL, Li QS, et al. Laparoscopic management of severe acute pancreatitis. Pancreas 2003;27:e46-e50.
19 Gorelick FS. Alcohol and zymogen activation in the pancreatic acinar cell. Pancreas 2003;27:305-310.
20 Pandol SJ, Gukovsky I, Satoh A, Lugea A, Gukovskaya AS. Emerging concepts for the mechanism of alcoholic pancreatitis from experimental models. J Gastroenterol 2003;38:623-628.
21 Cheon YK, Cho KB, Watkins JL, McHenry L, Fogel EL, Sherman S, et al. Frequency and severity of post-ERCP pancreatitis correlated with extent of pancreatic ductal opacification. Gastrointest Endosc 2007;65:385-393.
22 Cheng CL, Sherman S, Watkins JL, Barnett J, Freeman M, Geenen J, et al. Risk factors for post-ERCP pancreatitis: a prospective multicenter study. Am J Gastroenterol 2006;101: 139-147.
23 Buscaglia JM, Simons BW, Prosser BJ, Ruben DS, Giday SA, Magno P, et al. Severity of post-ERCP pancreatitis directly proportional to the invasiveness of endoscopic intervention: a pilot study in a canine model. Endoscopy 2008;40:506-512.
24 Guda N, Freeman M. Only if needed and as minimally as possible. Animal model for post-ERCP pancreatitis: a step in the right direction. Endoscopy 2008;40:521-522.
25 Testoni PA. Unresolved issues about post-ERCP pancreatitis: an overview. JOP 2002;3:156-161.
26 Freeman ML, Guda NM. Endoscopic Biliary and Pancreatic Sphincterotomy. Curr Treat Options Gastroenterol 2005;8:127-134.
27 Uomo G, Slavin J. Endoscopic sphincterotomy for acute pancreatitis: arguments in favour. Ital J Gastroenterol Hepatol 1998;30:557-561.
28 Trivedi CD, Pitchumoni CS. Drug-induced pancreatitis: an update. J Clin Gastroenterol 2005;39:709-716.
29 Gelrud A, Sheth S, Banerjee S, Weed D, Shea J, Chuttani R, et al. Analysis of cystic fibrosis gener product (CFTR) function in patients with pancreas divisum and recurrent acute pancreatitis. Am J Gastroenterol 2004;99:1557-1562.
30 Staritz M, Meyer zum Büschenfelde KH. Elevated pressure in the dorsal part of pancreas divisum: the cause of chronic pancreatitis? Pancreas 1988;3:108-110.
31 Ohshima Y, Tsukamoto Y, Naitoh Y, Hirooka Y, Furukawa T, Nakagawa H, et al. Function of the minor duodenal papilla in pancreas divisum as determined by duodenoscopy using indigo carmine dye and a pH sensor. Am J Gastroenterol 1994; 89:2188-2191.
32 Hartwig W, Schimmel E, Hackert T, Fortunato F, Bergmann F, Baczako A, et al. A novel animal model of severe pancreatitis in mice and its differences to the rat. Surgery 2008;144:394-403.
33 Boerma D, Straatsburg IH, Offerhaus GJ, Gouma DJ, van Gulik TM. Experimental model of obstructive, chronic pancreatitis in pigs. Dig Surg 2003;20:520-526.
34 Ding SP, Li JC, Jin C. A mouse model of severe acute pancreatitis induced with caerulein and lipopolysaccharide. World J Gastroenterol 2003;9:584-589.
35 Schneider A, Whitcomb DC, Singer MV. Animal models in alcoholic pancreatitis--what can we learn? Pancreatology 2002; 2:189-203.
36 San Román JI, De Dios I, Manso MA, Calvo JJ, López MA. Caerulein-induced acute pancreatitis in the rat. Pancreatic secretory response to cholecystokinin. Arch Int Physiol Biochim 1990;98:237-243.
37 Gronroos JM, Aho HJ, Hietaranta AJ, Nevalainen TJ. Early acinar cell changes in caerulein-induced interstitial acute pancreatitis in the rat. Exp Pathol 1991;41:21-30.
38 Dawra R, Sharif R, Phillips P, Dudeja V, Dhaulakhandi D, Saluja AK. Development of a new mouse model of acute pancreatitis induced by administration of L-arginine. Am J Physiol Gastrointest Liver Physiol 2007;292:G1009-G1018.
39 Rakonczay Z Jr, Hegyi P, Dósa S, Iványi B, Jármay K, Biczó G, et al. A new severe acute necrotizing pancreatitis model induced by L-ornithine in rats. Crit Care Med 2008;36:2117-2127.
40 Hue Su K, Cuthbertson C, Christophi C. Review of experimental animal models of acute pancreatitis. HPB(Oxford) 2006;8:264-286.
41 Dickson AP, Foulis AK, Imrie CW. Histology and bacteriology of closed duodenal loop models of experimental acute pancreatitis in the rat. Digestion 1986;34:15-21.
42 Goldenberg A, Romeo AC, Moreira MB, Apodaca FR, Linhares MM, Matone J. Experimental model of severe acute pancreatitis in rabbits. Acta Cir Bras 2007;22:366-371.
43 Toma H, Winston J, Micci MA, Shenoy M, Pasricha PJ. Nerve growth factor expression is up-regulated in the rat model of L-arginine-induced acute pancreatitis. Gastroenterology 2000; 119:1373-1381.
44 Schmitt M, Klonowski-Stumpe H, Eckert M, Lüthen R, Haussinger D. Disruption of paracellular sealing is an early event in acute caerulein-pancreatitis. Pancreas 2004;28:181-190.
45 Palazzo L, O'Toole D. Biliary stones: including acute biliary pancreatitis. Gastrointest Endosc Clin N Am 2005;15:63-82, viii.
46 Steer ML. How and where does acute pancreatitis begin? Arch Surg 1992;127:1350-1353.
47 Samuel I, Toriumi Y, Wilcockson DP, Turkelson CM, Solomon TE, Joehl RJ. Bile and pancreatic juice replacement ameliorates early ligation-induced acute pancreatitis in rats. Am J Surg 1995;169:391-399.
48 Samuel I, Chaudhary A, Fisher RA, Joehl RJ. Exacerbation of acute pancreatitis by combined cholinergic stimulation and duct obstruction. Am J Surg 2005;190:721-724.
49 Samuel I, Toriumi Y, Yokoo H, Wilcockson DP, Trout JJ, Joehl RJ. Ligation-induced acute pancreatitis in rats and opossums: a comparative morphologic study of the early phase. J Surg Res 1994;57:299-311.
50 Kaiser AM, Saluja AK, Sengupta A, Saluja M, Steer ML. Relationship between severity, necrosis, and apoptosis in five models of experimental acute pancreatitis. Am J Physiol 1995;269:C1295-C1304.
51 Tenner S, Dubner H, Steinberg W. Predicting gallstone pancreatitis with laboratory parameters: a meta-analysis. Am J Gastroenterol 1994;89:1863-1866.
52 Case RM. Is the rat pancreas an appropriate model of the human pancreas? Pancreatology 2006;6:180-190.
53 Lampel M, Kern HF. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol 1977;373:97-117.
54 Clemons AP, Holstein DM, Galli A, Saunders C. Ceruleininduced acute pancreatitis in the rat is significantly ameliorated by treatment with MEK1/2 inhibitors U0126 and PD98059. Pancreas 2002;25:251-259.
55 Yu JH, Yun SY, Lim JW, Kim H, Kim KH. Proteome analysis of rat pancreatic acinar cells: implication for cerulein-induced acute pancreatitis. Proteomics 2003;3:2446-2453.
56 Malleo G, Mazzon E, Genovese T, Di Paola R, Muià C, Crisafulli C, et al. Effects of thalidomide in a mouse model of cerulein-induced acute pancreatitis. Shock 2008;29:89-97.
57 Oruc N, Whitcomb DC. Theories, mechanisms, and models of alcoholic chronic pancreatitis.Gastroenterol Clin North Am 2004;33:733-750, v-vi.
58 Lund H, Tonnesen H, Tonnesen MH, Olsen O. Long-term recurrence and death rates after acute pancreatitis. Scand J Gastroenterol 2006;41:234-238.
59 Yadav D, Lowenfels AB. Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas 2006;33:323-330.
60 Uomo G, Molino D, Visconti M, Ragozzino A, Manes G, Rabitti PG. The incidence of main pancreatic duct disruption in severe biliary pancreatitis. Am J Surg 1998;176:49-52.
61 Lucas CE, McIntosh B, Paley D, Ledgerwood AM, Vlahos A. Surgical decompression of ductal obstruction in patients with chronic pancreatitis. Surgery 1999;126:790-797.
Received November 11, 2009
Accepted after revision March 6, 2010
Author Affiliations: Institute of Digestive Surgery and Department of General Surgery, West China Hospital, Sichuan University, Chengdu 610041, China (Wang GJ, Li Y, Zhou ZG, Wang C and Meng WJ)
Zong-Guang Zhou, PhD, MD, Institute of Digestive Surgery and Department of General Surgery, West China Hospital, Sichuan University, Chengdu 610041, China (Tel: 86-28-85164035; Fax: 86-28-85164035; Email: zhou767@163.com)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Pancreas transplantation in the mouse
- Adult-to-adult living donor liver transplantation for malignant metastatic melanoma to the liver
- Modified arteriaIization of orthotopic Iiver transpIantation in a mouse modeI
- Relationship between the expression of IP-10 and IP-10 mRNA in peripheral blood and HBV DNA level in patients with cirrhosis
- T29C genotype polymorphism of estrogen receptor alpha is associated with initial response to interferon-alpha therapy in chronic hepatitis B patients
- An effective model for predicting acute kidney injury after liver transplantation
