T29C genotype polymorphism of estrogen receptor alpha is associated with initial response to interferon-alpha therapy in chronic hepatitis B patients
2010-07-05
Hefei, China
T29C genotype polymorphism of estrogen receptor alpha is associated with initial response to interferon-alpha therapy in chronic hepatitis B patients
Ting-Ting Zhang, Zhen-Hua Zhang, Yu-Feng Gao, Ya-Fei Zhang, Dong-Liang Yang and Xu Li
Hefei, China
BACKGROUND: Virological clearance, delayed progression to cirrhosis or liver cancer, and increased survival are the long-term goals of antiviral therapy in chronic hepatitis B patients. Identification of host factors correlated with therapeutic response may contribute greatly to individual treatment. This study aimed at investigating whether T29C genotype polymorphism of estrogen receptor alpha (ESR1) is associated with the initial response to interferon-alpha (IFN-α) therapy in chronic hepatitis B patients.
METHODS: The initial responses of 100 patients to IFN-α therapy were evaluated and compared by classifying them into three groups according to T29C genotype polymorphism of ESR1: T/T, T/C, and C/C genotype groups. Polymerase chain reaction-restriction fragment length polymorphism was used to analyze the genotype polymorphism in T29C.
RESULTS: The frequency of initially combined response was markedly higher in both the T/T and T/C groups than in the C/C group (Z=10.326,P=0.006 andZ=26.247,P=0.000, respectively). In addition, the initial virological response was higher in the T/T and T/C groups than the C/C group (χ2=5.674,P=0.017 and χ2=4.980,P=0.026, respectively). In 78 initially HBeAg-positive patients, however, the frequency of initial e-antigen disappearance or seroconversion among the T/T, T/C, and C/C genotype groups was 34.15%, 27.78% and 15.79%, respectively, which were not significantly different.
CONCLUSION: The T29C genotype polymorphism of ESR1 is associated with the initial response to IFN-α in patients with chronic hepatitis B, and might be a significant marker for predicting the initial response to IFN-α, at least in this study population.
(Hepatobiliary Pancreat Dis Int 2010; 9: 275-279)
estrogen receptor; polymorphism; chronic hepatitis B; initial response; interferon-alpha
Introduction
Hepatitis B virus (HBV) infection constitutes a public health menace, and the number of HBV carriers worldwide is estimated to be 350 million. HBV-associated hepatitis, liver cirrhosis, and hepatocellular carcinoma lead to more than one million deaths annually and antiviral therapy plays an important role in prevention through persistent suppression of HBV replication.[1]Interferon-alpha (IFN-α) is one of the currently conventional drugs for anti-HBV treatment. However, some patients become non-responsive to antiviral treatment due to the influence of several factors such as age, gender, alanine aminotransferase (ALT), hepatitis B virus surface antigen (HBsAg) serum level, post-treatment response, HBV DNA level,[2-5]and host genetic factors including HLA-Ⅰ, Ⅱ, and Ⅲ, as well as polymorphisms of A (MxA)-88 and eIF-2a of regulatory region 2.[2,6-8]Therefore, there is an urgent need to find more factors related to the response to interferon therapy in order to select better individual treatment for chronic hepatitis B (CHB) patients. T29C genotypepolymorphism of ESR1 is associated with chronic HBV infection[9]and HBV-related hepatocellular carcinoma.[10]Our study aimed to evaluate whether the T29C gene polymorphism of ESR1 is associated with the response to IFN-α therapy in CHB patients.
Methods
Study population
Between June 2008 and February 2009, 100 patients with CHB from clinics and wards in our hospital were prospectively included in this study. Patients were not enrolled if they had ever received nucleoside or nucleotide analogue therapy. All selected patients volunteered to participate in the study and their initial response was evaluated after follow-up for 12 weeks from the beginning of the therapy. Informed consent was given by all patients who took part in the study.
Criteria for diagnosis and antivirotic response
All patients were confirmed according to the criteria in the2005 Guide on the Prevention and Treatment of Chronic Hepatitis B in China.[11]
There are several definitions of IFN-α therapy in patients with CHB: (1) initial response: response after 12 weeks of therapy; (2) virological response: serology HBV DNA level undetectable or ≥lg2 lower than baseline; (3) HBeAg seroconversion: disappearance of HBeAg and seroconversion to anti-HBe; and (4) combined response, which is divided into complete response (meeting the standard of serological response, virological response, and normalization of serum ALT levels at the same time in patients with HBeAg-positive CHB, or normalization of serum ALT levels and virological response at the same time in patients with HBeAg-negative CHB), partial response (between complete response and no response), and no response (patients who show none of the above responses).[12]
DNA extraction
From each patient before a meal, venous blood was drawn into vacuum tubes containing EDTA and stored at -20 ℃. Genomic DNA was extracted within one week using the DNA Extract kit (TaKaRa Biotechnology, Dalian, China) according to the manufacturer's instructions. HBV serologic markers were detected by ELISA (Kehua Biotech Co., Shanghai, China), HBV-DNA was detected by the quantitative polymerase chain reaction (PCR-fluorescence probing) (Da'an Biotechnology, Guangzhou, China), and liver function was assessed by a Modular-DPP automatic biochemical analyzer (Roche, Switzerland).
Genotyping
T29C genotype polymorphisms of ESR1 were characterized using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP).[13]PCR was performed in a volume of 25 μl containing 0.5 μl Tap, 0.5 μl sense primer, 0.5 μl anti-sense primer, 0.5 μl dNTP, 2.5 μl 10×PCR buffer, 2.5 μl DNA template, and 18 μl dH2O (Promega, USA). The sense primer (5'-GAC CAT GAC CCT CCA CAC CAA AGG ATC-3') and antisense primer (5'-ACC GTA GAC CTG CGC GTT G-3') were synthesized by Invitrogen Co., Shanghai, China. For PCR amplification, an initial denaturation at 94 ℃ for 5 minutes was followed by 35 cycles at 94 ℃ for 30 seconds, 61 ℃ for 40 seconds, and 72 ℃for 30 seconds, and a final extension at 72 ℃ for 5 minutes. The PCR product of 5 μl was digested for 1-2 hours with 1 μl of BamHⅠ(TaKaRa Biotechnology) at 30 ℃ according to the manufacturer's instructions and then separated on 3% agarose gels. RFLP bands were visualized under UV light with ethidium bromide staining. Digested amplicons from the C/C genotype appeared as 197bp bands on agarose gel electrophoresis, while the T/T allele appeared as a 220bp band. The T/C genotype had both of these bands[13](Fig.). To confirm the genotyping results, three genotypes of T29C were selected and PCR-amplified DNA samples were examined by DNA sequencing. DNA sequences of the PCR products were determined using the PCR sense primer with a GeneAmp PCR System 9700 (ABI, USA). The results of genotyping were 100% concordant.
Statistical analysis
Quantitative data were expressed as mean±SD. Statistical analysis was conducted using SPSS 10.0 (Chicago, IL). Genotypes were compared by using the Chi-square test or Fisher's exact test for categorical data and one-way analysis of variance (ANOVA) ornonparametric test for continuous data. APvalue less than 0.05 was considered significantly different, and a Bonfferoni correction was used to adjust for multiple amprisons, requiring aPvalue of 0.05/3=0.017 for statistical significance.
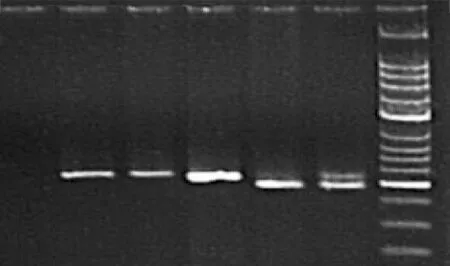
Fig. PCR-based restriction fragment length polymorphism genotyping of ESR1 T29C. Lane 1: negative control; lanes 2-4: T/T homozygotes; lane 5: T/C heterozygote; lane 6: C/C homozygote.
Results
Clinical and demographic characteristics of patients
One hundred and six patients with CHB were studied.Six patients were lost to follow-up because two of them were out of contact, two withdrew from the study, and another two discontinued therapy due to drug side effects.
The T29C genotype polymorphism of ESR1 was genotyped in 100 patients with CHB, of 55 had the T/T genotype, 22 had T/C, and 23 had C/C. No significant difference among the three genotypes was found in terms of the distribution of gender (χ2=1.148,P=0.563), age (F=1.153,P=0.320), initial ALT level (F=0.862,P=0.441), pretreatment HBV DNA (Z=1.961,P=0.161), pretreatment e-antigen status (χ2=0.055,P=0.973), and type of IFN-α (χ2=0.841,P=0.657). Overall, clinical and demographic characteristics showed no significant differences among the three genotypes in patients with CHB (Table 1).
Initial combined response among T/T, T/C, and C/C genotypes
The frequencies of the initial combined response in the T/T, T/C, and C/C groups are shown in Table 2. The respective frequency in no response, partial response, and complete response in the T/T group was 14.55%, 56.36% and 29.09%. This was 27.27%, 54.54% and 18.19% in the T/C group, and 73.91%, 17.40% and 8.69% in the C/C group. The initial combined response between the T/T and T/C groups was not significantly different (Z=2.078,P=0.354). However, the initial combined response among the T/T, T/C, and C/C groups was significantly different (Z=35.558,P=0.000; not shown in Table 2). Furthermore, the frequency ofthe initial combined response was markedly higher in the T/T and T/C groups than in the C/C group (P=0.000 andP=0.006, respectively) (Table 2). The results showed that patients with CHB who carried the T/T or T/C genotypes were more likely to achieve an initial combined response.
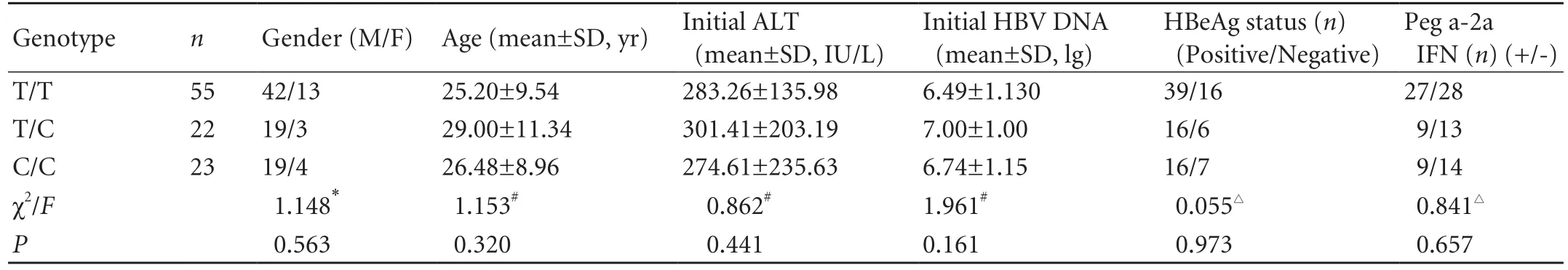
Table 1. Clinical and demographic characteristics of the study population
Initial virological response among T/T, T/C, and C/C genotypes
The respective frequencies of the initial virological response among the T/T, T/C, and C/C groups were 60.00%, 63.64% and 30.43%, respectively. No significant difference was found between the T/T and T/C groups (χ2=0.087,P=0.768). However, the initial virological response among the T/T, T/C, and C/C genotype groups was significantly different (χ2=6.751,P=0.034; not shown in Table 3). In addition, the frequencies of initial virological response in the T/T and T/C groups were higher than in the C/C group (χ2=5.674,P=0.017 and χ2=4.980,P=0.026, respectively) (Table 3). The dataindicated that patients with CHB who carried the T/T or T/C genotype were more likely to achieve an initial virological response.
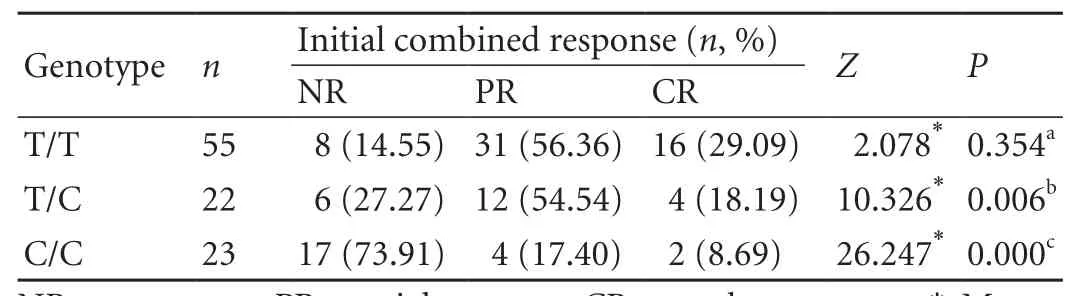
Table 2. Initial combined response to IFN-α therapy among the three genotype groups of patients with CHB (n, %)
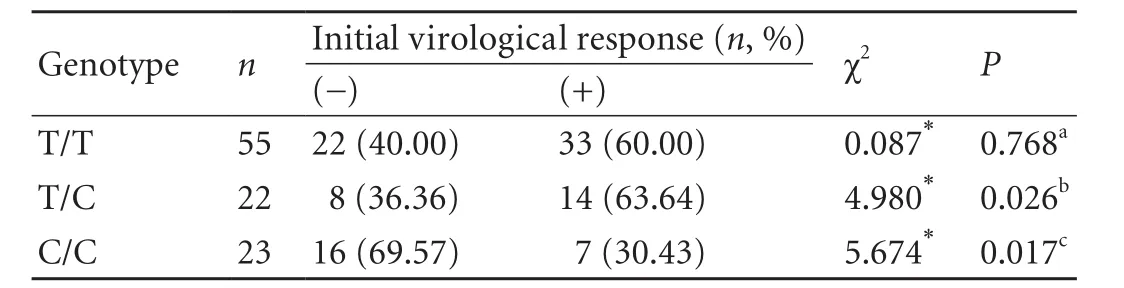
Table 3. Initial virological response to IFN-α therapy among the three genotype groups of patients with CHB (n, %)
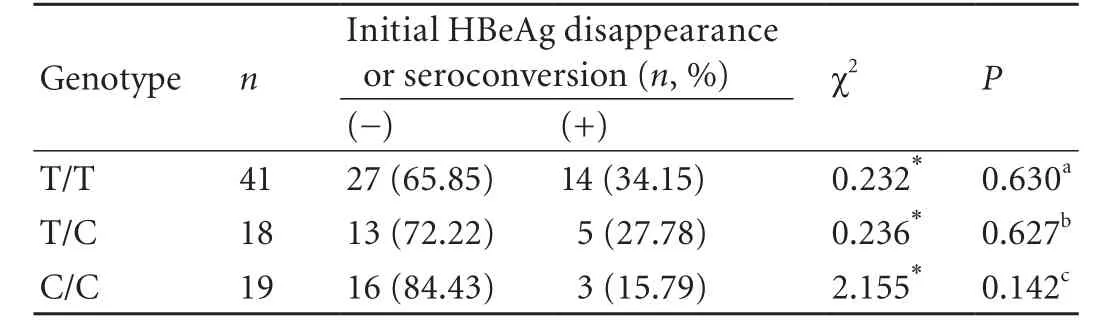
Table 4. Initial HBeAg disappearance or seroconversion with IFN-α therapy among the three genotype groups of patients with HBeAgpositive CHB (n, %)
Initial HBeAg disappearance or seroconversion
Of the 78 initially HBeAg-positive patients, only 22 achieved initial HBeAg disappearance or seroconversion, and the remaining 56 achieved neither (Table 4). The respective frequencies of initial HBeAg disappearance or seroconversion among the T/T, T/C, and C/C groups were 34.15%, 27.78%, and 15.79% (χ2=2.163,P=0.339; not displayed in Table 4). However, the initial HBeAg disappearance or seroconversion between the T/T and T/C groups was not significantly different (χ2=0.232,P=0.630). In addition, no significant difference was found between the T/C and C/C groups (χ2=0.236,P=0.627), and the difference between the T/T and C/C groups was not significant (χ2=2.155,P=0.142). The data indicated that the T29C genotype polymorphism of ESR1 was not related to initial HBeAg disappearance or seroconversion.
Discussion
As one of the important antiviral treatments, IFN-α therapy excerts its anti-viral effect indirectly through the immune system. However, patients react differently to IFN-α therapy. With regard to individualized treatment, it is desirable to predict the treatment response. In this study, we evaluated the initial response to IFN-α therapy in 100 patients and analyzed the genotype polymorphism in T29C by PCR-RFLP. All patients were classified into three groups according to the T29C genotype polymorphism of ESR1: T/T, T/C, and C/C genotype groups. Studies have shown that some characteristics of patients, i.e., age, gender, ALT, HBV DNA level, and pretreatment e-antigen status[2-5]may affect the response to IFN-α therapy. In our study, these factors were distributed uniformly among the T/T, T/C, and C/C groups, which may have removed them as confounding factors. Furthermore, the distribution of initial combined response and initial virological response were evidently different among the three genotypes. In conclusion, our results suggested that patients with CHB who possessed the T/T genotype were more likely to achieve an initial combined response, and patients with the T/C genotype were more likely to achieve an initial virological response, whereas those with the C/C genotype were less likely to develop these responses.
The association between T29C genotype polymorphism of ESR 1 and initial response to IFN-α therapy in CHB patients has never been reported. In addition, the exact mechanism of this association is not clear. The IFNs are a family of natural glycoproteins and regulatory cytokines with pleiotropic cellular functions, such as anti-viral, anti-proliferative, and immunomodulatory activities. It is known that IFN-α enhances anti-viral activity through immune regulation, which contributes to the result and clinical prognosis of HBV infection.[14]In addition, HBV dissemination is mainly limited by cellular immunity. Cytotoxic T lymphocytes play an important role in the host defense against HBV. T-cell receptors may also be activated by IFN-α. Higher absolute numbers of CD4+T-cells and higher levels of Th1 cytokines occur in females[15]and they show higher levels of antibodies and stronger T-cell activation than males after vaccination.[16]The estrogen/estrogen receptor axis can be simultaneously involved not only in the reproductive system, bone, and cardiovascular function but also in immune regulation.[17-21]The protective role of estrogen in the progress of chronic liver disease by inhibiting the replication of HBV has also been reported.[21]
Our study indicates that patients who have different T29C genotypes which react differently to IFN-α therapy, so we suggest that these genotypes may enhance the effect of IFN-α therapy by enhancing cellular immunity differently. This indicates a direct or indirect relationship and fine balance between the levels of ESR and IFN-α.
Estrogen carries out its function mainly through ESR1. The polymorphism of ESR1 affects the expression and function of estrogen.[22]The T29C genotype polymorphism of ESR1 which is caused by a C→T transition at codon 10 in exon 1[4]is a synonymous polymorphism and may not have functional consequences.[23]As there is no nonsynonymous SNP which results in amino acid change in the ESR1 gene, it is very likely that there exist(s) as-yet-unidentified causative regulatory polymorphism(s) associated with T29C.[9,24]The yet-unidentified causative regulatory polymorphism(s) may be in linkage disequilibrium with the observed associated T29C in the promoter introns. For example, in PvuⅡ and XbaⅠRFLP,[22]a(TA)n variable number of tandem repeats[18]can affect gene transcription.[23]Therefore, these polymorphisms cannot be excluded from linkage disequilibrium with the observed associated T29C. In that case, the association between ESR T29C genotype polymorphism and the initial response to IFN-α in patients with CHB may be a superficial phenomenon. More studies are required to fully understand the mechanism of the T29C genotype polymorphism of ESR1 in the initial response to IFN-α in patients with CHB.
Funding: This study was supported by grants from the National Natural Science Foundation of China (No. 30771907), and the Foundation of Pre-973 Program Projects (No. 2009CB526411).
Ethical approval: Not needed.
Contributors: ZTT and ZZH proposed the study and analyzed the data. ZTT wrote the first draft. All authors contributed to the design and interpretation of the study and to further drafts. LX is the guarantor.
Competing interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Shamliyan TA, MacDonald R, Shaukat A, Taylor BC, Yuan JM, Johnson JR, et al. Antiviral therapy for adults with chronic hepatitis B: a systematic review for a National Institutes of Health Consensus Development Conference. Ann Intern Med 2009;150:111-124.
2 Zhao H, Kurbanov F, Wan MB, Yin YK, Niu JQ, Hou JL, et al. Genotype B and younger patient age associated with better response to low-dose therapy: a trial with pegylated/ nonpegylated interferon-alpha-2b for hepatitis B e antigenpositive patients with chronic hepatitis B in China. Clin Infect Dis 2007;44:541-548.
3 Brunetto MR, Moriconi F, Bonino F, Lau GK, Farci P, Yurdaydin C, et al. Hepatitis B virus surface antigen levels: a guide to sustained response to peginterferon alfa-2a in HBeAgnegative chronic hepatitis B. Hepatology 2009;49:1141-1150.
4 Moucari R, Mackiewicz V, Lada O, Ripault MP, Castelnau C, Martinot-Peignoux M, et al. Early serum HBsAg drop: a strong predictor of sustained virological response to pegylated interferon alfa-2a in HBeAg-negative patients. Hepatology 2009;49:1151-1157.
5 de Andrade DR Jr, de Andrade DR. The influence of the human genome on chronic viral hepatitis outcome. Rev Inst Med Trop Sao Paulo 2004;46:119-126.
6 Han YN, Yang JL, Zheng SG, Tang Q, Zhu W. Relationship of human leukocyte antigen class II genes with the susceptibility to hepatitis B virus infection and the response to interferon in HBV-infected patients. World J Gastroenterol 2005;11:5721-5724.
7 Huang YX, Ma LN, Chen XY, Li Z, Huang YL, Shen CL, et al. Genetic polymorphisms of MxA protein and eIF-2a-reg2 and their responses to interferon treatment in patients with chronic hepatitis B. Zhonghua Gan Zang Bing Za Zhi 2007;15:187-191.
8 Chu RH, Ma LX, Wang G, Shao LH. Influence of HLA-DRB1 alleles and HBV genotypes on interferon-alpha therapy for chronic hepatitis B. World J Gastroenterol 2005;11:4753-4757.
9 Zhai Y, Zhou G, Deng G, Xie W, Dong X, Zhang X, et al. Estrogen receptor alpha polymorphisms associated with susceptibility to hepatocellular carcinoma in hepatitis B virus carriers. Gastroenterology 2006;130:2001-2009.
10 Deng G, Zhou G, Zhai Y, Li S, Li X, Li Y, et al. Association of estrogen receptor alpha polymorphisms with susceptibility to chronic hepatitis B virus infection. Hepatology 2004;40:318-326.
11 Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guideline on prevention and treatment of chronic hepatitis B in China (2005). Chin Med J (Engl) 2007; 120:2159-2173.
12 Liaw YF, Leung N, Guan R, Lau GK, Merican I, McCaughan G, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2005 update. Liver Int 2005;25:472-489.
13 Boroumand M, Ghaedi M, Mohammadtaghvaei N, Pourgholi L, Anvari MS, Davoodi G, et al. Association of estrogen receptor alpha gene polymorphism with the presence of coronary artery disease documented by coronary angiography. Clin Biochem 2009;42:835-839.
14 Rouet F, Chaix ML, Kpozehouen A, Inwoley A, Anaky MF, Fassinou P, et al. Relationship of CD4+T-cell counts and plasma HIV-1 RNA levels with serological HBeAg/anti-HBe patterns obtained in West-African HBV-HIV-1-co-infected children. J Trop Pediatr 2009;55:409-412.
15 Amadori A, Zamarchi R, De Silvestro G, Forza G, Cavatton G, Danieli GA, et al. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat Med 1995;1:1279-1283.
16 Michaels RM, Rogers KD. A sex difference in immunologic responsiveness. Pediatrics 1971;47:120-123.
17 Herynk MH, Fuqua SA. Estrogen receptor mutations in human disease. Endocr Rev 2004;25:869-898.
18 Albagha OM, Pettersson U, Stewart A, McGuigan FE, MacDonald HM, Reid DM, et al. Association of oestrogen receptor alpha gene polymorphisms with postmenopausal bone loss, bone mass, and quantitative ultrasound properties of bone. J Med Genet 2005;42:240-246.
19 Ban Y, Taniyama M, Tozaki T, Tomita M, Ban Y. Estrogen receptor alpha dinucleotide repeat polymorphism in Japanese patients with autoimmune thyroid diseases. BMC Med Genet 2000;1:1.
20 Yuen MF, Tanaka Y, Fong DY, Fung J, Wong DK, Yuen JC, et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol 2009;50:80-88.
21 Shimizu I, Ito S. Protection of estrogens against the progression of chronic liver disease. Hepatol Res 2007;37:239-247.
22 Anna P, Katarzyna MR, Maryna KR, Anna G, Andrzej R, Malgorzata SZ. Puvll and Xbal gene polymorphisms of estrogen receptor alpha in children and young adults with cancer from north-eastern region of Poland. Pol Merkur Lekarski 2008;25:137-140.
23 Cohn CS, Sullivan JA, Kiefer T, Hill SM. Identification of an enhancer element in the estrogen receptor upstream region: implications for regulation of ER transcription in breast cancer. Mol Cell Endocrinol 1999;158:25-36.
24 Gold B, Kalush F, Bergeron J, Scott K, Mitra N, Wilson K, et al. Estrogen receptor genotypes and haplotypes associated with breast cancer risk. Cancer Res 2004;64:8891-8900.
Received July 13, 2009
Accepted after revision March 4, 2010
Author Affiliations: Department of Infectious Diseases, First Affiliated Hospital, Anhui Medical College, Hefei 230022, China (Zhang TT, Zhang ZH, Gao YF, Zhang YF and Li X); Division of Clinical Immunology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China (Zhang ZH and Yang DL)
Xu Li, MD, Department of Infectious Diseases, First Affiliated Hospital, Anhui Medical College, Hefei 230022, China (Tel: 86-551-2922912; Email: aylixu@yahoo.com.cn)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Pancreas transplantation in the mouse
- Modified arteriaIization of orthotopic Iiver transpIantation in a mouse modeI
- Relationship between the expression of IP-10 and IP-10 mRNA in peripheral blood and HBV DNA level in patients with cirrhosis
- Integrity of the pancreatic duct-acinar system in the pathogenesis of acute pancreatitis
- Adult-to-adult living donor liver transplantation for malignant metastatic melanoma to the liver
- An effective model for predicting acute kidney injury after liver transplantation
