Adult-to-adult living donor liver transplantation for malignant metastatic melanoma to the liver
2010-07-05
Chengdu, China
Adult-to-adult living donor liver transplantation for malignant metastatic melanoma to the liver
Ji Zhao, Lu-Nan Yan and Bo Li
Chengdu, China
BACKGROUND: Metastases from malignant melanoma to the liver are rare in China, and surgical resection may be of potential benefit. Liver transplantation for this disease has never been reported.
METHODS: We report a case of adult-to-adult living donor liver transplantation (A-A LDLT) for metastatic melanoma. With a surgical history of ocular melanoma, the recipient presented with emaciation from a large right hepatic mass which also probably had portal vein invasion. A-A LDLT was successfully performed and no postoperative complications were observed in either the donor or the recipient. Postoperative pathology confirmed the diagnosis of metastatic malignant melanoma; however no adjuvant chemotherapy was employed after transplantation. We also reviewed the literature on the surgical treatment of metastatic malignant melanoma to the liver and discussed the LDLT indications.
RESULT: Recurrence occurred 6 months after surgery and the patient died from recurrence of the disease 8 months posttransplant.
CONCLUSIONS: Review of the literature suggested that only a small subset of selected patients may benefit from liver resection. Large metastatic disease in the liver potentially involving a major vessel, as in this case, should be contraindicated for liver transplantation.
(Hepatobiliary Pancreat Dis Int 2010; 9: 329-332)
liver transplantation; living donor; melanoma; indication
Introduction
Metastases from malignant melanoma to the liver are diagnosed in 10% to 20% of patients, with poor prognosis.[1]Chemotherapy and immunotherapy have limited impact on such patients. Patients with the disease localized to the liver are few, but accumulated experience in surgical management suggests that liver resection may improve overall survival and occasionally long-term cure in selected patients whose metastatic tumor can be completely resected.[2-4]However, the criteria for selection of patients suitable for resection remain unclear.
We present a case of a large right hepatic mass and disseminated nodules confined to the liver with probable portal vein invasion without extra-hepatic metastases. The lesion could be radically resected using complete hepatectomy. The patient underwent adultto-adult living donor liver transplantation (A-A LDLT) which was followed by early recurrence and death. To our knowledge, this is the first case of A-A LDLT for metastatic melanoma to the liver reported worldwide. This case cautions transplant surgeons that LDLT should not be done for patients with large lesions and major vascular invasion because of the dismal prognosis.
Case report
The patient was a 56-year-old woman admitted for progressive wasting and a solid mass in the right hepatic lobe revealed by CT. The family history was negative for cutaneous lesions; however there was a surgical history of left eyeball excision about 42 months before presentation. Malignant melanoma pathologically was diagnosed of the ciliary body without invasion of the surrounding tissues. After the surgery, she received no adjuvant therapy. On admission examination of skin surfaces including oral mucosa was negative. Physical examination was otherwise normal.
Abdominal CT revealed a 10×8 cm solid mass in the anterior sector of the right hepatic lobe as well asmultiple smaller nodules (0.3-1.5 cm) in other parts of the liver. In addition, the anterior branch of the right portal vein and the distal portion of the middle hepatic vein were compressed and irregular in shape. No ascites, intra-abdominal metastasis and abdominal or retroperitoneal enlarged lymph nodes were found (Fig. 1). Nuclide bone scan revealed no signs of bone metastasis. Chest X-ray and other laboratory findings were normal.
Metastatic malignant melanoma was diagnosed tentatively. In consideration of the size of the major lesion, multiple intrahepatic nodules and probable major vascular invasion, it was impossible to perform a partial hepatectomy and ensure tumor-free margins. Total hepatectomy and transplantation were the only treatment with curative potential. However, prognosis was not good, with a high recurrence rate and poor long-term survival because of the malignant nature of the lesion. After careful interview with family members of the patient about her treatment and the possible poor prognosis, her 31-year-old elder son determined to donate his right liver to his mother.
The donor operation took about 8.5 hours and the right liver graft weighing 550 g was harvested without the middle hepatic vein. The graft-recipient body weight rate was calculated to be 0.99%. The son was discharged 10 days after surgery without any complications. The recipient weighed 55.5 kg and was 153 cm tall. The operation took 11 hours and 35 minutes, with a blood loss of 1700 ml. A large dark mass in the right hepatic lobe and multiple dark nodules in the rest of the liver were found during the operation (Fig. 2). No other lesions were seen in the abdomen. The subsequent procedures were performed.[5]
Her postoperative course was uneventfully. No adjuvant chemotherapy was used. The immunosuppressive regimen, included prednisone, tacrolimus and mycophenolic acid. With no signs of acute graft rejection and infection, the recipient recovered and was discharged 30 days after transplantation. Microscopic examination after operation showed a large atypical melanocytic proliferation in the resected liver. Immunohistochemical staining revealed HMB45(+), HEPA(-), and EMA(-), confirming the diagnosis of multiple metastatic malignant melanoma of the liver (Fig. 3).

Fig. 1. CT before LDLT showing irregular enhancement in the major lesion during the portal vein phase. The anterior branch of the right portal vein was compressed and irregular in shape.
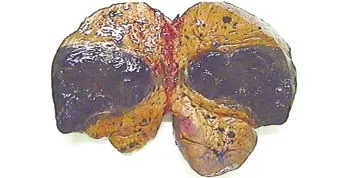
Fig. 2. When the resected liver was split, a large dark mass in the right hepatic lobe and multiple dark nodules in the rest of the liver were found.
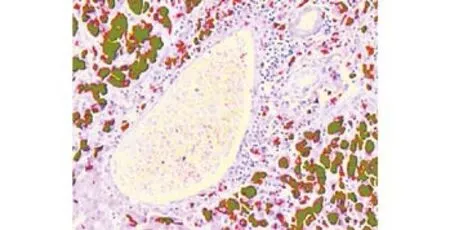
Fig. 3. Photomicrograph showing large atypical melanocytic proliferation near a hepatic lobule (HE, original magnification ×50).
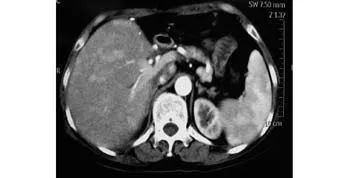
Fig. 4. CT 6 months after transplantation revealed multiple nodules in the liver graft with enhancement during the hepatic arterial phase, which was probably recurrent disease.
The recipient was followed up every month after discharge. Six months after transplantation, the patientcomplained of fatigue, wasting, loss of appetite and vomiting after meal. CT revealed multiple nodules in the liver graft with enhancement in the hepatic arterial phase and relatively lower enhancement in the portal vein phase, indicating there was probably a recurrent disease (Fig. 4). The liver function of the patient was normal. The patient received symptomatic treatment and nutritional support except adjuvant therapy. More seriously she gradually became cachexic and finally died 8 months after transplantation.
Discussion
Malignant melanoma often arises as a primary ocular or cutaneous tumor, and metastatic lesions to the liver are diagnosed in 10% to 20% of such patients. Hepatic metastases can lead to medical emergencies such as massive bleeding from the ruptured lesion and acute liver failure.[6-9]Resection is the most frequently adopted treatment option. The largest reported series of 40 patients with hepatic melanoma metastases who underwent the resection suggested that 75% had tumor recurrence, and that the median time to recurrence after resection was 8.3 months. The 5-year survival rate for patients with a primary ocular melanoma was 20.5%, whereas there were no 5-year survivors among patients with cutaneous melanoma. In conclusion, recurrence is common, and primary ocular melanoma seems to have a better survival rate than primary cutaneous melanoma.[10]Rose et al[1]reported 1750 patients with metastatic melanoma to the liver, of whom 34 (2%) underwent exploratory celiotomy. Twenty-four (71%) underwent hepatic resection and 10 (29%) underwent exploration but no resection. Eighteen patients (75%) underwent complete surgical resection, while the remaining 6 underwent palliative or debulking procedures with incomplete resection. The median disease free survival (DFS) and overall survival (OS) estimates in the 24 patients who underwent surgical resection were 12 months (range 0-147 months) and 28 months (range 2-147 months), respectively. Patients rendered surgically free of disease also tended to have improved OS (P=0.06). The median OS was 28 months for patients who underwent surgical resection compared with 4 months for patients who underwent exploration only (P<0.001). It was suggested that liver resection of metastatic melanoma to the liver may improve DFS and OS in selected patients, especially when complete resection of the tumor and histologically negative resection margins could be achieved. However, the subset of patients who were suitable for hepatectomy was small (less than 2%), and only selected patients may benefit from liver surgery. The indications are not yet clarified. Herman et al[11]proposed the following criteria for patients for possible surgical resection: absence of extra-hepatic disease after evaluation with CT/MRI and FDG-PET scans; diseasefree interval longer than 24 months after the resection of primary melanoma; presumed completely resectable lesions; and absence of clinical co-morbidities. Patients fitting the criteria may benefit from liver resection.
In our patient, primary melanoma was ocular. The disease-free interval was 42 months and there were no signs of extra-hepatic disease and co-morbidities; these were in accordance with Herman's criteria. However, partial hepatectomy would probably not ensure a complete disease resection. Therefore we adopted A-A LDLT because it was the only modality with the possibility of radical excision. To our knowledge, A-A LDLT for metastatic melanoma to the liver has never been reported. Criteria for selection of patients suitable for the operation remain unclear. The attempt was risky because of the high recurrence rate and probable poor prognosis of this malignant tumor, which make transplantation controversial. The dismal outcome of the present case cautions us that large disease and major vascular invasion should be contraindicated despite the strong will and determination of the donors who strive for even a slim chance to save their family members. Besides, immunosuppressive therapy is associated with increased cancer risk after transplantation and may have a negative impact on recipients.[12]In consideration of the shortage of donors, however, transplantation is not an optimal treatment. In the meantime, the safety of both donors and recipients cannot be overemphasized, with an ultimate goal of minimizing postoperative complications. Especially, the donors who undergo major surgery without any health benefit should be as little injured as possible.[13-15]
Acknowledgements
Special thanks are due to Zhe-Yu Chen, Zuo-Jin Liu, and Wei-Ya Wang, for their help in writing the paper. I would also like to extend my gratitude to Professor Tian-Fu Wen, Professor Ji-Chun Zhao, Dr. Wen-Tao Wang, Dr. Jia-Yin Yang and Dr. Ming-Qing Xu for their constructive suggestions.
Funding: None.
Ethical approval: Not needed.
Contributors: ZJ wrote the first draft of this case report. All authors contributed to the intellectual context and approved the final version. YLN is the guarantor.
Competing interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Rose DM, Essner R, Hughes TM, Tang PC, Bilchik A, Wanek LA, et al. Surgical resection for metastatic melanoma to the liver: the John Wayne Cancer Institute and Sydney Melanoma Unit experience. Arch Surg 2001;136:950-955.
2 Wood TF, DiFronzo LA, Rose DM, Haigh PI, Stern SL, Wanek L, et al. Does complete resection of melanoma metastatic to solid intra-abdominal organs improve survival? Ann Surg Oncol 2001;8:658-662.
3 Hsueh EC, Essner R, Foshag LJ, Ye X, Wang HJ, Morton DL. Prolonged survival after complete resection of metastases from intraocular melanoma. Cancer 2004;100:122-129.
4 Vahrmeijer AL, van de Velde CJ, Hartgrink HH, Tollenaar RA. Treatment of melanoma metastases confined to the liver and future perspectives. Dig Surg 2008;25:467-472.
5 Yan LN, Li B, Zeng Y, Wen TF, Zhao JC, Wang WT, et al. Modified techniques for adult-to-adult living donor liver transplantation. Hepatobiliary Pancreat Dis Int 2006;5:173-179.
6 Cooperman AM, Weiland LH, Welch JS. Massive bleeding from a ruptured metastatic hepatic melanoma treated by hepatic lobectomy. Case report and review of the literature. Mayo Clin Proc 1976;51:167-170.
7 Dousei T, Miyata M, Yamaguchi T, Nagaoka M, Takahashi E, Kawashima Y. Rupture of liver metastasis of malignant melanoma--a case of hepatic resection. Jpn J Surg 1991;21:480-484.
8 Montero JL, Muntané J, de las Heras S, Ortega R, Fraga E, De la Mata M. Acute liver failure caused by diffuse hepatic melanoma infiltration. J Hepatol 2002;37:540-541.
9 Kaplan GG, Medlicott S, Culleton B, Laupland KB. Acute hepatic failure and multi-system organ failure secondary to replacement of the liver with metastatic melanoma. BMC Cancer 2005;5:67.
10 Pawlik TM, Zorzi D, Abdalla EK, Clary BM, Gershenwald JE, Ross MI, et al. Hepatic resection for metastatic melanoma: distinct patterns of recurrence and prognosis for ocular versus cutaneous disease. Ann Surg Oncol 2006;13:712-720.
11 Herman P, Machado MA, Montagnini AL, D'Albuquerque LA, Saad WA, Machado MC. Selected patients with metastatic melanoma may benefit from liver resection. World J Surg 2007;31:171-174.
12 Haagsma EB, Hagens VE, Schaapveld M, van den Berg AP, de Vries EG, Klompmaker IJ, et al. Increased cancer risk after liver transplantation: a population-based study. J Hepatol 2001;34:84-91.
13 Haga J, Shimazu M, Wakabayashi G, Tanabe M, Kawachi S, Fuchimoto Y, et al. Liver regeneration in donors and adult recipients after living donor liver transplantation. Liver Transpl 2008;14:1718-1724.
14 Yan L, Li B, Zeng Y, Wen T, Zhao J, Wang W, et al. Preliminary experience for reducing biliary complication in adult-toadult living donor liver transplantation using right lobe graft. Hepatol Res 2007;37:305-309.
15 Taner CB, Dayangac M, Akin B, Balci D, Uraz S, Duran C, et al. Donor safety and remnant liver volume in living donor liver transplantation. Liver Transpl 2008;14:1174-1179.
Received September 13, 2009
Accepted after revision December 1, 2009
Author Affiliations: Department of Liver and Vascular Surgery, West China Hospital, Sichuan University, Chengdu 610041, China (Zhao J, Yan LN and Li B); Multiple Organ Transplant Center, Sichuan Provincial People's Hospital, Chengdu 610072, China (Zhao J)
Lu-Nan Yan, MD, PhD, Department of Liver and Vascular Surgery, West China Hospital, Sichuan University, Chengdu 610041, China (Tel: 86-28-85422476; Fax: 86-28-85423724; Email: yanlvnan@163. com)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Pancreas transplantation in the mouse
- Modified arteriaIization of orthotopic Iiver transpIantation in a mouse modeI
- Relationship between the expression of IP-10 and IP-10 mRNA in peripheral blood and HBV DNA level in patients with cirrhosis
- Integrity of the pancreatic duct-acinar system in the pathogenesis of acute pancreatitis
- T29C genotype polymorphism of estrogen receptor alpha is associated with initial response to interferon-alpha therapy in chronic hepatitis B patients
- An effective model for predicting acute kidney injury after liver transplantation
