不同氧疗方案对小鼠急性肾损伤保护作用的比较
2024-11-29徐峰夏静邵雅楠李丹吴新贺

[摘" "要]" "目的:比较不同给氧方案对脂多糖(lipopolysaccharide, LPS)诱导的急性肾损伤(acute kidney injury, AKI)模型小鼠肾组织炎症性损伤及外周血炎症因子的影响。方法:采用ICR雌性小鼠,腹腔注射LPS(5 mg/kg)建立小鼠AKI模型,随机分成:(1)空白组(Control组),注射同等量的生理盐水;(2)模型组(LPS组);(3)纯氧治疗组(LPS+100%O2组);(4)2.0 绝对大气压(atmosphere absolute, ATA)高压氧(hyperbaric oxygen, HBO)治疗组(LPS+2.0 ATA HBO组);(5)3.0 ATA HBO治疗组(LPS+3.0 ATA HBO组),治疗后24 h,通过流式微球技术及实时荧光定量PCR(real-time PCR, RT-PCR)实验分别检测肾组织和外周血中促炎因子IL-1β、IL-6和TNF-α,以及抑炎因子IL-10水平。此外,通过HE染色法观察肾组织炎症性损伤的组织形态变化。结果:IL-1β、IL-6和TNF-α在LPS诱导24 h后均显著增加;100%O2和2.0 ATA HBO治疗均可抑制促炎因子IL-1β、IL-6和TNF-α的释放,且2.0 ATA HBO抑制作用更显著(Plt;0.05或Plt;0.01);此外,LPS+2.0 ATA HBO组抑炎因子IL-10水平显著增高(Plt;0.01)。HE染色结果表明,与LPS组相比,100%O2和2.0 ATA HBO治疗后,肾组织炎症性损伤均有所减轻,且LPS+2.0 ATA HBO组改善效果最好(Plt;0.05或Plt;0.01),而LPS+3.0 ATA HBO组肾组织损伤未改善。结论:100%O2和2.0 ATA HBO治疗均可明显改善AKI小鼠炎症性损伤,且2.0 ATA HBO改善作用更好。
[关键词]" "急性肾损伤;氧疗;高压氧;常压氧;炎症;细胞因子;小鼠
[中图分类号]" "R692" " " " " " " "[文献标志码]" "A" " " " " " " "[文章编号]" "1674-7887(2024)02-0154-05
Comparison of the protective effects of different oxygen therapy regimens against
acute kidney injury in mice*
XU Feng1**, XIA Jing2, SHAO Yanan2, LI Dan2, WU Xinhe2***" " " " (1Department of Nephrology, 2Department of Rehabilitation Medicine, the Affiliated Rehabilitation Hospital of Nantong University, Jiangsu 226002)
[Abstract]" "Objective: Comparison of the effects of different oxygen therapy regimens on renal tissue inflammatory injury and peripheral blood inflammatory factors in a mouse model of lipopolysaccharide(LPS)-induced acute kidney injury(AKI). Methods: ICR female mice were injected intraperitoneally with 5 mg/kg of LPS to establish a model of AKI. They were randomly divided into the following groups: (1)Control group(injected with the same amount of physiological saline); (2)Model group(LPS group); (3)Pure oxygen treatment group(injected with LPS and 100%O2); (4)2.0 atmosphere absolute(ATA) hyperbaric oxygen(HBO) treatment group(LPS+2.0 ATA HBO group); (5)3.0 ATA HBO treatment group LPS+3.0 ATA HBO group). The pro-inflammatory cytokines IL-1β, IL-6, and TNF-α and the anti-inflammatory cytokine IL-10 in peripheral blood and kidney tissue were detected by flow cytometric bead array and real-time PCR(RT-PCR) assays. The histomorphological changes of kidney tissue were observed by HE staining. Results: IL-1β, IL-6, and TNF-α levels were significantly increased after 24 hours of LPS induction. 100%O2 and 2.0 ATA HBO could inhibit the release of IL-1β, IL-6, and TNF-α, and 2.0 ATA HBO had a more pronounced impact, with a significantly increased IL-10 level in this treatment group. HE staining showed that compared with LPS, 100%O2 and 2.0 ATA HBO could alleviate the inflammatory injury of kidney, and 2.0 ATA HBO showed a better effect. Nevertheless, 3.0 ATA HBO did not reverse the damage to the kidney tissue. Conclusion:" The above results suggested that both 100%O2 and 2.0 ATA HBO treatments significantly improved inflammatory injury in AKI mice. Moreover, 2.0 ATA HBO had a superior effect on inhibiting tissue inflammation.
[Key words]" "acute kidney injury; oxygen therapy; hyperbaric oxygen; normobaric oxygen; inflammation; cytokines; mouse
急性肾损伤(acute kidney injury, AKI)是临床上常见的危急重症之一,具有高发病率和高死亡率的特点;其发病机制与炎症反应有关,即促炎因子与抑炎因子的不平衡引起组织损伤,最终导致病情恶化及死亡率升高[1-3]。在脂多糖(lipopolysaccharide, LPS)诱导的AKI模型中,观察到多种促炎因子浸润导致肾脏组织损伤;因此,抑制机体炎症过度反应对LPS诱导的AKI的保护作用极为重要。
越来越多的研究[4]表明,氧疗可调控炎症反应,对组织炎症性损伤具有保护作用。高压氧(hyperbaric oxygen, HBO)治疗是一种基于在增强大气压下暴露于纯氧的治疗方法,通常作为感染等不同疾病的主要或辅助疗法,特别是深部或顽固性感染,如坏死性筋膜炎、骨髓炎和慢性软组织感染以及感染性心内膜炎等[5-7]。实验动物研究[8]表明,2.0 绝对大气压(atmosphere absolute, ATA)HBO治疗可减轻砷诱导的肝脏和肾脏组织损伤;2.5 ATA HBO治疗可有效改善LPS诱导的小鼠急性肺损伤[9];100%O2和3.0 ATA HBO预处理均可减轻脓毒血症模型大鼠急性肝组织损伤[10]。临床研究[11]表明,2.0 ATA HBO治疗减少AKI患者的住院时间,改善了肾血流动力学;2.5 ATA HBO治疗可加速伤口愈合,改善糖尿病足溃疡并提高患者的生存率[12];3.0 ATA HBO可有效治疗颈部坏死性感染[13];但当压力>3.0 ATA时,通常报道更多的不利影响[5]。然而AKI临床常需依靠医师经验选择治疗方案,关于肾组织损伤对于不同氧疗方案的应答反应是否有差异也缺乏研究。因此,需要探究肾脏器官组织对不同氧疗应答反应,为辅助肾脏器官组织损伤治疗摸索最佳氧疗方案提供实验依据。
目前临床使用的氧疗方法逐渐多样化,如面罩给氧、机械通气给氧和高压氧等[14-16]。因此,采用LPS诱导AKI小鼠模型,模拟临床上不同的氧疗方法进行干预,通过分析外周血和肾脏组织器官的炎症因子表达以及器官损伤评分,从抑制炎症角度比较不同吸氧方案对AKI模型小鼠肾组织损伤的治疗效果,为肾组织器官炎症损伤治疗时氧疗方案的选择提供初步实验依据。
1" "材料和方法
1.1" "动物来源与模型建立" "雌性ICR小鼠,体质量22~26 g,购自南通大学实验动物中心(机构许可证:SYXK(SU)-2012-0030)。所有动物置于塑料笼中,室温控制在(24±2)℃、日光灯照明(12 h/12 h明暗)、自由饮食,持续3 d。将LPS(货号:L2630, Sigma Aldrich)粉末溶于生理盐水制备成1 mg/mL的药液避光保存,ICR小鼠按照5 mg/kg剂量腹腔注射形成脓毒血症AKI模型,并立即对模型小鼠进行不同氧疗方案治疗。
1.2" "实验分组与处理" "动物随机分成5组:空白组(Control组)、模型组(LPS组)、纯氧治疗组(LPS+100%O2组)、2.0 ATA HBO治疗组(LPS+2.0 ATA HBO组)、3.0 ATA HBO治疗组(LPS+3.0 ATA HBO组),每组3~6只。Control组注射等量生理盐水,与LPS组均未进行治疗;LPS+100%O2组:将模型小鼠置于密封的带有进(出)气口的玻璃树脂箱内,在常压下接受纯氧治疗60 min;LPS+2.0 ATA HBO组与LPS+3.0 ATA HBO组:将模型小鼠置于100 L的舱内(安徽省芜湖潜水装备厂),分别在2个绝对大气压和3个绝对大气压下接受纯氧治疗60 min,且升压(减压)时间各5 min。LPS诱导后24 h,对各组小鼠腹腔注射复合麻醉剂2.5%Avertin(2-甲基-2-丁醇,货号152463;2, 2, 2-三溴乙醇,货号T48402,Sigma),深度麻醉后处死并进行取材。本实验方案获南通大学实验动物中心伦理审查批准(S20230315-003)。
1.3 " 实验方法
1.3.1" "实时荧光定量PCR(real-time PCR, RT-PCR)" "按试剂盒说明书,使用TRIzol试剂(Invitrogen,CA,美国)从肾组织中分离总RNA,并逆转录成cDNA。使用SYBR qPCR Master Mix荧光定量PCR仪进行反应,β-actin(小鼠中的Actb)用于标准化目标基因的相对转录水平。引物序列如表1。
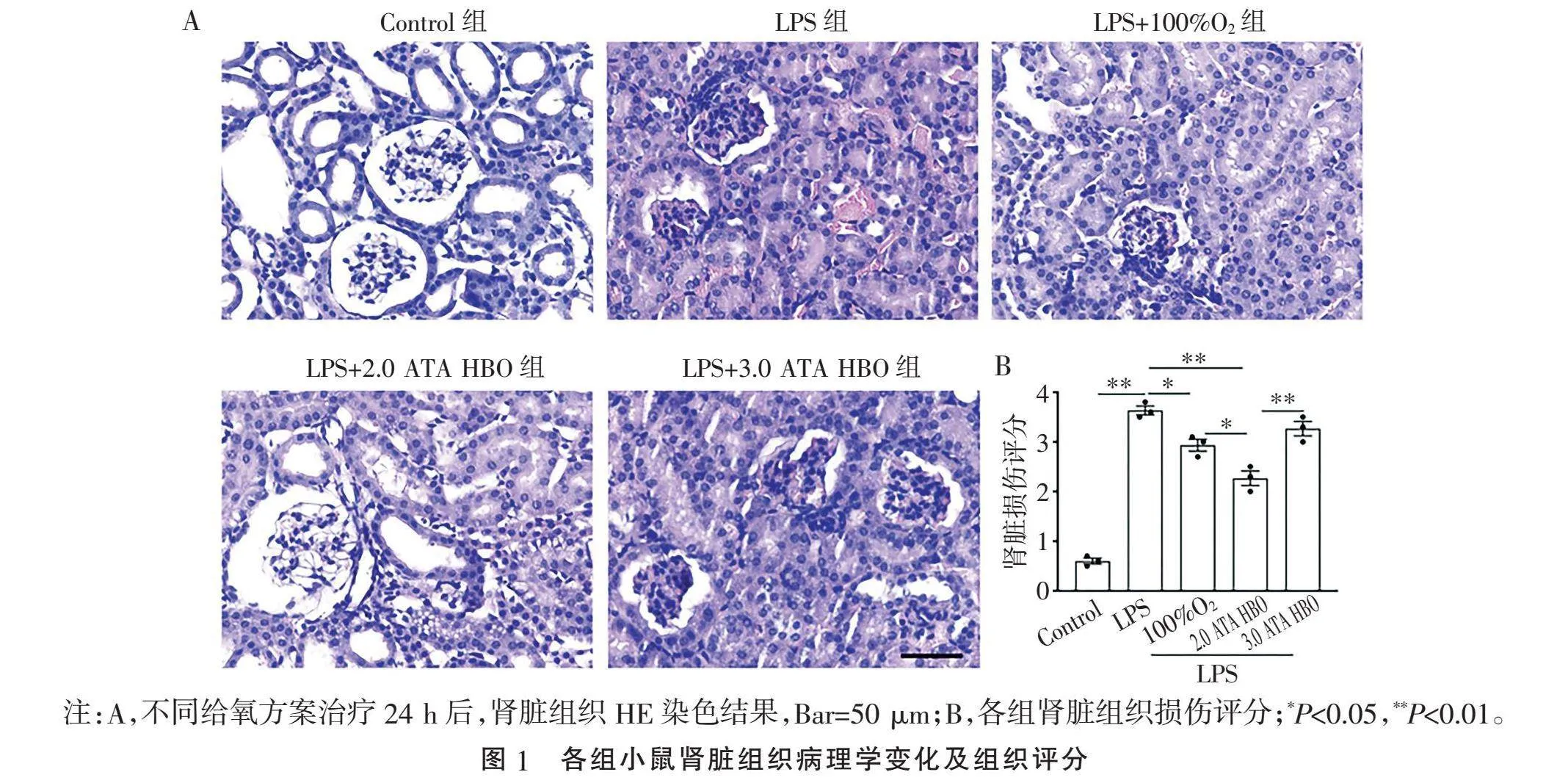
1.3.2" "HE染色及评分" "将10%甲醛固定的肾组织包埋在石蜡中,切片并行HE染色形态学检查。肾组织的病理评分由专业病理科医师进行评估。肾小管损伤程度,包括肾小管扩张、变平和空泡形成,分为5个等级(0,无;1,≤25%;2,>25%~50%;3,>50%~75%;4,gt;75%),基于光学显微镜下高倍视野中受影响的肾小管百分比;各分数相加,将总分的平均值作为肾损伤评分[17-18]。
1.3.3" "流式细胞微球技术(cytometric bead array, CBA)" "使用BDTM CBA Flex Set试剂盒和BDTM Mouse Enhance Sensitivity Master Buffer Kit试剂盒(货号562246,BD Bioscience)测量血清中4种不同炎症因子的浓度水平;IL-1β(货号562278,BD Bioscience),IL-6(货号562236,BD Bioscience),TNF-α(货号562336,BD Bioscience),IL-10(货号562263,BD Bioscience)。使用BriCyteE6(迈瑞,中国)流式细胞仪设置珠进行校准,按照试剂制造商的说明倍比稀释制备标准品,每个样本采集200个细胞,将不同目的蛋白捕获微球在待测样品中的检测荧光强度代入各自的标准品曲线方程中,计算每组样品中各种炎症因子的浓度。
1.4" "统计学方法" "使用SPSS 25.0软件进行统计分析,所有实验数据均以均数±标准误(mean±standard error, SEM)形式表示。通过单因素方差分析,差异有统计学意义后再行两两t检验,Plt;0.05表示差异有统计学意义。
2" "结" " " 果
2.1" "不同给氧方案对AKI模型小鼠肾脏组织病理损伤的减轻程度不同" "与Control组相比,肾组织的病理损伤有肾小球细胞增多,肾小管上皮细胞浑浊肿胀,间质水肿充血,中性粒细胞浸润。LPS+100%O2组治疗后,肾小管上皮细胞浑浊肿胀减轻,中性粒细胞浸润减少;LPS+2.0 ATA HBO组肾组织病理损伤均显著改善,而LPS+3.0 ATA HBO组肾脏组织病理损伤无明显改善(Plt;0.05或Plt;0.01,图1)。结果表明,在一定的氧分压范围内,氧疗对于组织病理损伤的改善效果随着氧分压的升高而升高,但超过一定阈值后,改善效果消失。
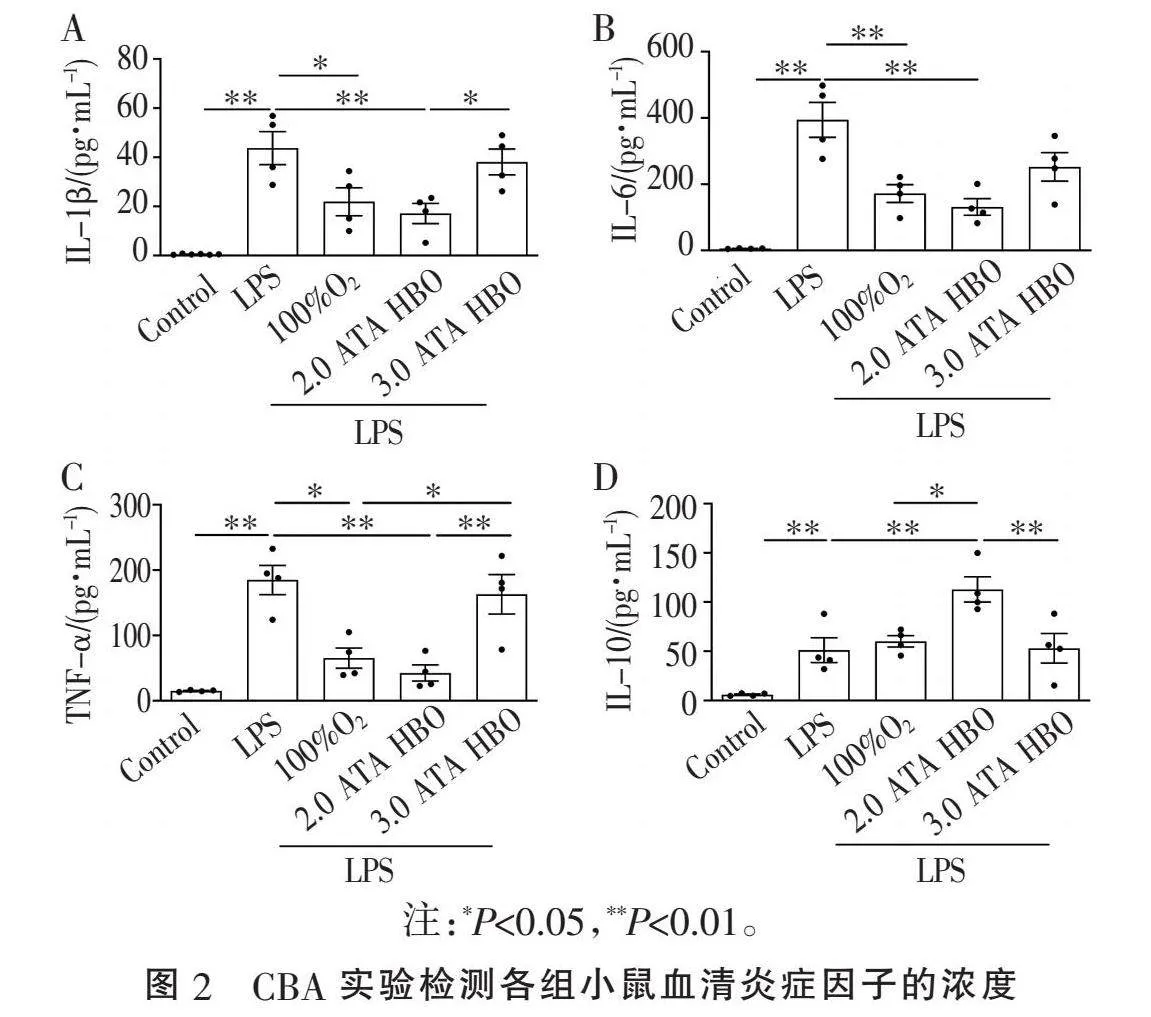
2.2" "不同给氧方案对LPS诱导的AKI模型小鼠外周血炎症因子的影响" "通过CBA实验检测各组小鼠血清中炎症因子水平。与Control组相比,LPS诱导24 h后IL-1β、IL-6和TNF-α(Plt;0.01,图2)水平均显著升高。与LPS组相比,LPS+100%O2组和LPS+2.0 ATA HBO组均抑制LPS诱导的IL-1β、IL-6和TNF-α升高,LPS+2.0 ATA HBO组作用更显著(Plt;0.01或Plt;0.05, 图2A~C),但LPS+3.0 ATA HBO组治疗前后炎症因子水平差异无统计学意义。LPS+2.0 ATA HBO组治疗后血清IL-10水平显著提高(Plt;0.01, 图2D)。结果表明,LPS诱导的小鼠AKI模型中,100%O2和2.0 ATA HBO治疗均能不同程度地抑制促炎因子的释放,2.0 ATA HBO抑制炎症因子的效果最佳。
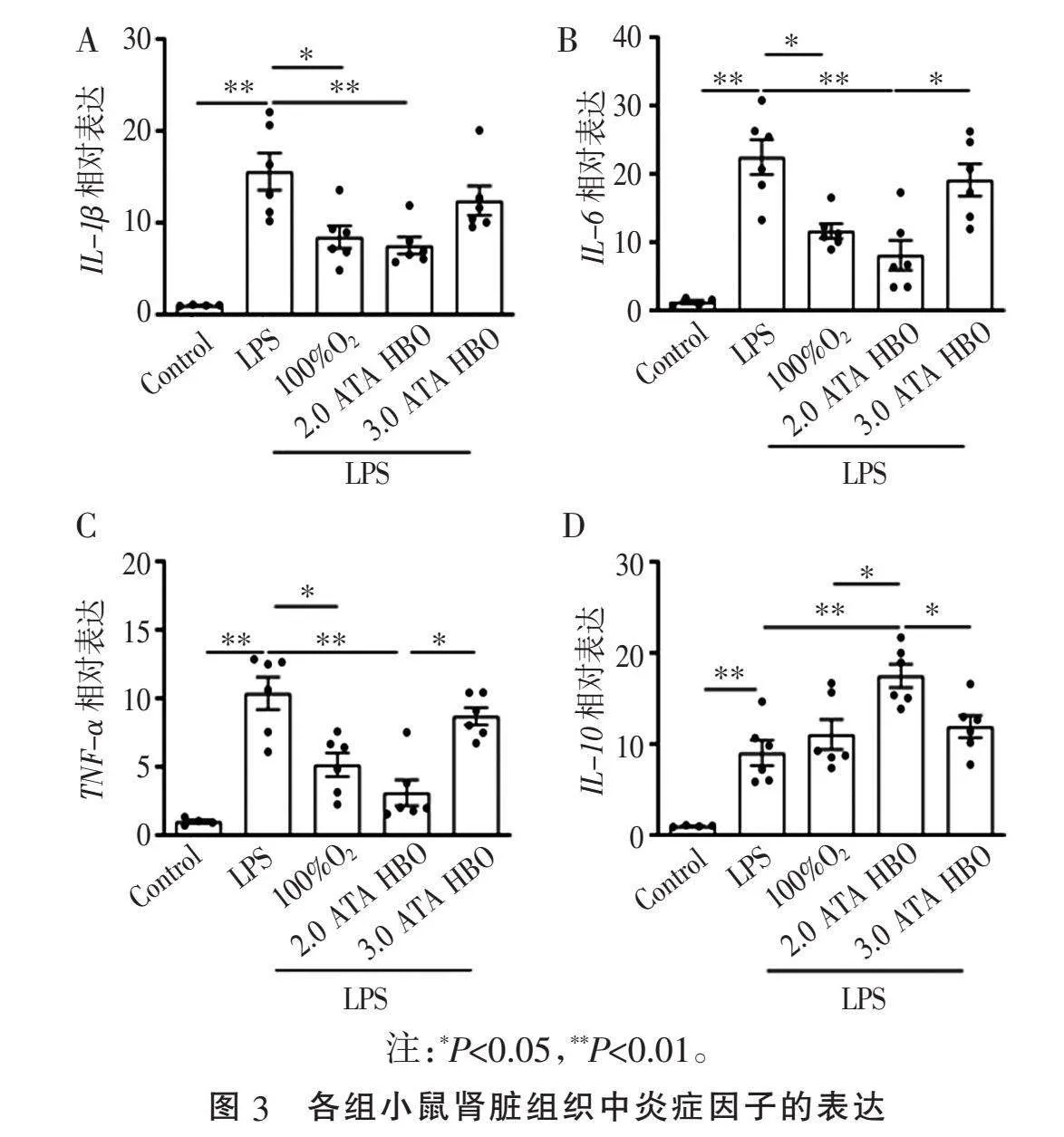
2.3" "不同给氧方案对AKI模型小鼠肾脏组织炎症因子的影响" "LPS诱导后,肾组织中促炎因子IL-1β、IL-6、TNF-α和IL-10的mRNA水平均显著上调(Plt;0.01, 图3A~D);与LPS组相比,LPS+100%O2组和LPS+2.0 ATA HBO组IL-1β、IL-6和TNF-α水平下降,LPS+2.0 ATA HBO组下降幅度更大(Plt;0.05或Plt;0.01, 图3A~C);而LPS+3.0 ATA HBO组炎症因子水平治疗前后差异无统计学意义。与LPS组相比,LPS+2.0 ATA HBO组治疗后IL-10 mRNA水平显著升高(Plt;0.01, 图3),其他组相比差异无统计学意义(P>0.05)。结果表明,不同的给氧方案对于肾脏组织炎症因子的表达影响不同,2.0 ATA HBO治疗既抑制了肾脏组织促炎因子的表达,也促进了肾脏组织抑炎因子的表达。
3" "讨" " " 论
本研究探讨了不同给氧方案对LPS诱导的AKI模型小鼠肾组织炎症性损伤的保护作用。100%O2和2.0 ATA HBO治疗均抑制了促炎因子IL-1β、IL-6和TNF-α的表达,减轻了AKI模型小鼠肾组织的损伤,仅2.0 ATA HBO治疗上调抑炎因子IL-10的表达,且改善肾组织损伤的效果更好,为HBO治疗AKI的临床应用提供了重要的实验依据。
炎症的过度反应导致组织炎症性缺氧[19],缺氧可激活炎症细胞并释放大量促炎因子,如TNF-α、IL-1β、IL-6和IL-8等,加剧组织损伤[20-21]。本研究中,100%O2和2.0 ATA HBO治疗均能抑制促炎因子的表达,减轻肾组织的炎症性损伤,这与J.L.HALBACH等[22-23]的研究结果相似,其中2.0 ATA HBO改善组织损伤效果最好,这可能与改善组织缺氧效率有关。众所周知,随着氧浓度以及氧分压的增加,血液中的氧含量增加,而 HBO通过增加血浆中的溶解氧和氧气的扩散距离更有效改善组织缺氧[5, 19],这种作用已在局灶性脑组织缺血中被证实[24]。此外,无论是在外周血还是在肾组织中,LPS+2.0 ATA HBO组的IL-10水平均明显高于其他给氧治疗组,说明不同的给氧方案对组织中炎症因子水平的影响不同。已有文献[25]表明,抑炎因子IL-10通过抑制IL-6的分泌降低脓毒血症相关AKI模型小鼠的死亡率,而HBO可上调IL-10的表达。文献[8-9, 11]报道,HBO通过抑制促炎因子的释放和增加抑炎因子的分泌来减轻小鼠急性肾组织损伤,这与本研究结果一致。但是当压力升到3.0 ATA时,促炎因子(IL-1β、IL-6和TNF-α)和抑炎因子IL-10的表达水平与LPS组均无差异,同时3.0 ATA HBO治疗对LPS诱导的多器官组织损伤均无改善作用,这可能与氧毒性水平有关。越来越多的研究[26-27]表明,氧毒性会诱导细胞死亡和疾病发生。而HBO产生的氧毒性与暴露的压力和持续时间有关,O.SUKRU等[28]发现,随着暴露时间的延长,3.0 ATA HBO治疗会导致大鼠肺和脑组织氧化应激损伤。本研究表明氧疗对肾组织炎症性损伤具有保护作用,且2.0 ATA HBO的保护作用最好。
本研究结果表明,单次2.0 ATA HBO治疗虽明显改善了肾组织炎症性损伤,但未观察其远期效果。因此,下一步计划采用生化以及病理指标对小鼠肾损伤进行长期检测,并比较不同疗程的治疗效果。此外,还可考虑在大动物模型上开展临床前的疗效观察及机制探讨。
总之,在AKI时,2.0 ATA HBO治疗在调节机体炎症反应和减轻肾组织损伤方面效果最好。这不仅加深了对 HBO肾组织炎症性损伤保护作用的认识和理解,也为拓展HBO的临床适用范围以及AKI的救治提供了重要的实验依据。
[参考文献]
[1]" "张宇慧, 杨莉. 脓毒症相关急性肾损伤[J]. 临床内科杂志, 2022, 39(6):372-376.
[2]" "LI C, WANG W, XIE S S, et al. The programmed cell death of macrophages, endothelial cells, and tubular epithelial cells in sepsis-AKI[J]. Front Med, 2021, 8:796724.
[3]" "ZARBOCK A, NADIM M K, PICKKERS P, et al. Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative workgroup[J]. Nat Rev Nephrol, 2023, 19(6):401-417.
[4]" "WOO J, MIN J H, LEE Y H, et al. Effects of hyperbaric oxygen therapy on inflammation, oxidative/antioxidant balance, and muscle damage after acute exercise in normobaric, normoxic and hypobaric, hypoxic environments: a pilot study[J]. Int J Environ Res Public Health, 2020, 17(20):7377.
[5]" "ORTEGA M A, FRAILE-MARTINEZ O, GARC?魱A-MONTERO C, et al. A general overview on the hyperbaric oxygen therapy: applications, mechanisms and translational opportunities[J]. Medicina, 2021, 57(9):864.
[6]" "KIRBY J P, SNYDER J, SCHUERER D J E, et al. Essentials of hyperbaric oxygen therapy: 2019 review[J]. Mo Med, 2019, 116(3):176-179.
[7]" "KRP1NAR , UZUN H. The effects of hyperbaric oxygen at different pressures on oxidative stress and antioxidant status in rats[J]. Medicina, 2019, 55(5):205.
[8]" "EROLU H A, BYK B,ZTOPUZ , et al. Effects of hyperbaric oxygen treatment on liver and kidney tissue in chronic arsenic toxicity[J]. Undersea Hyperb Med, 2022, 49(4):467-477.
[9]" "HAN G, MA L, GUO Y, et al. Hyperbaric oxygen therapy palliates lipopolysaccharide-induced acute lung injury in rats by upregulating AQP1 and AQP5 expression[J]. Exp Lung Res, 2015, 41(8):444-449.
[10]" "BEKTAS A, ULUSOY M, MAS R M. Do late phase hyperbaric and normobaric oxygen therapies have effect on a liver damage? An experimental sepsis model[J]. Gen Med(Los Angel), 2019, 7(3):1-6.
[11]" "SOKOLOVA K A. The role of hyperbaric oxygenation in combined treatment of acute pyelonephritis[J]. Urologiia, 2010(5):10-14.
[12]" "SHARMA R, SHARMA S K, MUDGAL S K, et al. Efficacy of hyperbaric oxygen therapy for diabetic foot ulcer, a systematic review and meta-analysis of controlled clinical trials[J]. Sci Rep, 2021, 11(1):2189.
[13]" "NEDREB"T, BRUUN T, SKJSTAD R, et al. Hyperbaric oxygen treatment in three cases of necrotizing infection of the neck[J]. Infect Dis Rep, 2012, 4(1):e21.
[14]" "ALLARDET-SERVENT J, SICARD G, METZ V, et al. Benefits and risks of oxygen therapy during acute medical illness: just a matter of dose![J]. Rev Med Interne, 2019, 40(10):670-676.
[15]" "BRANSON R D. Oxygen therapy in COPD[J]. Respir Care, 2018, 63(6):734-748.
[16]" "LEWIS R S, BAKER P E, PARKER R, et al. High-flow nasal cannulae for respiratory support in adult intensive care patient[J]. Emergencies, 2022, 34(3):222-224.
[17]" "YOO J Y, CHA D R, KIM B, et al. LPS-induced acute kidney injury is mediated by Nox4-SH3YL1[J]. Cell Rep, 2020, 33(3):108245.
[18]" "龚琴, 王木兰, 何鹿玲, 等. 比较四种因素所致实验性急性肾损伤及炎性坏死因子的表达[J]. 中药药理与临床, 2019, 35(3):180-185.
[19]" "CHOUDHURY R. Hypoxia and hyperbaric oxygen therapy: a review[J]. Int J Gen Med, 2018, 11:431-442.
[20]" "HENDRICKSON C M, MATTHAY M A. Endothelial bio-markers in human sepsis: pathogenesis and prognosis for ARDS[J]. Pulm Circ, 2018, 8(2):2045894018769876.
[21]" "HE M Y, SHI W, YU M, et al. Nicorandil attenuates LPS-induced acute lung injury by pulmonary endothelial cell protection via NF-κB and MAPK pathways[J]. Oxid Med Cell Longev, 2019, 2019:4957646.
[22]" "HALBACH J L, PRIETO J M, WANG A W, et al. Early hyperbaric oxygen therapy improves survival in a model of severe sepsis[J]. Am J Physiol Regul Integr Comp Physiol, 2019, 317(1):R160-R168.
[23]" "HAUSER B, BARTH E, BASSI G, et al. Hemodynamic, metabolic, and organ function effects of pure oxygen ventilation during established fecal peritonitis-induced septic shock[J]. Crit Care Med, 2009, 37(8):2465-2469.
[24]" "KJELLBERG A, ABDEL-HALIM L, HASSLER A, et al. Hyperbaric oxygen for treatment of long COVID-19 syndrome(HOT-LoCO): protocol for a randomised, placebo-controlled, double-blind, phase II clinical trial[J]. BMJ Open, 2022, 12(11):e061870.
[25]" "SCH?譈TTLER J, NEUMANN S. Interleukin-6 as a prognostic marker in dogs in an intensive care unit[J]. Vet Clin Pathol, 2015, 44(2):223-228.
[26]" "CHECA J, ARAN J M. Reactive oxygen species: drivers of physiological and pathological processes[J]. J Inflamm Res, 2020, 13:1057-1073.
[27]" "CHEN R L, LAI U H, ZHU L L, et al. Reactive oxygen species formation in the brain at different oxygen levels: the role of hypoxia inducible factors[J]. Front Cell Dev Biol, 2018, 6:132.
[28]" "SUKRU O, AHMET K, TURGUT T, et al. Correlation between hyperbaric oxygen exposure pressures and oxidative parameters in rat lung, brain, and erythrocytes[J] .Clin Biochem, 2005, 38(8):706-711.
[收稿日期] 2023-12-27
