日粮添加表皮生长因子对断奶仔猪肠道抗氧化能力、炎症反应和免疫状态的影响
2024-04-13王茜茜王丽霞刘东李雅丽李建中杨焕胜
王茜茜 王丽霞 刘东 李雅丽 李建中 杨焕胜


收稿日期:2023-07-01;修回日期:2023-09-03。
基金項目:国家自然科学基金重点项目(32130099);湖南省自然科学基金项目(2020JJ5635);湖南省科技创新平台与人 才计划项目(2022RC3060)。
作者简介:王茜茜,硕士研究生。
* 通信作者:杨焕胜,教授,主要从事动物营养生理相关研究。E-mail: yhs@hunnu.edu.cn。
Received date: 2023-07-01; Revised date: 2023-09-03.
Foundation items: Key Project of National Natural Science Foundation of China (32130099); Hunan Provincial Natural Science Foundation (2020JJ5635); Hunan Provincial Science and Technology Innovation Platform and Talent Project (2022RC3060).
Biography: WANG Qianqian, master student.
* Corresponding author: YANG Huansheng, professor, mainly engaged in the research of animal nutrition and physiology.
E-mail: yhs@hunnu.edu.cn.
Effects of Dietary Supplementation with Epidermal Growth Factor on Intestinal Antioxidant Capacity, Inflammatory Response, and Immune Status in Weaning Piglets
WANG Qianqian1, WANG Lixia1, 2, LIU Dong1, 2, LI Yali1, LI Jianzhong1, YANG Huansheng1, 2*
(1. Hunan International Joint Laboratory of Animal Intestinal Ecology and Health, Changsha 410081, China; 2. Chinese Academy of Science, Institute of Subtropical Agriculture, Changsha 410125, China)
Abstract: Epidermal growth factor (EGF) is a cytoprotective peptide that plays a crucial role in gut growth and health. The study mainly explored the effects of EGF on the intestinal antioxidant capacity, inflammatory response, immune status in weaning piglets. Forty-two 21-day-old weaned piglets were randomly assigned to three treatments consisting of a same basic diet containing 0 (control), 200, or 400 ?g/kg EGF, respectively. There were 14 replicates per treatment, and 7 piglets per treatment were sampled on days 7 and 14 of the experiment. Dietary supplementation of 200 ?g/kg EGF increased the activity of superoxide dismutase (SOD) during the entire experimental period. This supplementation decreased malondialdehyde (MDA) content whereas it increased serum immunoglobulin A (IgA) content on day 7 post-weaning. Animals receiving the diet supplemented with 400 ?g/kg EGF decreased concentration of tumor necrosis factor-α (TNF-α) and tended to increase the level of secretory immunoglobulin A (SIgA) in the overall experimental period. In addition, the phosphorylation level of nuclear factor-κB (NF-κB) p65 was higher for piglets fed EGF diet. In summary, EGF can enhance intestinal antioxidant capacity, decrease inflammatory response, and increase immune status in weaned piglets, suggesting that EGF has a positive role in piglet gut health.
Key words: epidermal growth factor (EGF); antioxidant capacity; immunity; inflammation; weaning piglets
CLC number: S828.9 Document code: ADOI:10.3969/j.issn.1007-7146.2024.01.007
摘 要:表皮生長因子(EGF)作为一种细胞保护肽,在肠道生长和健康中起着重要的作用。本研究主要探讨EGF对断奶仔猪肠道抗氧化能力、炎症反应和免疫状态的影响。选取42头21日龄的断奶仔猪随机分配到3个处理组中,这些处理组均由相同的基础日粮组成,分别含有0(对照)、200或400 ?g/kg EGF。每个处理14个重复,并且在试验的第7天和第14天对每个处理的7头仔猪进行采样。在整个试验期间,膳食补充200 ?g/kg EGF增加了超氧化物歧化酶(SOD)的活性。这种补充也降低了断奶后第7天丙二醛(MDA)的含量,但增加了血清免疫球蛋白A(IgA)的含量。在整个试验期间,补充400 ?g/kg EGF降低了肿瘤坏死因子-α(TNF-α)的浓度,并倾向于提高分泌型免疫球蛋白A(SIgA)的水平。此外,饲喂EGF日粮的仔猪核因子-κB(NF-κB)p65的磷酸化水平相对更高。综上,EGF可以提高断奶仔猪的肠道抗氧化能力,减少炎症反应,并提高免疫状态,这表明EGF对仔猪肠道健康有积极作用。
关键词:表皮生长因子;抗氧化能力;免疫力;炎症;断奶仔猪
中图分类号:S828.9 文献标志码:ADOI:10.3969/j.issn.1007-7146.2024.01.007
(Acta Laser Biology Sinica, 2024, 33(1): 057-064)
Weaning is possibly the most stressful event for swine in commercial production [1], because those animals experience multiple psychosocial and environmental stressors during the transition from breast milk to solid food [2]. Consequently, the gastrointestinal tract of newly weaned piglets is often accompanied by architectural disorders and functional alterations, which are pathologically characterized by villous atrophy, immune system dysfunctions and severe inflammatory diseases [3-4]. Mammalian milk contains many important growth factors that directly affect gut maturation and immunologic defense [5-7]. After early weaning, the decreased intake of milk-borne growth factors may be a major cause of impairment of immune system and inflammatory response.
The epidermal growth factor (EGF) is one of the most abundant growth factors found in maternal milk [8]. It has a maturing, nourishing, and healing effect on the intestinal mucosa [3]. For example, studies have shown that EGF can accelerate wound healing by down-regulating nitric oxide content and lipid peroxidation, so it is considered to be an oxygen free radical scavenger [9]. Several studies also demonstrated that administration of exogenous EGF reduced the incidence of disease and inhibited production of pro-inflammatory cytokines in diverse experimental models [10-12]. In addition, through EGF receptor (EGFR) signaling, EGF is effective in triggering the nuclear factor-κB (NF-κB) activation, which plays a pivotal role in inflammatory responses and adaptive immune regulation in macrophage [13-14].
There have been many attempts in pigs to confirm that EGF can stimulate gut growth and nutrient digestion [8, 15-16]. However, there are few related studies on the effect of EGF on the immune system of weaned piglets. Therefore, the objective of this study was to investigate the influences of dietary supplementation with EGF on intestinal antioxidant capacity, inflammatory response, immune status in weaned piglets and to reveal the underlying mechanism.
1 Materials and methods
1.1 Ethics statements
Animal experiments in the present study were approved by the Institutional Animal Care and Use Committee of Hunan Normal University, with the approval number of DASC -2013-020 [17].
1.2 Animals and experimental treatments
Forty-two piglets (Duroc×Landrace×Yorkshire) at (6.40±0.12) kg were weaned at 21 days old and randomly divided into 3 groups. The control group was fed with basic diets, and the experimental group was fed with experimental feed supplemented with 200 ?g/kg EGF and 400 ?g/kg EGF, respectively. There were 14 replicates per treatment. All piglets were purchased from Hunan Baodong Animal Feed Development Co., Ltd (Hunan, China). The diet formulations (Tab. 1) met the corresponding nutritional standards and had been mentioned in a previous study [18-19]. The feeding process is carried out in accordance with the standard procedures of the farm. EGF in this study was purchased from Zyme Fast Biotechnology Co., Ltd. (Hunan, China).
1.3 Tissue samples collection
On day 7 and 14 in the experiment, seven pigs from each treatment were randomly selected for sampling as described by Yang et al [20] . Serum was prepared by centrifuging the blood at 3 500×g, for 12 min and then stored at -80℃. After blood sampling, piglets were sacrificed using 4% sodium pentobarbital solution (50 mg/kg BW) and then pigs abdomen was immediately dissected to remove intestine. The jejunum was resected and washed carefully with 0.9% NaCl solution for collecting mucosa. The mucosa layer (approximately 2 g) was scraped away from each intestinal tissue and quickly frozen in liquid nitrogen to provide samples for analyzing antioxidant indexes contents, inflammatory factors concentrations, and protein synthesis.
1.4 Immune parameters, antioxidant indexes, and inflammatory cytokines analysis
The jejunal mucosa sample was ground in liquid nitrogen and mixed with 0.9% saline evenly, and centrifuged (3 200×g, 4℃, 10 min) to collect the supernatant, which was stored until the next analysis. Immunoglobulin A (IgA) was measured by kits (Yonghe Sunshine Technology Co., Ltd., Hunan). The antioxidant indicators were determined using superoxide dismutase (SOD; A001-3), malondialdehyde (MDA; A003-4) and total antioxidant capacity (T-AOC; A015-2) kits (Nanjing Built Bioengineering Research Institute, Nanjing). Based on the manufacturing and commercial description (Hubei Klass Biotechnology Co., Ltd., China), mucosal cell factor interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α) and secretory immunoglobulin A (SIgA) levels were measured using immunosorbent assay (ELISA) test agents. Bicinchoninic Acid Assay (BCA) protein concentration reagent kit (Beyotime, Shanghai) is used to determine protein concentration. The antioxidant index, pro-inflammatory cytokines and SIgA in the intestinal mucosa were calculated based on the protein concentration in the sample.
1.5 Western blot analysis
The jejunal mucosa sample was ground in liquid nitrogen, and added to a centrifuge tube containing 10 ?L phenylmethanesulfonyl fluoride (PMSF, Beyotime Biotechnology) and 1 mL RIPA buffer for lysing. The Western blot method was described previously [21]. After centrifugation at 4℃ and 12 000×g for 12 min, the supernatant was taken to determine the protein concentration. The loading amount was adjusted according to the protein concentration of different samples, mixed thoroughly with SDS sample buffer (Beoytime Biotechnology), boiled in 95℃ water for 10~12 min to denature. First, the denatured proteins were separated by SDS-polyacrylamide gel electrophoresis according to their size, and then transferred to a polyvinglidene fluoride membrane (Millipore, Billerica, MA) at a constant flow of 200 mA for 50 min. The membrane was blocked in 5% skim milk solution prepared by TBST (Applygen Technologies Inc., Beijing, China) for 2 h, and then incubated with the primary antibody [NF-κB p65, phosphorylated NF-κB p65 (p-NF-κB p65, abcam) and β-actin (Santa Cruz Biotechnology), diluted 1:1 000] overnight at 4℃. After washing 4 times with TBST, they were incubated with secondary antibodies [mouse anti-β-actin (Santa Cruz) or rabbit anti-NF-κB, p-NF-κB (Cell Signalling)] with 1:3 000 dilution for 2 h on a shaker. After incubation, the secondary antibody was removed, washed 4 times with TBST and edetected using chemiluminescence (Applygen Technologies Inc., Beijing, China). Images were subsequently acquired by autoradiography with an analytical imaging system (GeneGnome XRQ; Syngene, Cambridge, UK). The gray value of the target protein band was calculated using the β-actin band as an internal reference to obtain its protein expression level.
1.6 Statistical analysis
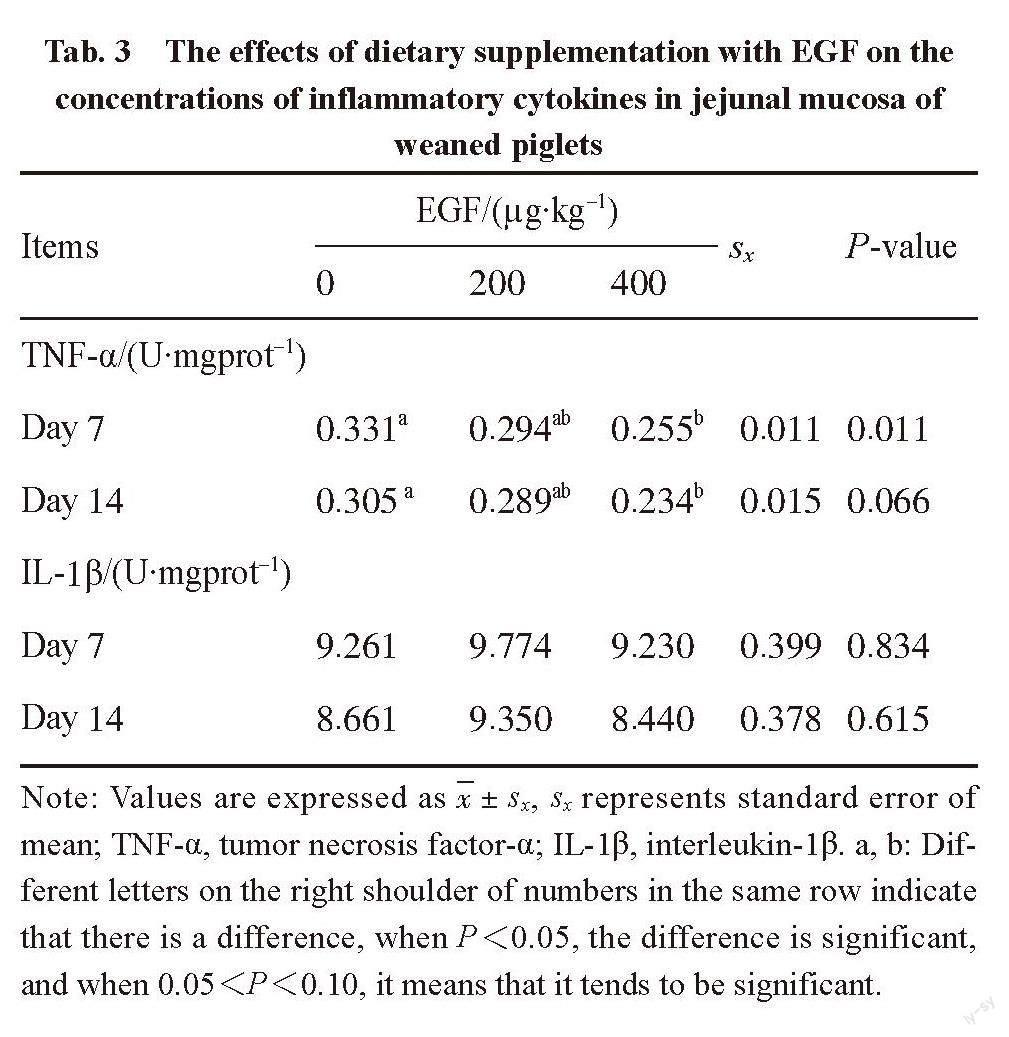
The data were examined by one-way ANOVA using SPSS software (version 22, SPSS Inc., Chicago, IL, USA). The fact of P<0.05 indicates significant difference, and the fact of 0.05 2 Results and analysis 2.1 Antioxidant indexes contents SOD activity, MDA and T-AOC content can reflect the antioxidant capacity of the tissue. The activity of SOD significantly increased (P<0.05) in the piglets fed 200 ?g/kg EGF diet on day 7 post-weaning and tended to increase (P<0.10) for those animals on day 14 post-weaning (Tab. 2). Dietary supplementation with 200 ?g/kg EGF significantly decreased (P<0.05) MDA concentration on day 7 post-weaning (Tab. 2). Meanwhile, MDA concentration tended to increase (0.05 Tab. 2 The effects of dietary supplementation with EGF on the contents of antioxidant indexes in jejunal mucosa of weaned piglets Items EGF/(?g·kg-1) sx P-value 0 200 400 SOD/(U·mL-1) Day 7 5.792b 9.010a 5.222b 0.654 0.024 Day 14 8.886b 10.096a 6.700b 0.757 0.074 MDA/(nmol·mgprot-1) Day 7 0.794a 0.290b 0.464b 0.075 0.015 Day 14 0.573ab 0.247 b 0.762a 0.097 0.058 T-AOC/(U·mgprot-1) Day 7 0.141 0.103 0.133 0.020 0.711 Day 14 0.280 0.376 0.262 0.353 0.378 Note: Values are expressed as x±sx, sx represents standard error of mean; SOD, superoxide dismutase; MDA, malondialdehyde; T-AOC, total antioxidant capacity. a, b: Different letters on the right shoulder of numbers in the same row indicate that there is a difference, when P<0.05, the difference is significant, and when 0.05 2.2 Inflammatory cytokines in jejunal mucosa TNF-α and IL-1β in the mucosa as inflammatory cytokines can reflect the level of inflammation in the body. Tab. 3 shows the effects of dietary supplementation with EGF on the concentrations of inflammatory cytokines in jejunal mucosa of weaned piglet. Dietary supplementation with 400 ?g/kg EGF decreased (P<0.05) TNF-α concentration on day 7 post-weaning. Meanwhile, TNF-α content had a decreased tendency (0.05 Tab. 3 The effects of dietary supplementation with EGF on the concentrations of inflammatory cytokines in jejunal mucosa of weaned piglets Items EGF/(?g·kg-1) sx P-value 0 200 400 TNF-α/(U·mgprot-1) Day 7 0.331a 0.294ab 0.255b 0.011 0.011 Day 14 0.305 a 0.289ab 0.234b 0.015 0.066 IL-1β/(U·mgprot-1) Day 7 9.261 9.774 9.230 0.399 0.834 Day 14 8.661 9.350 8.440 0.378 0.615 Note: Values are expressed as x±sx, sx represents standard error of mean; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β. a, b: Different letters on the right shoulder of numbers in the same row indicate that there is a difference, when P<0.05, the difference is significant, and when 0.05 2.3 Serum IgA and mucosal SIgA concentrations The concentration of IgA and SIgA in serum reflects the immune status of the body to a certain extent. Tab. 4 shows the effects of dietary supplementation with EGF on serum IgA and mucosal SIgA contents in weaned piglets. Dietary supplementation with EGF at 200 ?g/kg markedly increased (P<0.05) serum IgA concentration on day 7 post-weaning. Piglets received 400 ?g/kg EGF tended to increase (P<0.10) SIgA levels during the entire experimental period. And there was a dose-dependent increase in serum IgA and SIgA concentration on day 14 post-weaning. 2.4 The activation of transcription factor NF-κB To investigate whether NF-κB pathway was activated after EGF treatment, the protein abundance and phosphorylation state of NF-κB p65 in weaned pigs were measured by Western blot analysis. It can be concluded in Fig. 1, although the protein abundance of NF-κB p65 did not differ significantly (P>0.10) among treatment groups, the phosphorylation level of NF-κB p65 was greater (P<0.05) in the 200 ?g/kg EGF group on day 7 post-weaning. However, the phosphorylation level of NF-κB p65 was higher in the 400 ?g/kg EGF group on day 14 post-weaning. 3 Discussion The EGF is a cytoprotective peptide that plays a crucial role in gut growth, maturation or repair [10]. Previous studies have shown that EGF can increase villus height, digestive enzymes activities, and the expression of nutrient transports in weaned piglets [15, 22], which contributed to the enhancement of intestinal digestive and absorptive functions. In present study, we found that dietary supplementation with EGF elevated intestinal antioxidant capacity, decreased inflammatory response and increased immune response in weaned piglets. Furthermore, our results further suggested that EGF-mediated those effects may be associated with the activation of NF-κB signaling molecule. These findings may constitute a way to promote the maturation of the immune system in piglets and resist weaning stress in the no antibiotic era. It was reported that uncontrolled production of some damaging free radical metabolites could cause lipid peroxidation and oxidative damage, eventually resulting in severe injuries of gastrointestinal tract [23]. As an important member of antioxidant system, SOD provides a major defense mechanism through preventing or reducing free radical accumulation and functioning in ROS-mediated injury [3]. Additionally, MDA is involved in lipid peroxidation and DNA strand breaks [24]. Cui et al [25]has reported that weaning led to the lower SOD activity and higher MDA content in the small intestine. However, our study showed that after 200 ?g/kg EGF stimulation, the increase of SOD and T-AOC activities reached the maximum on day 14, and the concentration of MDA reached the minimum on day 14, suggesting an enhanced intestinal antioxidant capacity, which could help pigs to attenuate weaning-associated oxidative stress. However, piglets fed 400 ?g/kg EGF had higher MDA content and lower SOD activity in the mucosa. This may be due to the increased antioxidant capacity of the organism after EGF supplementation, and the decrease in ROS concentration leads to a compensatory decrease in SOD enzyme activity [26], whereas more studies are needed to investigate the underlying reasons. Moreover, the study performed by Rocourt et al[27]suggested that heparin-binding EGF, decreased the mRNA expression and protein production of IL-6, IL-1β and TNF-α. Therefore, we postulated that EGF may be implicated in inflammatory response. In this study, the concentration of pro-inflammatory cytokines IL-1β and TNF-α were detected. After EGF treatment, the content of IL-1β increased on day 7 but decreased on day 14, TNF-α content decreased on day 7 and 14 in response to 200 and 400 ?g/kg EGF stimulation. It was shown that EGF reduced the production of pro-inflammatory cytokines in piglets on day 14, suggesting that EGF may function by affecting TNF-α and IL-1β concentration to participate in the regulation of inflammatory response. Immunoglobulin A is critical for protecting the gut against dietary and microbial antigens invasion and maintaining intestinal health [28]. The SIgA is a predominant immunoglobulin in mucosal surfaces and can be used to assess intestinal mucosal immunity [29]. Hence, the contents of serum IgA and mucosal SIgA were determined in the present experiment to evaluate whether EGF was effective in the regulation of immune capacity for piglets during the transition phase. We found that serum IgA and SIgA concentrations were higher or tended to be higher for pigs receiving diets supplemented with EGF compared with unsupplemented pigs, with SIgA concentrations peaking when response to 400 ?g/kg EGF stimulation on day 7, which was in close agreement with earlier report [30]. And IgA is more obviously stimulated by 200 ?g/kg EGF on day 7. This suggested that EGF may have beneficial effect on the systemic immunity in weaning piglets. It is now commonly accepted that the nuclear transcription factor NF-κB plays central roles in modulating immunological status, inflammatory responses, and oxidative stress [31-32]. Accumulating evidences, investigating NF-κB as a key regulator of inflammation and stress, demonstrated that loss or inhibition of NF-κB at sites of inflammation may have additional, possibly undesirable consequences [33], which may suggest a protective effect for this transcription factor. At the present study, EGF increased the phosphorylation level of NF-κB p65 on day 7 and day 14. Similar result was also found by Sethi et al [34] at the same time, EGF also increased the protein expression level of NF-κB on day 7, and it increased with the increase of EGF concentration. On day 14, the protein expression level of NF-κB remained basically unchanged, which may be because the inflammatory response was weak at this time. Thus, the activation of this transcription factor may positively regulate EGF-mediated effects on oxidant damage, and immune function [34]. In summary, our study demonstrated that dietary supplementation with EGF enhanced intestinal antioxidant capacity, decreased inflammatory response, and increased immune status of weaned piglets, which may be partly associated with NF-κB signaling pathway. This research will provide guidance for the application of EGF in pig feed to promote the earlier maturation of immune system. References: [1] YANG H S, XIONG X, WANG X C, et al. Effects of weaning on intestinal upper villus epithelial cells of piglets[J]. PLoS One, 2016,11(3): e0150216. [2] CAMPBELL J M, CRENSHAW J D, POLO J. The biological stress of early weaned piglets[J]. Journal of Animal Science and Biotechnology, 2013, 4(1): 19. [3] ARDAPIRINCCI P, BOLKENT S. The role of epidermal growth factor in prevention of oxidative injury and apoptosis induced by intestinal ischemia/reperfusion in rats[J]. Acta Histochemica, 2014, 116(1): 167-175. [4] ZHANG Y, ZHENG P, YU B, et al. Dietary spray-dried chicken plasma improves intestinal barrier function and modulates immune status in weaning piglets[J]. Journal of Animal Science, 2016, 94(1): 173-184. [5] YANG H S, XIONG X, WANG X C, et al. Effects of weaning on intestinal crypt epithelial cells in piglets[J]. Scientific Reports, 2016, 6(1): 36939. [6] BALLARD O, MORROW A L. Human milk composition: nutrients and bioactive factors[J]. Pediatric Clinics of North America, 2013, 60(1): 49-74. [7] PLAYFORD R J, MACDONALD C E, JOHNSON W S. Colostrum and milk-derived peptide growth factors for the treatment of gastrointestinal disorders[J]. American Journal of Clinical Nutrition, 2000, 72(1): 5-14. [8] XU S, WANG D, ZHANG P, et al. Oral administration of lactococcus lactis-expressed recombinant porcine epidermal growth factor (rpEGF) stimulates the development and promotes the health of small intestines in early-weaned piglets[J]. Journal of Applied Microbiology, 2015, 119(1): 225-235. [9] COSKUN S, GULEC E G, BALABANLI B, et al. Effects of epidermal growth factor on lipid peroxidation and nitric oxide levels in oral mucosal ulcer healing: a time-course study[J]. Surgery Today, 2007, 37(7): 570-574. [10] DVORAK B, HALPERN M D, HOLUBEC H, et al. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model[J]. American Journal of Physiology-gastrointestinal and Liver Physiology, 2002, 282(1): G156-G164. [11] HALPERN M D, DOMINGUEZ J A, DVORAKOVA K, et al. Ileal cytokine dysregulation in experimental necrotizing enterocolitis is reduced by epidermal growth factor[J]. Journal of Pediatric Gastroenterology and Nutrition, 2003, 36(1): 126-133. [12] CLARK J A, GAN H, SAMOCHA A J, et al. Enterocyte-specific epidermal growth factor prevents barrier dysfunction and improves mortality in murine peritonitis[J]. American Journal of Physiology-gastrointestinal and Liver Physiology, 2009, 297(3): G471-G479. [13] YAN F, CAO H, SHI Y, et al. Epidermal growth factor (EGF) receptor activation suppresses macrophage inflammatory cytokine production[J]. Gastroenterology, 2011, 140(1): S491. [14] ALBERTI C, PINCIROLI P, VALERI B, et al. Ligand-dependent EGFR activation induces the co-expression of IL-6 and PAI-1 via the NF-κB pathway in advanced-stage epithelial ovarian cancer[J]. Oncogene, 2012, 31(37): 4139-4149. [15] BEDFORD A, CHEN T, HUYNH E, et al. Epidermal growth factor containing culture supernatant enhances intestine development of early-weaned pigs in vivo: potential mechanisms involved[J]. Journal of Biotechnology, 2015, 196: 9-19. [16] SCHWEIGER M, STEFFL M, AMSELGRUBER W M. Differential expression of EGF receptor in the pig duodenum during the transition phase from maternal milk to solid food[J]. Journal of Gastroenterology, 2003, 38(7): 636-642. [17] ZONG E Y, HUANG P F, YANG H S, et al. The effects of dietary sulfur amino acids on growth performance, intestinal morphology, enzyme activity, and nutrient transporters in weaning piglets[J]. Journal of Animal Science, 2018, 96(3): 1130-1139. [18] COUNCIL N R. Nutrient requirements of swine: eleventh revised edition[M]. Washington, DC: The National Academies Press, 2012. [19] WANG L X, ZHU F, YANG H S, et al. Epidermal growth factor improves intestinal morphology by stimulating proliferation and differentiation of enterocytes and mTOR signaling pathway in weaning piglets[J]. Science China Life Science, 2019, 63(1): 259-268. [20] YANG H S, FU D Z, SHAO H, et al. Impacts of birth weight on plasma, liver and skeletal muscle neutral amino acid profiles and intestinal amino acid transporters in suckling Huanjiang mini-piglets[J]. PLoS One, 2012, 7(12): e50921. [21] HE L Q, LI H, HUANG N, et al. Alpha-ketoglutarate suppresses the NF-κB-mediated inflammatory pathway and enhances the PXR-regulated detoxification pathway[J]. Oncotarget, 2017, 8(61): 102974-102988. [22] YESILIRMAK D C, KUMARL A, TUZUN F, et al. Milk-borne epidermal growth factor modulates bilirubin levels in neonatal rats[J]. Clinical Nutrition, 2015, 3(1): 1-7. [23] VECCHIO M, CURRO M, TRIMARCHI F, et al. The oxidative stress response in elite water polo players: effects of genetic background[J]. Biomed Research International, 2017, 2017: 7019694. [24] WANG H F, ZHONG X H, SHI W Y, et al. Study of malondialdehyde (MDA) content, superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities in chickens infected with avian infectious bronchitis virus[J]. African Journal of Biotechnology, 2011, 10(1): 9213-9217. [25] CUI Z, HANG L, ZHUANG C, et al. Dietary zinc oxide modulates antioxidant capacity, small intestine development, and jejunal gene expression in weaned piglets[J]. Biological Trace Element Research, 2016, 175(2): 331-338. [26] CHEN L, ZHONG Y, WANG C, et al. Effects of β-alanine on intestinal development and immune performance of weaned piglets[J]. Animal Nutrition, 2022, 12(1): 398-408. [27] ROCOURT D V, MEHTA V B, BESNER G E. Heparin-binding EGF-like growth factor (HB-EGF) decreases inflammatory cytokine expression after intestinal ischemia/reperfusion injury[J]. Journal of Surgical Research, 2007, 139(2): 269-273. [28] REN W, ZOU L, LI N, et al. Dietary arginine supplementation enhances immune responses to inactivated Pasteurella multocida vaccination in mice[J]. British Journal of Nutrition, 2013, 109(5): 867-872. [29] BAKKER-ZIERIKZEE A M, VAN TOL E A F, KROES H, et al. Faecal SIgA secretion in infants fed on pre- or probiotic infant formula[J]. Pediatric Allergy and Immunology, 2006, 17(2): 134-140. [30] LEE D N, KUO T Y, CHEN M C, et al. Expression of porcine epidermal growth factor in Pichia pastoris and its biology activity in early-weaned piglets[J]. Life Sciences, 2006, 78(6): 649-654. [31] NEURATH M F, BECKER C, BARBULESCU K. Role of NF-kappaB in immune and inflammatory responses in the gut[J]. Gut, 1998, 43(6): 856-860. [32] VAN DEN BERG R, HAENEN G R, VAN DEN BERG H, et al. Transcription factor NF-kappaB as a potential biomarker for oxidative stress[J]. British Journal of Nutrition, 2001, 86(1): S121-S127. [33] HAUSSLER U, VON W G, SCHMID R M, et al. Epidermal growth factor activates nuclear factor-kappaB in human proximal tubule cells[J]. American Journal of Physiology-renal Physiology, 2005, 289(4): F808-F815. [34] SETHI G, AHN K S, CHATURVEDI M M, et al. Epidermal growth factor (EGF) activates nuclear factor-κB through IκBα kinase-independent but EGF receptor-kinase dependent tyrosine 42 phosphorylation of IκBα[J]. Oncogene, 2015, 34(42): 5407.
