Synthesis and Characterization of 9,9-diethyl-1-phenyl-1,9-dihydrofluoreno[2,3-d]imidazole-ended Fluorophores
2024-01-03LISaijunWANGTianqiTANQitaoLINHaixiaCUIYongmei
LI Saijun, WANG Tianqi, TAN Qitao, LIN Haixia, CUI Yongmei*
(1. College of Science, Shanghai University, Shanghai 200444, China; 2. School of Medicine, Shanghai University, Shanghai 200444, China)
Abstract: A series of π-conjugated compounds ending with 9,9-diethyl-1-phenyl-1,9-dihydrofluoreno[2,3-d]imidazole were conveniently synthesized by condensation of the key intermediate 9,9-diethyl-N2-phenyl-9H-fluorene-2,3-diamine with the corresponding symmetric aryl phthalaldehydes under very mild conditions.The structures of these compounds were confirmed by 1H NMR, 13C NMR, and HRMS.Their UV-Vis spectroscopy data, fluorescent spectroscopy data, and further details of the electronic properties from cyclic voltammetry measurements and theoretical calculations were studied.Most compounds possess good fluorescence-emitting ability with quantum yield of fluorescence values in the region of 0.36-0.92 and display emission within 449-513 nm depending on the molecular nature.
Key words: fluorene; imidazole; synthesis; UV absorption; fluorescence
1 Introduction
Fluorene has been used as a functional building block in the fabrication of materials for applications in organic light-emitting diodes, field-effect transistors and organic solar cells (OSCs)[1-4].The molecular and photophysical properties of fluorenes can be tuned by structural modifications at the 2, 3, 6, 7,and 9- positions, which makes them one of the most important classes of advanced materials being used in organic electronics[5-9].Fluorene derivatives have found application in electroluminescent devices as emitting materials due to their excellent electric or hole transporting ability, luminescence efficiency, thermal stability, and attractive optoelectronic properties[10-17].However, small-molecule fluorene derivatives are still rarely reported in the practical applications in OLEDs[18-20].
Fluorescent characteristic relies largely on molecular structure and molecular assembly.Clarification the structure-property relationship of fluorescence would allow us to design more useful fluorescent reagents and probes which might be applied in other fields such as analytical and biological chemistry in the future.
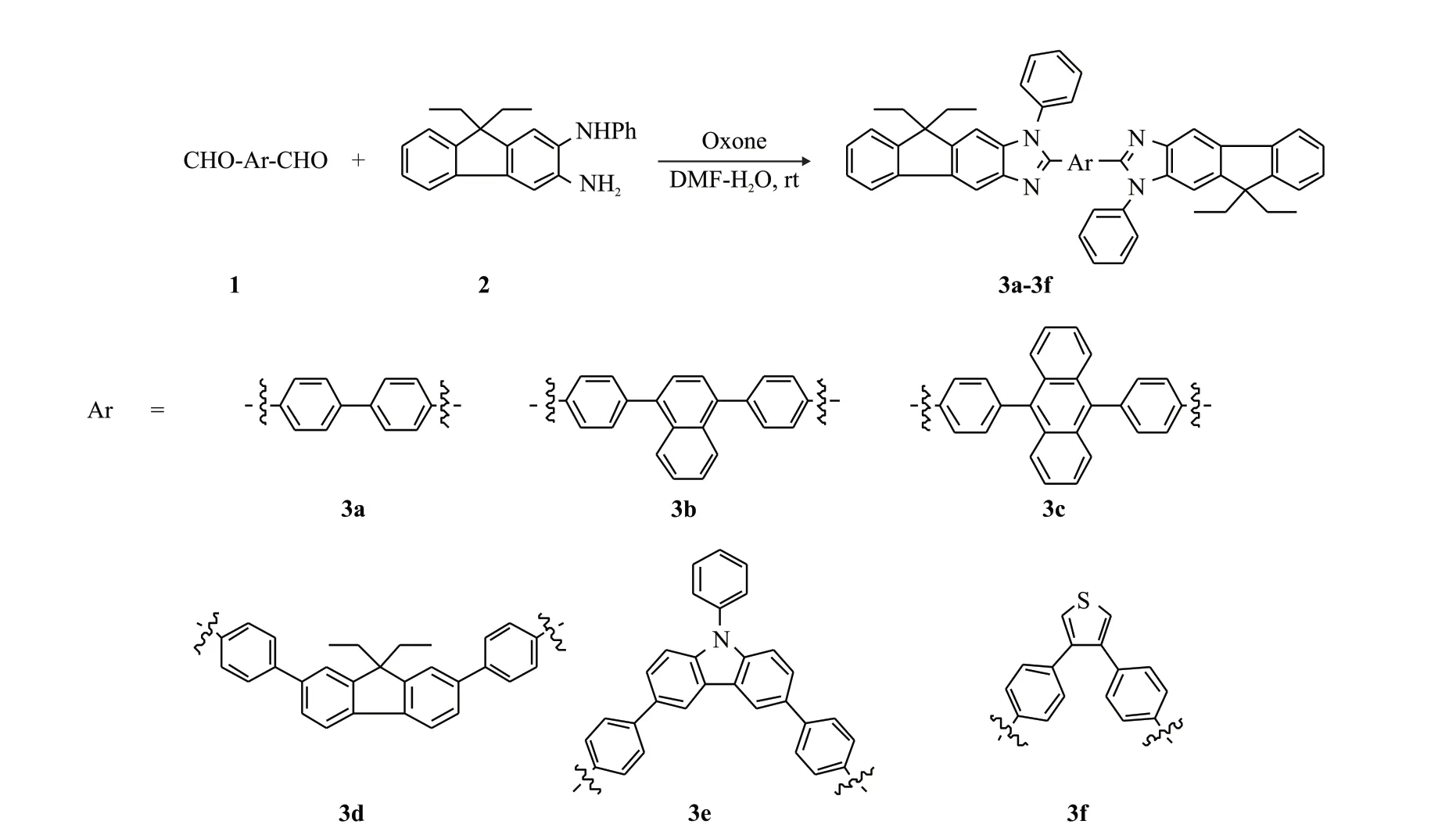
Based on our continued research on fluoreno[2,3-d]imidazole systems with specific optoelectronic properties[21-23].We reported the efficient synthesis and characterization of a series of compounds 3a-f ending with 9,9-diethyl-1-phenyl-1,9-dihydrofluoreno[2,3-d]imidazole groups.
2 Experimental
2.1 Reagents and instrument
Commercially available starting chemicals were purchased and used as received without further purification.Thin-layer chromatography (TLC) was conducted on silica gel GF254 plates.1H NMR and13C NMR spectra were recorded on a Bruker Avance (500 MHz) spectrometer in CDCl3, unless otherwise noted.Chemical shifts were reported in the scale relative to CHCl3(7.26 ppm) for1H NMR, and to CDCl3(77.16 ppm) for13C NMR, as internal references.High-resolution mass spectrometer (HRMS) spectra were recorded on Agilent 6540/6538.UV-Vis spectra were recorded on UV-2501Pc spectrophotometer.Fluorescent measurements were recorded on RF-5301 spectrophotometer.
2.2 General procedure for the synthesis of compounds 3a-3f
The compound 9,9-diethyl-N2-phenyl-9Hfluorene-2,3-diamine (1000 mg, 3.04 mmol) and the corresponding aldehyde (1.38 mmol) were dissolved in a mixed solvent of DMF and H2O (VDMF:VH2O=30:1),oxone (1.1 g, 1.8 mmol) was added, and the mixture was stirred at room temperature until the reaction was complete.Then the mixture was extracted with ethyl acetate, evaporated and purified with ethyl acetate and petroleum ether to afford the target compounds 3a-3f.
2.2.1 4,4’-b i s(9,9-d i e t h y l-1-p h e n y l-1,9-
dihydrofluoreno[2,3-d]imidazol-2-yl)-1,1’-biphenyl (3a)
Light yellow solid, Yield: 46.3%.M.p.= 303-305 ℃;1H-NMR (500 MHz, CDCl3) δ (ppm) 8.16 (s,2H), 7.82 (d,J= 7.5 Hz, 2H), 7.63 (d,J= 8.4 Hz, 4H),7.60-7.51 (m, 10H), 7.41 (d,J= 7.1 Hz, 4H), 7.38-7.33 (m, 2H), 7.32-7.28 (m, 4H), 7.11 (s, 2H), 2.08-1.91 (m, 8H), 0.31 (t,J= 7.3 Hz,12H);13C-NMR(125 MHz, CDCl3) δ (ppm) 151.89, 149.55, 146.86,142.85, 141.70, 140.75, 137.92, 137.62, 137.21,130.05, 129.81, 129.40, 128.65, 127.60, 126.93,126.82, 126.66, 122.79, 119.53, 110.19, 104.41, 55.83,33.53, 8.54.HR-MS: m/z calcd for C60H50N4([M+H]+)827.4114, found 827.4101.
2.2.2 1,4-b i s(4-(9,9-d i e t h y l-1-p h e n y l-1,9-dihydrofluoreno[2,3-d]imidazol-2-yl)phenyl)naphthalene (3b)
Light yellow solid, Yield: 28.5%.M.p.= 305-307 ℃;1H-NMR (500 MHz, DMSO-d6) δ (ppm)8.08 (s, 2H), 7.83 (d,J= 2.8 Hz, 2H), 7.72 (d,J= 7.1 Hz, 2H), 7.60 (d,J= 7.6 Hz, 4H), 7.48 (t,J= 7.1 Hz,4H), 7.44-7.40 (m, 2H), 7.39-7.29 (m, 12H), 7.25 (t,J= 6.5 Hz, 2H), 7.18 (d,J= 7.9 Hz, 4H), 7.03 (s,2H),1.90 (m, 8H), 0.21 (t,J= 7.3 Hz, 12H);13C-NMR (125 MHz, CDCl3) δ (ppm) 152.11, 149.57, 146.86, 142.87,141.73, 141.69, 139.29, 137.92, 137.65, 137.25,131.72, 130.11, 130.08, 129.29, 129.16, 128.71,127.66, 126.95, 126.66, 126.43, 126.24, 126.15,122.81, 119.56, 110.23, 104.47, 55.84, 33.54, 8.58.
2.2.3 9,10-bis(4-(9,9-diethyl-1-phenyl-1,9-dihydrofluoreno[2,3-d]imidazol-2-yl)phenyl)anthracene (3c )
White solid, Yield: 19%.M.p.= 305-308 ℃;1H-NMR (500 MHz, DMSO-d6) δ (ppm) 8.09 (d,J=1.5 Hz, 2H), 7.91 (m, 4H), 7.86-7.83 ( m, 6H), 7.60 (d,J= 7.6 Hz, 4H), 7.56 (s, 2H), 7.48 (t,J= 7.1 Hz, 4H),7.44-7.40 (m, 2H), 7.39-7.29 (m, 12H), 7.25 (t,J= 6.5 Hz, 2H), 2.06-1.90 (m, 8H), 0.21 (t,J= 7.3 Hz, 12H);13C-NMR (125 MHz, CDCl3) δ (ppm) 159.13, 151.45,150.70, 141.13, 138.64, 136.70, 135.84, 133.05,130.89, 129.75, 129.36, 128.47, 128.41, 126.95,126.56, 125.94, 123.42, 119.79, 118.39, 114.71,104.23, 55.85, 33.51, 8.56.
2.2.4 2,2’-((9,9-diethyl-9H-fluorene-2,7-diyl)bis(4,1-phenylene))bis(9,9-diethyl-1-phenyl-1,9-dihydrofluoreno[2,3-d]imidazole) (3d)
Yellow solid, Yield: 40.4%.M.p.= 310-313 ℃;1H-NMR (500 MHz, DMSO-d6) δ (ppm) 8.19 (s, 2H),7.83 (d,J= 7.4 Hz, 2H), 7.77 (d,J= 7.9 Hz, 2H), 7.66(dd,J= 21.2, 8.4 Hz, 8H), 7.57 (dt,J= 18.5, 7.2 Hz,10H), 7.45 (d,J= 7.2 Hz, 4H), 7.39-7.35 (m, 2H),7.33-7.29 (m,4H), 7.12 (s, 2H), 2.14-1.93 (m, 12H),0.38 (t,J= 7.3 Hz, 6H), 0.32 (t,J= 7.3 Hz, 12H);13C-NMR (125 MHz, CDCl3) δ (ppm) 152.10, 150.97,149.55, 146.78, 142.85, 142.21, 141.74, 140.84,139.16, 137.89, 137.67, 137.32, 130.06, 129.78,128.64, 127.66, 126.93, 126.88, 126.16, 122.78,121.35, 120.17, 119.53, 110.16, 104.40,57.96, 55.82,33.54, 8.55.HR-MS: m/z calcd for C77H66N4([M+H]+)1047.5366, found 1047.5357.
2.2.5 2,2’-((9-phenyl-9H-carbazole-3,6-diyl)bis(4,1-phenylene))bis(9,9-diethyl-1-phenyl-1,9-dihydrofluoreno[2,3-d]imidazole) (3e)
Light yellow solid, Yield: 39.2%.M.p.= 303-306℃;1H-NMR (500 MHz, DMSO-d6) δ (ppm) 8.41 (d,J= 1.5 Hz, 2H), 8.18 (s, 2H), 7.83 (d,J= 7.5 Hz, 2H),7.71-7.67 (m, 10H), 7.66-7.62 (m, 3H), 7.60 (d,J= 7.6 Hz, 5H), 7.56 (t,J= 7.3 Hz, 2H), 7.51 (t,J= 7.3 Hz,1H), 7.48-7.45 (m, 6H), 7.39-7.35 (m, 2H), 7.33-7.28(m, 4H), 7.13 (s, 2H), 2.02 (m, 8H), 0.32 (t,J= 7.3 Hz,12H);13C-NMR (125 MHz, CDCl3) δ (ppm) 151.90,149.56, 149.56, 146.86, 146.71, 142.87, 142.83,142.48, 141.77, 141.69, 141.07, 140.74, 137.91,137.84, 137.67, 137.61, 137.33, 137.18, 130.05,129.83, 129.81, 128.64, 127.67, 127.59, 126.97,126.93, 126.82, 122.79, 119.52, 110.33, 110.17,110.12, 104.41, 55.82, 33.53, 8.55.HR-MS: m/z calcd for C78H61N5([M+H]+) 1068.5006, found 1068.4997.
2.2.6 3,4-b i s(4-(9,9-d i e t h y l-1-p h e n y l-1,9-dihydrofluoreno[2,3-d]imidazol-2-yl)phenyl thiophene (3f)
Light yellow solid, Yield: 33.4%.M.p.= 245-248 ℃;1H-NMR (500 MHz, CDCl3) δ (ppm) 8.14 (d,J= 0.6 Hz, 2H), 7.81 (d,J= 7.5 Hz, 2H), 7.63 (dd,J=8.0, 6.3 Hz, 1H), 7.57 (dd,J= 12.9, 5.1 Hz, 1H), 7.53-7.49 (m, 4H), 7.45-7.39 (m, 6H), 7.38-7.32 (m, 7H),7.29 (dt,J= 6.4, 3.4 Hz, 4H), 7.11-7.07 (m, 5H), 2.06-1.90 (m, 8H), 0.30 (t,J= 7.3 Hz, 12H);13C-NMR (125 MHz, CDCl3) δ (ppm) 149.42, 147.88, 141.09, 140.04,138.26, 136.91, 136.33, 134.56, 130.95, 129.67,128.22, 126.73, 124.31, 123.28, 122.19, 121.66,115.18, 57.33, 35.42, 8.93.HR-MS: m/z calcd for C64H52N4S ([M+H]+) 909.3992, found 909.3983.
3 Results and discussion
3.1 Synthesis
As shown in scheme 1, condensation of the corresponding symmetric aryl phthalaldehyde (1)with the key intermediate 9,9-diethyl-N2-phenyl-9H-fluorene-2,3-diamine (2)[21]in wet DMF at room temperature under oxone-mediated oxidative conditions afforded the target compounds 3a-f in 19%-46.3%yields.All target compounds were isolated and purified by column chromatography on silica gel with high purity and their molecular structures were confirmed by1H NMR spectroscopy,13C NMR spectroscopy,and HRMS, which are shown detailed in experiment section.
3.2 Optical properties
The UV-Vis absorption spectra of compounds 3a-f were measured in CH2Cl2and DMSO solutions with the concentration of 1 × 10-5mol/dm3at room temperature,and the optical characteristics are summarized in Table 1.All compounds displayed similar absorption characteristics, with two absorption peaks in the ranges of 250-277 and 345-376 nm, which could be attributed to different intramolecular π-π* transitions.Compounds 3d and 3e with the absorption maxima at 366 and 376 nm, respectively, had longer absorption wavelengths, as the conjugated length extended, while,3f showed the shortest λmaxat 345 nm because of the increased steric hinderance and less planar structures.The UV-Vis absorption spectra measured in DMSO solutions indicated that all compounds showed small red shifts no more than 3 nm, compared with the corresponding spectra in CH2Cl2, suggesting that the ground states of all these compounds are rather nonpolar in nature.
Scheme 1 Synthesis of conjugated compounds 3a-f
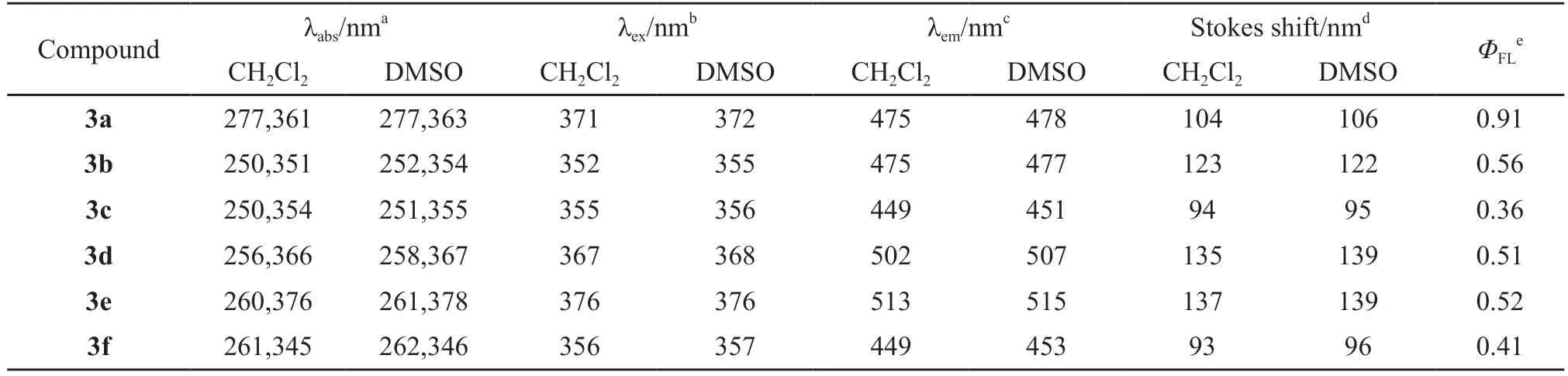
Table 1 Optical properties and ΦFL of compounds 3a-f
The photoluminescence (PL) spectra were recorded upon excitation at each excitation wavelength(λex) in CH2Cl2solution (1×10-7M) at room temperature.In CH2Cl2solution, the fluorescence spectra of compounds 3a and 3b were very similar with emission peaks at 475 nm.The fluorescence spectra of 3d (peaked at 502 nm), and 3e (peaked at 513 nm)were both red-shifted relative to that of 3a due to the elongation of the conjugation length.However, the anthracene inserted derivative (3c) with emission peak at 449 nm was blue-shifted by 26 nm relative to 3a, this might be attributed to the highly twisted conformation between the anthracene and the 9,9-diethyl-1,2-diphenyl-1,9-dihydrofluoreno[2,3-d]imidazole units.Similar to that of 3c, the derivative 3f showed blue emission, with the emission peak at 449 nm.In addition,the solvent effect on the fluorescence characteristics was also observed.All compounds were slightly red-shifted in DMSO relative to those in CH2Cl2.
Quantum yields were calculated on the basis of quinine sulfate (ΦFL= 0.55).3a showed the highest quantum yield in solutions and relatively lower quantum yields were observed for all the other compounds and compounds 3c and 3f showed the lowest emission intensities maybe because of the increased torsional strain by inserting an anthracene or 1,2-thiophene into biphenyl.In all, the emission efficiency in dilute solution largely depends on the molecular structure and can be tuned by structural modification.
3.3 Electrochemical properties
The redox potentials of all compounds were determined by cyclic voltammetry (CV) measurements which were carried out in a three-electrode cell setup with 0.1 M tetrabutylammonium perchlorate(Bu4NClO4) as a supporting electrolyte in anhydrous CH2Cl2to probe the electrochemical behavior of the materials.As seen from Table 2, the onset potentials of oxidation (Eox) of these compounds were at about 0.92-0.97 eV.The band gaps (Eg) of about 2.88-3.12 eV were estimated from the optical edge.Compounds 3c and 3f exhibited a relatively larger band gap by comparison with those of compounds 3a-b, 3d-e, which derived from the more distorted π-conjugation structures.The LUMO energy levels for compounds 3a-f were determined to be around 2.42-2.67 eV by combining the HOMO energy levels together with the band gaps.
Density functional theory (DFT) calculations have also been applied to characterize the three-dimensional geometries and the frontier molecular orbital energy levels of 3a-f at the B3LYP/6-31G* level by using the Gaussian 03 program.The orbital plots of the HOMOLUMO are illustrated in Fig.1.In most of the above compounds (3a-b and 3d-e), the HOMOs were located on the entire molecular backbones consisting of the two fluorenoimidazole skeletons and the bridging aryl ring.Their LUMOs, however, were mainly concentrated on the interconnected aryl ring.In the case of compound 3c, the anthracene was nearly perpendicular to the 9,9-diethyl-1,2-diphenyl-1,9-dihydrofluoreno[2,3-d]imidazole units, the HOMO and LUMO were localized mainly on the anthracene as expected.For compound 3f, the HOMO was mainly constituted by one fluorenoimidazole skeleton and a bridging phenyl ring,however, LUMO was localized on the other side.
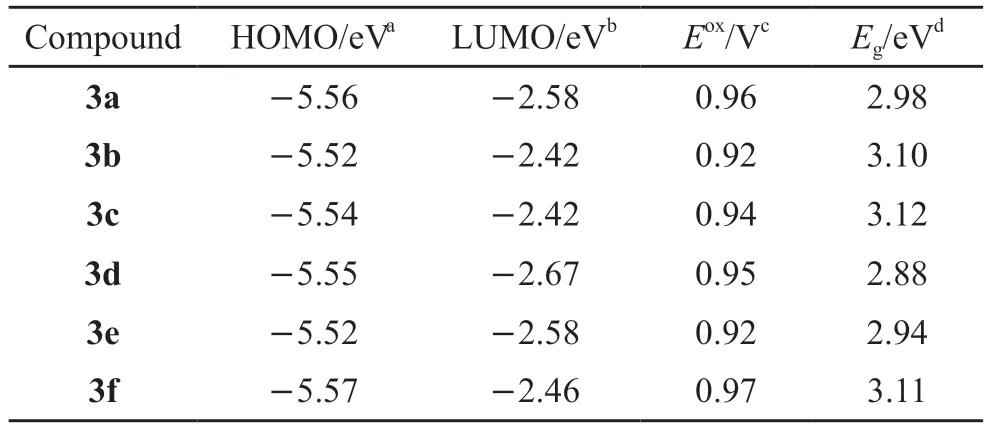
Table 2 Optical properties of compounds 3a-3f
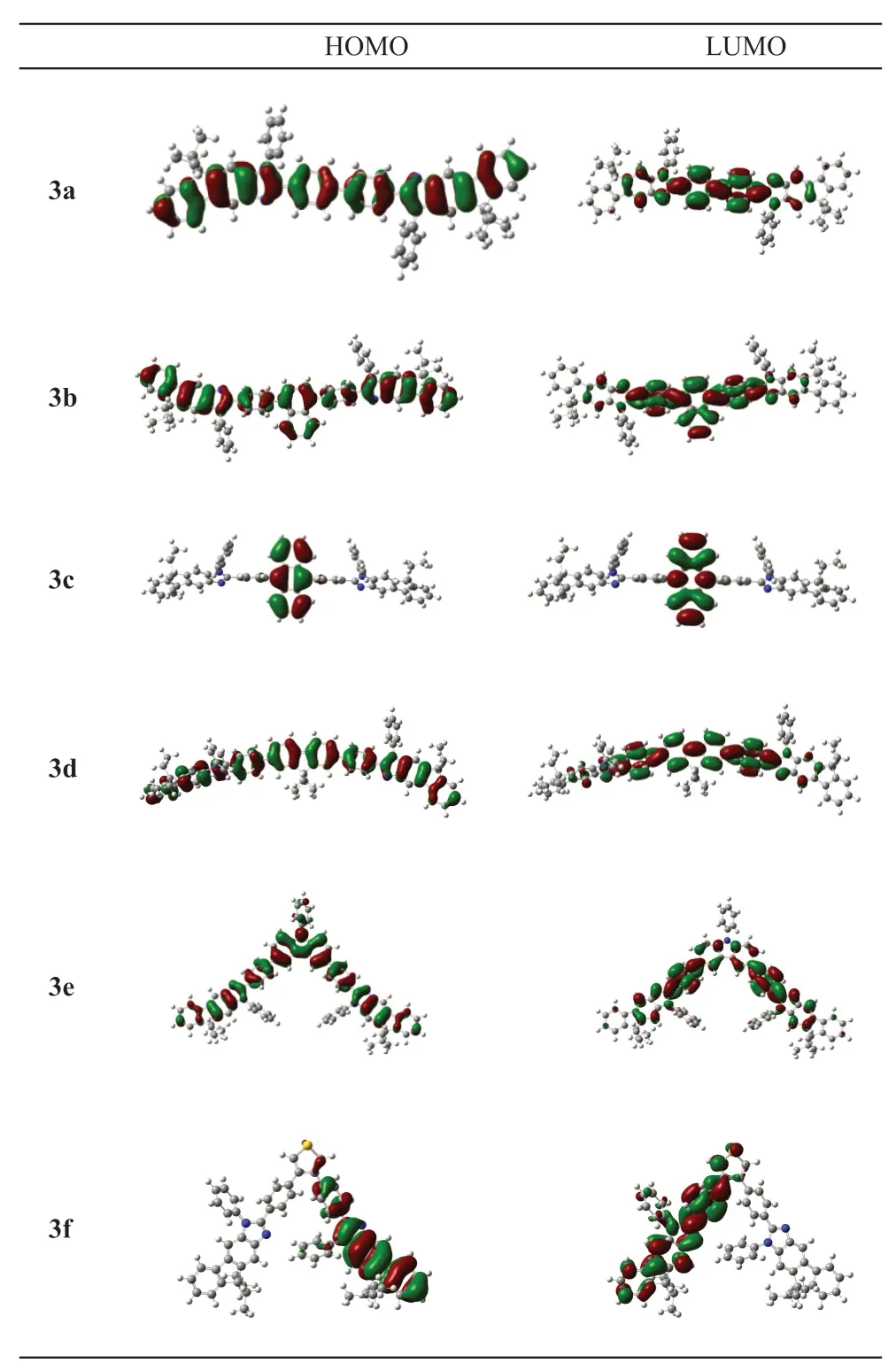
Fig.1 HOMO and LUMO electronic density distributions of compounds 3a-3f
4 Conclusions
The synthesis and structure-property relationship of a series of π-conjugated compounds ending with 9,9-diethyl-1-phenyl-1,9-dihydrofluoreno[2,3-d]imidazole groups was studied in detail.The absorption spectra and fluorescence characteristics show dependence on their molecular structure and the luminescent efficiencies can be tuned by structural modification.This study demonstrates that systematic study of these organic emitters can provide an efficient way to produce excellent candidates for the potential applications in optoelectronics.Further research regarding the incorporation of these systems usable as organic light-emitting diodes will be reported in due course.
Conflict of interest
All authors declare that there are no competing interests.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Enhanced Electrochemical Performances of Ni Doped Cr8O21 Cathode Materials for Lithium-ion Batteries
- Design on the Prestressed Concrete Frame Beam-column
- Synthesis and Flocculation of Polyacrylamide with Low Water Absorption for Non-dispersible Underwater Concrete
- Experimental Behavior of Recycled Aggregate Concrete Filled Steel Tubular Columns
- Impact-abrasive Wear Behavior of ZTA and NbC Reinforced Fe60 Matrix Composites
- Synthesis and Characterization of Hollow Strontium Carbonate Pompons by Composite Soft Template Method
