Enhanced Electrochemical Performances of Ni Doped Cr8O21 Cathode Materials for Lithium-ion Batteries
2024-01-03TANGGuoliLIUHanxingYUZhiyongYANGBoKONGLinghua
TANG Guoli, LIU Hanxing,2,3, YU Zhiyong*, YANG Bo, KONG Linghua
(1. School of Materials Science and Engineering, Wuhan University of Technology, Wuhan 430070, China; 2. International School of Materials Science and Engineering, Wuhan University of Technology, Wuhan 430070, China; 3. State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan 430070, China)
Abstract: Cathode materials, nickel doped Cr8O21, were synthesized by a solid-state method.The effects of Ni doping on the electrochemical performances of Cr8O21 were investigated.The experimental results show that the discharge capacities of the samples depend on the nickel contents, which increases firstly and then decreases with increasing Ni contents.Optimized Ni0.5Cr7.5O21 delivers a first capacity up to 392.6 mAh·g-1 at 0.1 C.In addition, Ni doped sample also demonstrates enhanced cycling stability and rate capability compared with that of the bare Cr8O21.At 1 C, an initial discharge capacity of 348.7 mAh·g-1 was achieved for Ni0.5Cr7.5O21,much higher than 271.4 mAh·g-1 of the un-doped sample, with an increase of more than 28%.Electrochemical impedance spectroscopy results confirm that Ni doping reduces the growth of interface resistance and charge transfer resistance, which is conducive to the electrochemical kinetic behaviors during charge-discharge.
Key words: Cr8O21; cathode material; doping; electrochemical performances; lithium-ion batteries
1 Introduction
Rechargeable lithium-ion batteries (LIBs) acted as energy providers are a significant component in various portal electronic devices, such as smart phones,digital cameras and notebook computers[1].Recently,the increasing application in large-scale energy storage devices has produced further requirements for higher energy density of LIBs[2,3].As an important part of LIBs, the cathode materials instrumentally affect the energy density of the battery[4-7].However, the intrinsic low specific capacities (140-170 mAh·g-1) of traditional cathode materials (such as LiCoO2, LiFePO4or LiNixMnyCo1–x–yO2) limit the energy densities of LIBs[8,9].
Some chromium oxides, such as Cr3O8, Cr2O5,and CrO2, exhibited high initial discharge specific capacity and high voltage platforms, and thus have been regarded as promising cathode materials for LIBs[10-15].The oxidation states of chromium oxides played a vital role in their cycling reversible properties,in which the Cr3O8exhibited the most excellent electrochemical performances.The Cr3O8obtained by Aroraet al[10]showed high initial discharge capacity more than 250 mAh·g-1with the voltage range from 2.0 to 4.2 V.Moreover, the phase-pure Cr3O8prepared by Ramasamyet alvia optimizing the synthesis conditions provided a higher initial discharge capacity of over 320 mAh·g-1[12], which far exceeded the specific discharge capacity of current commercial cathode materials.Norbyet al[13]determined that the Cr8O21should be the true chemical formula of the previously-named Cr3O8by neutron diffraction technique, which consisted of a pair of Cr(III)-O octahedrons with common edges combined with two single Cr(VI)-O tetrahedrons to form layers.Although Cr8O21showed high initial specific capacity, its cycling reversibility and rate performance should been further improved for potential commercial applications[15,16].
Doping foreign elements has been proved to be efficient to optimize the electrochemical performances of cathode materials in lithium-ion batteries.Partial substitutions of the transition metal sites by Al, Mn,Co, Sn, Cu, Nb have been attempted for enhancing the structural stability and electronic conductivity of various oxides[17-21].In this paper, Ni was selected to improve the electrochemical performances of Cr8O21due to the fact the ion radius of Ni2+is close to that of Cr3+.This may ensure that the Cr8O21structure will not be damaged due to the doping of Ni.Besides, the incorporation of nickel ion can help to regulate the electronic conductivity[22,23].Previous work has proved that nickel doping can improve the electrochemical properties of TiO2, Li3VO4, CuFe2O4, LiMn2O4,and LiCoO2[22-26].Nevertheless, as far as we know,there have been no reports on nickel doping Cr8O21cathode until now.Here, the effects of the Ni doping on the phase structure, morphological feature and electrochemical properties of Cr8O21were investigated.The results showed that the Ni-doped Cr8O21significantly improved initial discharge capacity, rate performance and cycling reversibility.
2 Experimental
2.1 Materials synthesis and characterizations
Firstly, NixCr8-xO21(x=0.25, 0.5, and 0.75)materials were prepared by heating a mixture of CrO3(Alfa, 99.9%) and CoC2O4(Alfa, 99.5%) according to the designed stoichiometric ratio of Cr/Ni for 30 h at 290℃.Then, the as-prepared products were washed several times with distilled water until the liquid became clear.Finally, the materials were dried at 120 ℃ for 12 h in air.The Cr8O21was also synthesized by the same method except the heating temperature was hold at 270 ℃.
The crystal structures of all as-prepared products were characterized by X-ray diffraction (XRD,PANalytical X’Pert PRO) with Cu-Kα radiation,and the 2θrange was selected from 5° to 80° with a scanning rate of 1 °/min and a step size of 0.02°.The morphology of the sample was investigated via a scanning electron microscopy (SEM, HITACHI S-4800).X-ray photoelectron spectroscopy (XPS) was performed on ESCALAB 250Xi, taking Al K-Alpha as the radiation source to determine the valence of the samples.
2.2 Electrochemical characterizations
The CR2025 coin-type cells were assembled to evaluate the electrochemical performances of the cathode samples.All coin cells were composed of the working cathode electrode (active material: acetylene black: polyvinylidene fluoride binder = 80:10:10,weight ratio), the Celgard 2 400 separator, the lithium foil anode electrode, the stainless steel gasket and spring gasket.The electrolyte consisted of 1M LiPF6in ethylene carbonate (50vol%) and dimethyl carbonate(50vol%).The charge and discharge measurements of as-prepared cells were implemented with a battery charger (CT2001A, Land) at room temperature.The data of electrochemical impedance spectroscopy (EIS)in the frequency range of 10-2-105Hz were collected by an electrochemical workstation (CHI660B, Chenhua)at voltage amplitude of 5 mV, and all of the cells were charged to 3.8 V on the CT2001A battery charger before the impedance measurements.The cyclic voltammetry (CV) experiments were performed with the voltage range of 2.0-4.2 Vvs.Li/Li+at a scan rate of 0.1 mV/s on CHI660B.
3 Results and discussion
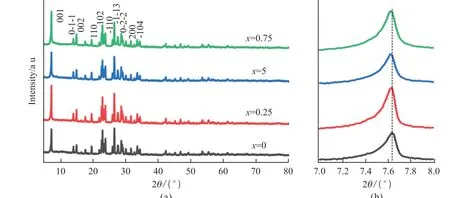
Fig.1 (a) XRD patterns of NixCr8-xO21 (x = 0, 0.25, 0.5, and 0.75); (b) Enlarged view of (001) peak
The X-ray diffraction patterns of the as-prepared samples with various Ni contents are shown in Fig.1.The diffraction peaks of all Ni doped materials can be matched with un-doped Cr8O21(JCPDS No.80-2473), where no secondary phase is observed within the precision limit of the XRD.Compared with undoped Cr8O21, minor shift is detected in the peak position of Ni doped Cr8O21(Fig.1(b)).The diffraction peaks related to the (001) crystal plane gradually shift to a lower angle, which indicates an increase in the experimental lattice parameters with the increase of Ni content.This is because the ionic radius of Ni2+(69 pm) is larger than that of Cr3+(61.5 pm).In addition,the morphological features of the NixCr8-xO21(x= 0,0.25, 0.5, and 0.75) samples were detected by Scanning Electron Microscopy (SEM) (Fig.2).It is easy to find that all of the samples present similar secondary particles agglomerated by numerous primary particles with irregular flake shape, where no apparent change on the particle morphology is observed.
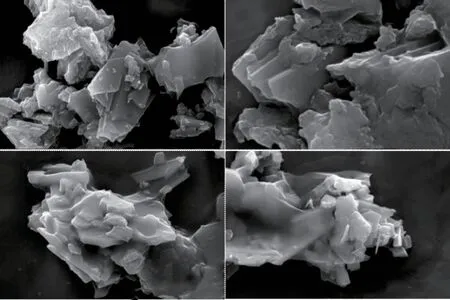
Fig.2 SEM images of NixCr8-xO21 samples with different Ni contents: (a) Cr8O21, (b) Ni0.25Cr7.75O21, (c) Ni0.5Cr7.5O21 and (d)Ni0.75Cr7.25O21
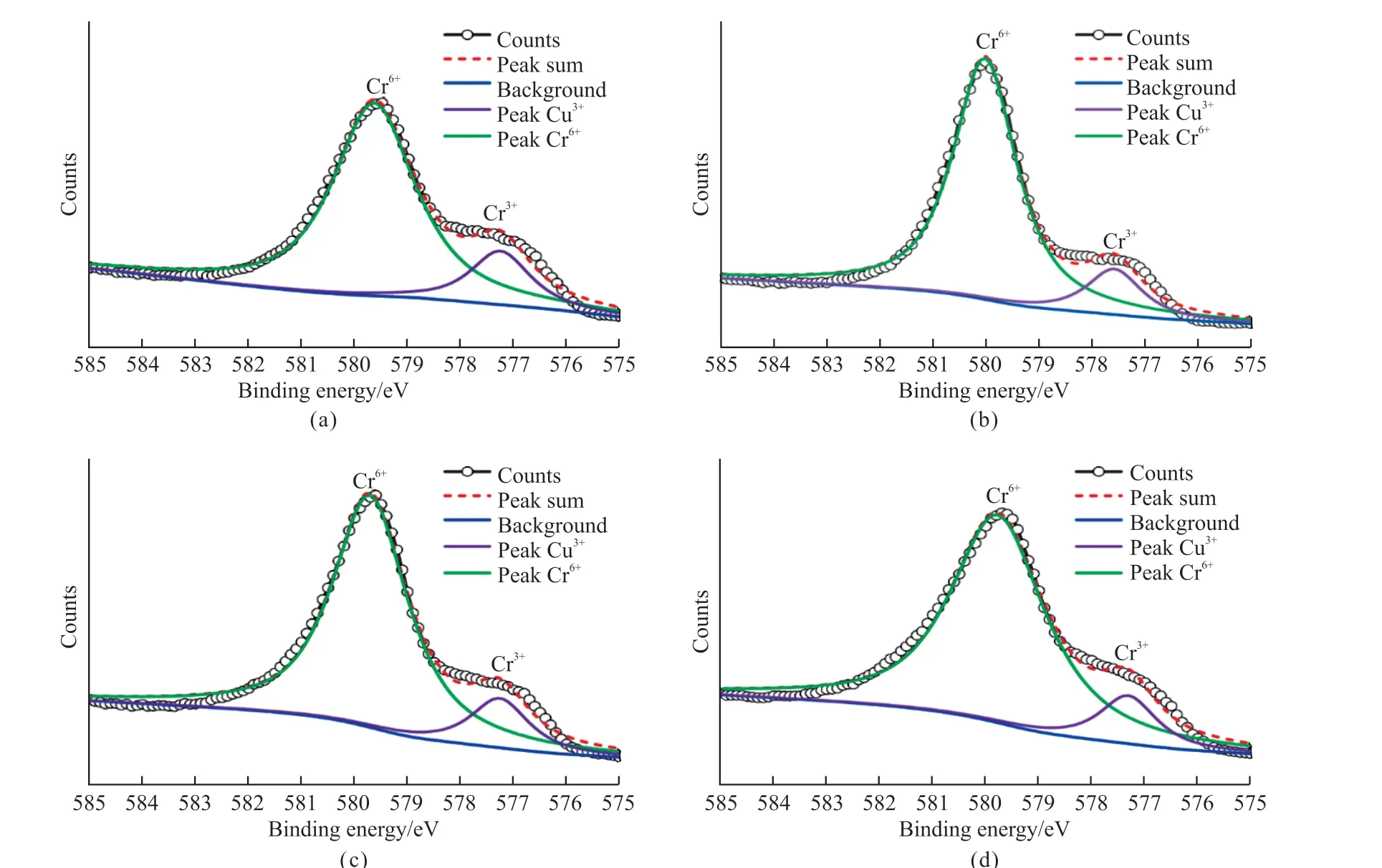
Fig.3 The XPS spectra of Cr2p of NixCr8-xO21 samples: (a) Cr8O21; (b) Ni0.25Cr7.75O21; (c) Ni0.5Cr7.5O21; (d) Ni0.75Cr7.25O21
Fig.3 depicts the XPS spectra of Cr 2p of NixCr8-xO21(x= 0, 0.25, 0.5, and 0.75) samples.The Cr 2p peaks can be divided into Cr3+and Cr6+with binding energies at 576.5 and 578.8 eV, respectively.Compared with the Cr3+/Cr6+ratio of 27.4% in the sample without Ni, the Cr3+/ Cr6+ratio in the doped samples seems to gradually decrease with increasing nickel content, which are 26.3% of Ni0.25Cr7.75O21, 24.6% of Ni0.5Cr7.5O21and 23.4% of Ni0.75Cr7.25O21, respectively.These results suggest that Ni2+replaces Cr3+rather than Cr6+, which is consistent with our prediction.
The comparison of the initial discharge capacities for the samples with various Ni contents at 0.1 C is shown in Fig.4(a).It can obviously be perceived that the initial discharge capacities increase first and then decrease with increasing Ni contents, which indicates that Ni contents make an important contribution to the improvement of discharge specific capacities of the samples.Among them, the Ni0.5Cr7.5O21sample de livers the highest specific capacity of 392.6 mAh·g-1.Figs.4(b) and 4(c) exhibit the discharge curves of two representative samples of Cr8O21and Ni0.5Cr7.5O21at selected cycles under 0.1 C.As shown, the discharge specific capacities of both samples decrease significantly between the second cycle and the first cycle, which results from the irreversible phasetransition during the first discharge[13,14].However, Ni doping obviously enhances the cycling performnces of the sample in the following cycles.The capacity decay of bare Cr8O21sample is as high as 65.6 mAh·g-1from second cycle to 100th cycle, while that of Ni0.5Cr7.5O21sample is less than 12 mAh·g-1.
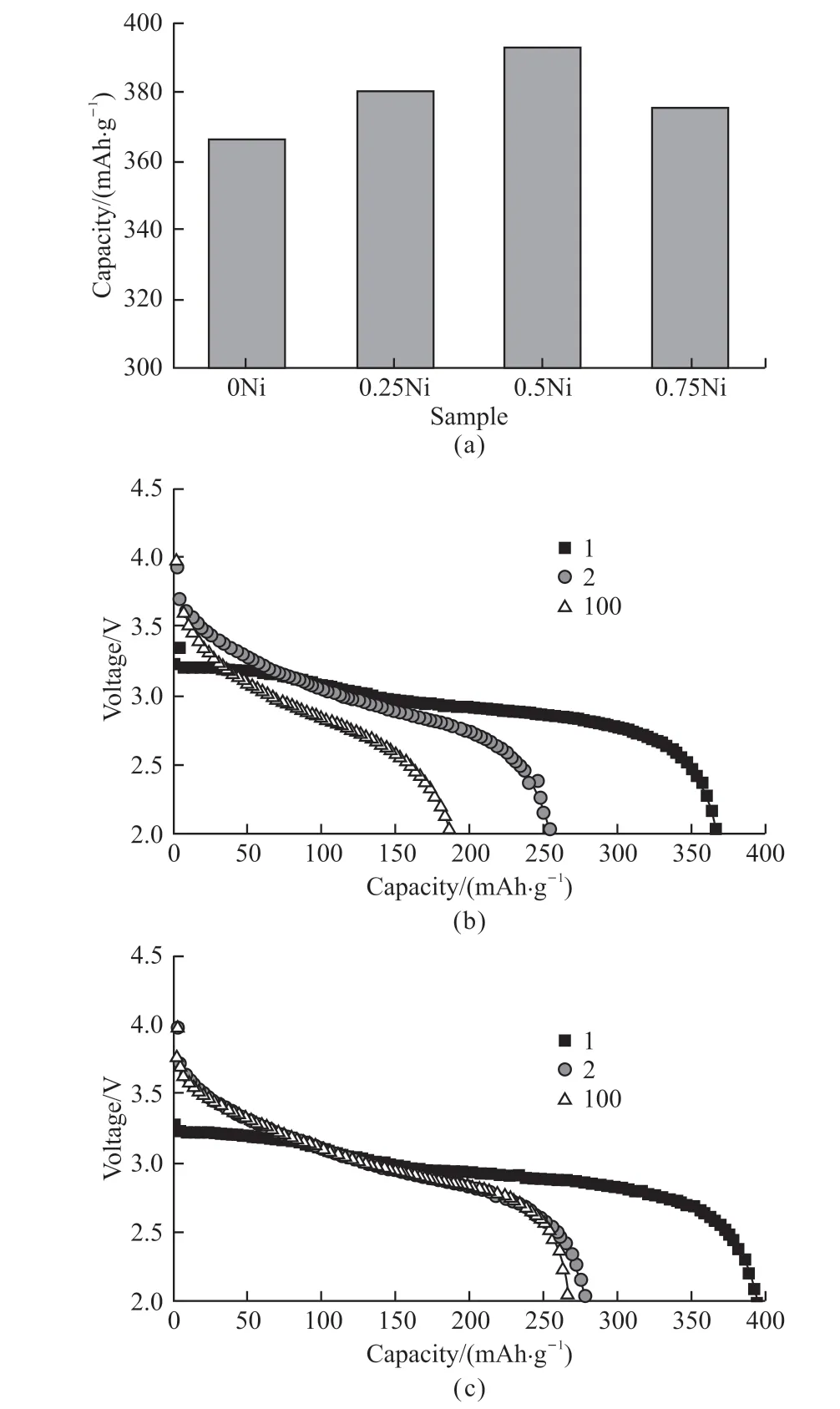
Fig.4 (a)The initial discharge capacities of NixCr8-xO21 samples at 0.1 C (x=0, 0.25, 0.5, and 0.75); The discharge profiles of (b)Cr8O21 and (c) Ni0.5Cr7.5O21 at selected cycles under 0.1 C
Fig.5 presents the cycling performances of Cr8O21and Ni0.5Cr7.5O21samples with the voltage window from 2 to 4.2 V under 0.1 C.The discharge capacities of the first and 100th cycles of the un-doped Cr8O21are 366.4 and 187.7 mAh·g-1, respectively.Obviously, both the first discharge capacity and the 100th discharge capacity of the Ni0.5Cr7.5O21sample are higher than those of un-doped Cr8O21.In addition, the specific discharge capacity of un-doped Cr8O21decreases rapidly upon cycling, and only 51.2% specific discharge capacity is remained after 100th cycle.On the contrary,the capacity retention of Ni0.5Cr7.5O21demonstrates a significant improvement, which could reach up to 67.9% after 100 cycles.The above observations imply that the better cycling performance can be effectively achieved via introducing Ni into Cr8O21.
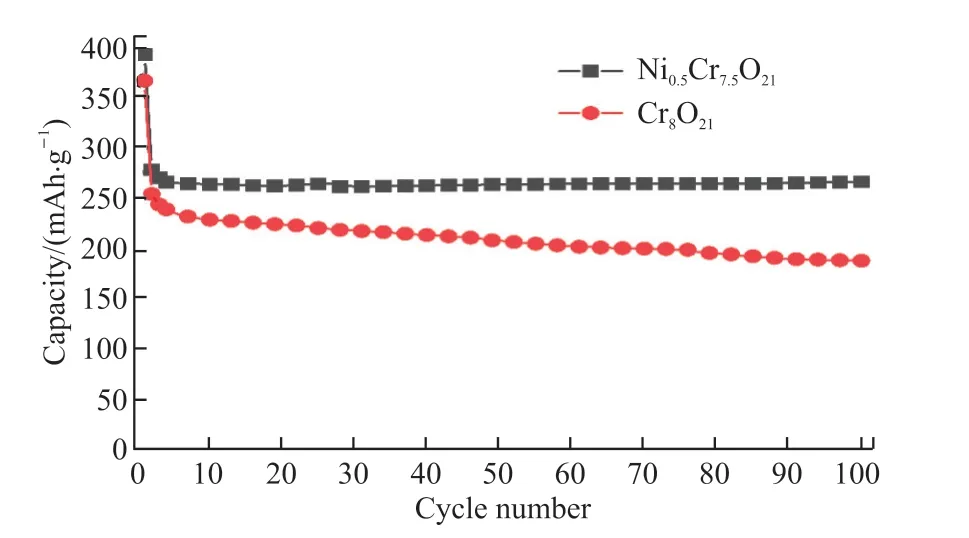
Fig.5 The cycling performances of Cr8O21 and Ni0.5Cr7.5O21 at 0.1 C
Fig.6 reveals the first discharge curves of the samples at 0.1, 0.5, and 1 C with a cut-off voltage 2 V, respectively.All the samples illustrate similar first discharge curves at 0.1 C, which contains two voltage platforms at around 3.2 and 3.0 V.However, at higher rates, such as 0.5 and 1 C, the voltage plateaus seem to disappear and become a sloping region from 3.2 to 2.8 V for un-doped sample.With the increase of current densities, the initial discharge capacities of all samples decrease, however the Ni0.5Cr7.5O21sample still exhibits higher discharge capacities.For example,the discharge capacity of Cr8O21rapidly decays to only 271.4 mAh·g-1at 1 C, while Ni0.5Cr7.5O21sample can still provide a good discharge capacity of 348.7 mAh·g-1, with an increase of more than 28%.The capacity retention ratio of 1vs0.1 C for Ni0.5Cr7.5O21is about 88.8%, while only about 74.1% for Cr8O21.Obviously, the Ni doping can apparently improve the rate properties of the Cr8O21.Compared with the reported Cr-based cathode materials in some previous work, Ni0.5Cr7.5O21still exhibits a significant advantage in the initial discharge capacity (Table 1).
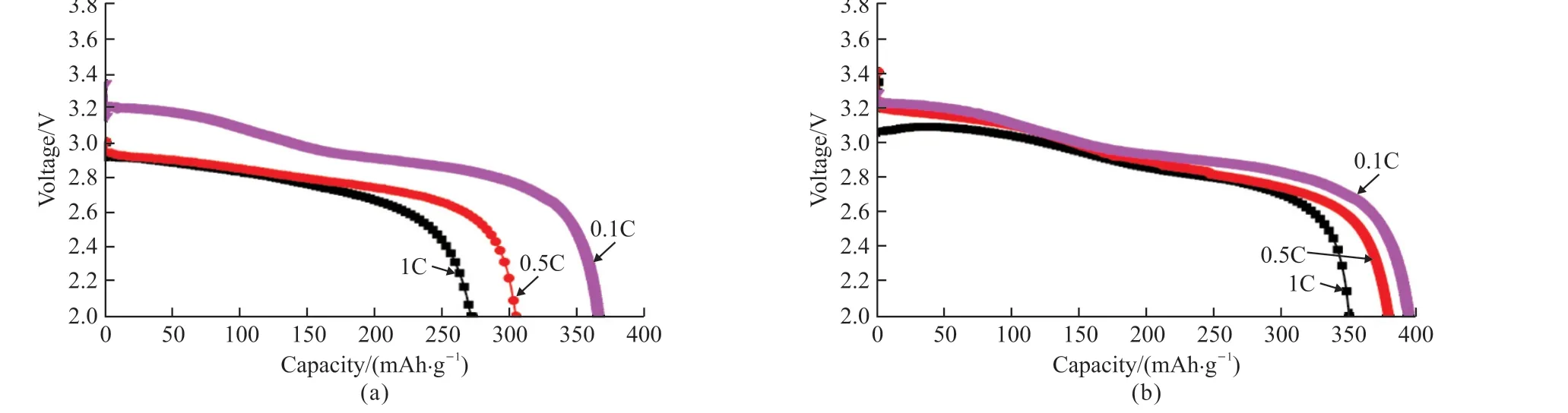
Fig.6 The initial discharge curves of (a) Cr8O21 and (b) Ni0.5Cr7.5O21 at different rates
The CV curves of Cr8O21electrode and Ni0.5Cr7.5O21electrode for the first three cycles are described in Fig.7, which are collected at the scan rate of 0.1 mV/s and over the voltage range 2-4.2 V.In the initial cycle, two reduction peaks and one oxidation peak can be observed.In the subsequent intercalation and de-intercalation processes, both the Cr8O21sample and Ni0.5Cr7.5O21sample perform comparable CV curves with a pair of redox peaks.For the Ni0.5Cr7.5O21sample,the reduction peak and oxidation peak appear at 2.82 and 3.56 V, respectively, while they are located at 2.69 and 3.53 V for bare Cr8O21sample.The difference of the redox peaks potential ∆E(0.84 V) for un-doped Cr8O21is higher than that for Ni0.5Cr7.5O21sample(0.84 V), which indicates that Ni doping inhibits the polarization and improves the cycling stability of undoped Cr8O21.Moreover, the CV curves of Ni doped sample deliver excellent overlap between two cycles,which also verifies that Ni doping contributes to the improvement of cycling performance.
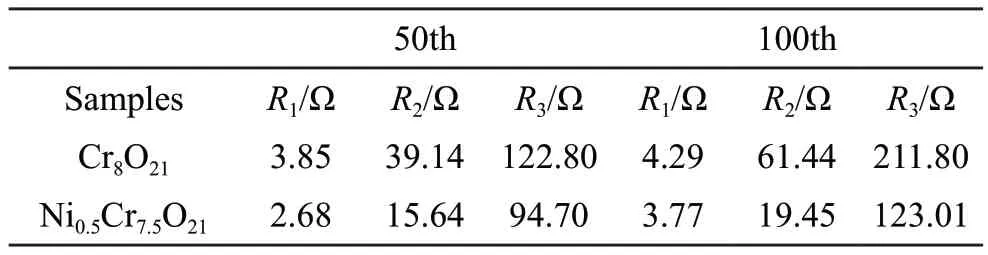
Table 2 The fitting EIS results of Cr8O21 and Ni0.5Cr7.5O21 samples
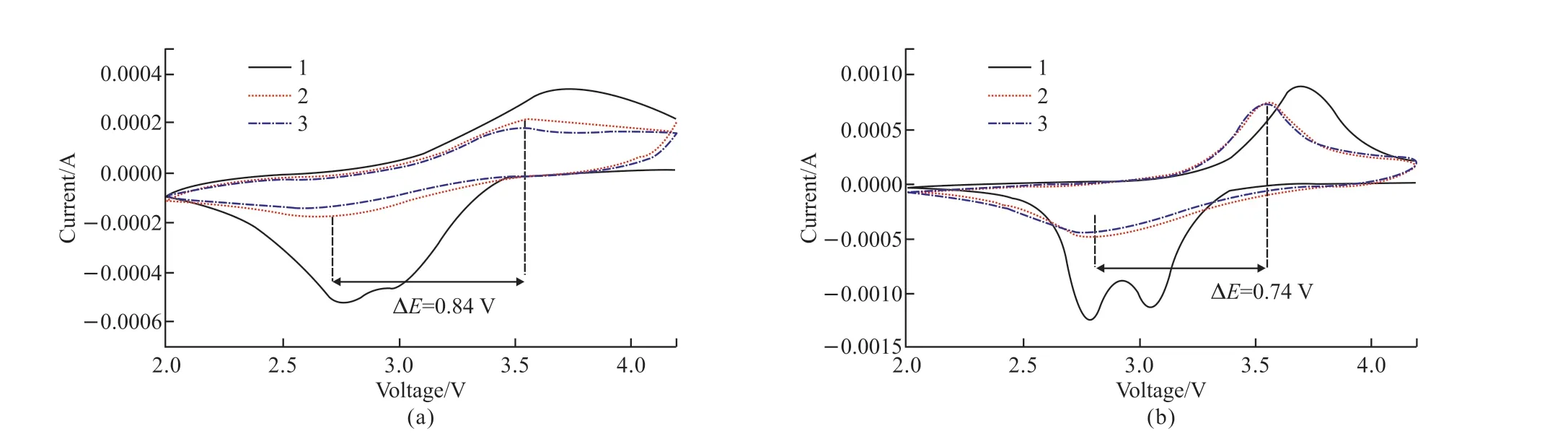
Fig.7 The cycling CV curves at scan rate of 0.1 mV/s with voltage range 2-4.2 V of (a) Cr8O21 and (b) Ni0.5Cr7.5O21
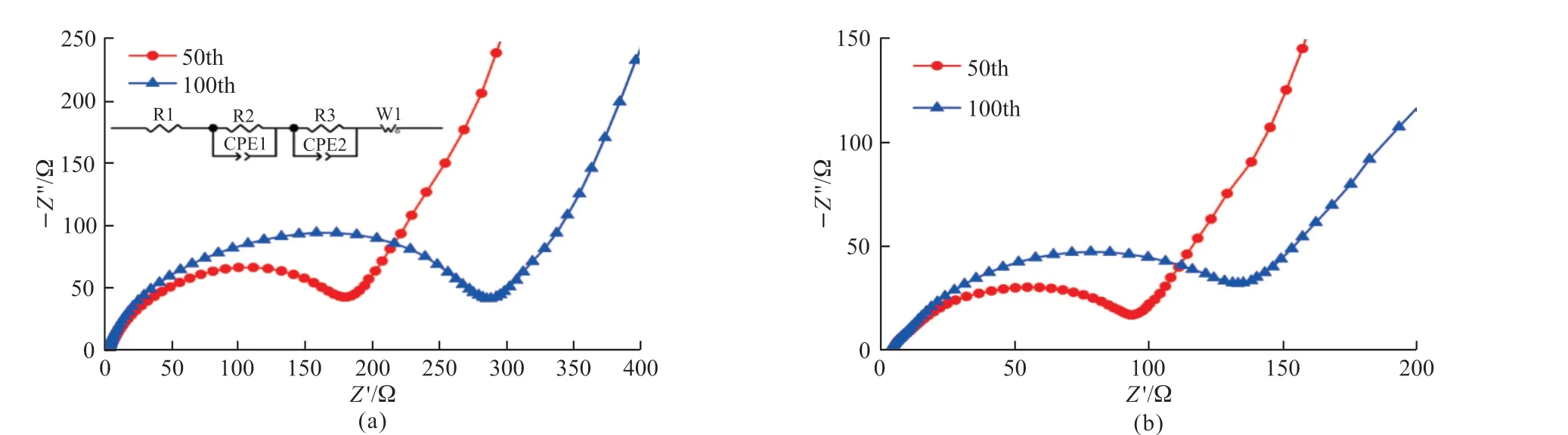
Fig.8 The EIS spectra for Cr8O21 and Ni0.5Cr7.5O21 between 10-2 to 105 Hz
EIS measurements of the cells with Cr8O21and Ni0.15Cr7.85O21samples are shown in Fig.8.The matched equivalent circuit is shown as inset in Fig.8(a)[26,27],and the fitting results of the impedance parameters are listed in Table 2.It is easy to be found that both samples show almost sameRs(solution resistance),while theRf(interface impedance) andRct(chargetransfer resistance) values of un-doped sample at the 50th cycle are higher than those of Ni doped sample.In addition, the growth ofRfandRctvalues of Ni doped Cr8O21after 100 cycles is apparently less than that of un-doped Cr8O21sample compared with those after 50th cycle.It can be concluded that Ni doping can effectively promote the electrochemical kinetics of electrode during cycling, which also fits well the above rate performance results.
4 Conclusions
In summary, Ni doped Cr8O21were successfully synthesized and investigated as cathode materials for lithium-ion batteries.Obviously, Ni doping contributed to enhancing the electrochemical performances of Cr8O21.Ni contents acted a significant role in determining the reversible capacity of the prepared samples.Ni0.5Cr7.5O8exhibited a highest initial capacity of 392.6 mAh·g-1, compared to 366.4 mAh·g-1for Cr8O21at 0.1 C.A reversible capacity of over 266 mAh·g-1after 100 cycles at 0.1 C could be obtained for Ni0.5Cr7.5O8sample, while only 187.7 mAh·g-1was left for un-doped sample.Moreover, Ni doped Cr8O21was characterized by better rate capability compared with bare material.The capacity retention of 1 vs 0.1 C for Ni0.15Cr7.85O21was over 88%, much higher than that of Cr8O21(74%).
Conflict of interest
All authors declare that there are no competing interests.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Design on the Prestressed Concrete Frame Beam-column
- Synthesis and Flocculation of Polyacrylamide with Low Water Absorption for Non-dispersible Underwater Concrete
- Experimental Behavior of Recycled Aggregate Concrete Filled Steel Tubular Columns
- Impact-abrasive Wear Behavior of ZTA and NbC Reinforced Fe60 Matrix Composites
- Synthesis and Characterization of Hollow Strontium Carbonate Pompons by Composite Soft Template Method
- Preparation of Co/CoOx Derived from a Lowtemperature Etching of ZIF-67 for Oxygen Reduction and Oxygen Evolution Catalytic Reaction
