Synthesis and Characterization of Hollow Strontium Carbonate Pompons by Composite Soft Template Method
2024-01-03LIBiboDONGXiufangLIYuSONGJianXIEWuxiZHANGWeiLIUYunfei
LI Bibo, DONG Xiufang*, LI Yu, SONG Jian, XIE Wuxi, ZHANG Wei, LIU Yunfei
(1. School of Environment and Safety Engineering, North University of China, Taiyuan 030051, China; 2. School of Chemical Engineering and Technology, North University of China, Taiyuan 030051, China; 3. Xi’an Modern Chemistry Research Institute, Xi’an 710065, China)
Abstract: The hollow strontium carbonate pompons was synthesized for the first time by a controlled reaction precipitation method with sodium dodecyl benzene sulfonate (SDBS) and polyvinyl pyrrolidone (PVP)work together as template.The sampled particles were characterized by scanning electron microscopy (SEM),transmission electron microscopy (TEM), nitrogen adsorption-desorption measurement, X-ray diffraction(XRD), Energy dispersive X-Ray spectroscopy (EDX), Fourier transform infrared spectroscopy (FTIR),Thermogravimetric analysis and differential scanning calorimetry (TGA-DSC), etc.It is shown that the assynthesized hollow strontium carbonate pompons with the size of about 2 μm consist of flake-like particles under the optimal reaction conditions.The formation mechanism of hollow strontium carbonate pompons was preliminarily explored.
Key words: strontium carbonate; hollow structure; pompons; composite soft template method
1 Introduction
Strontium carbonate (SrCO3) is a kind of inorganic compound, which is one of the important chemical raw materials and the most important strontium salt.The products prepared with strontium carbonate are widely used in chemical industry, light industry, military industry, electronics, metallurgy and more than dozens of industries[1-3].Strontium carbonate products with different morphologies have different application values.Strontium carbonate of spherical and high purity can be used as a production raw material for electronic ceramics.Threadiness strontium carbonate has a good reinforcing effect.And needle-like strontium carbonate can be used in microelectronics industry and plastic, rubber, coating and other fillers.However, ribbon-like strontium carbonate particles have higher activity and are easy to be mixed with titanium dioxide to produce evenly dispersed strontium titanate powder[4-9].Therefore, the research and development of new morphology and structural strontium carbonate powder have significant for social production and people’s lives.
At present, the control of particle size and morphology of strontium carbonate is widely studied at home and abroad.Most of the synthesis methods of strontium carbonate are to control the particle size and morphology of strontium carbonate by adjusting reaction conditions or adding crystal shape control agent.The synthesis methods of strontium carbonate includes reaction precipitation method[10], ultrasonic synthesis method[11], carbonization method[12], solvent thermal method[13]and microbial oxidation synthesis method[14],etc.These preparation methods directly or indirectly use high purity strontium chloride, strontium nitrate and recrystallized strontium hydroxide as raw materials to react with high purity carbonization agent and assist in the synthesis of specific morphology of strontium carbonate in morphology agent and different reactors.And a variety of products with spherical[15],ellipsoid[16], branchlet-like[17], flower-like[18], ribbonlike[19], needle- like[20]and threadiness[21]have been synthesized.However, the synthesis and morphology control methods of hollow strontium carbonate are less studied[22,23].
In this paper, the synergism between sodium dodecyl benzene sulfonate and polyvinyl pyrrolidone is used to control the morphology of strontium carbonate.The hollow strontium carbonate pompons was synthesized for the first time by composite soft template controlled reactive precipitation method.The synthesized hollow strontium carbonate pompons has the characteristics of uniform size and good dispersion.
2 Experimental
2.1 Materials
Strontium chloride hexahydrate (SrCl2·6H2O)was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd.Ammonium bicarbonate(NH4HCO3) was purchased from Shanghai Macklin Biochemical Co., Ltd.Sodium dodecyl benzene sulfonate (SDBS) and Polyvinylpyrrolidone (PVP) were purchased from Sinopharm Chemical Reagent Co.,Ltd.Anhydrous ethanol (C2H5OH) was purchased from Tianjin Hengxing Chemical Reagent Manufacturing Co., Ltd.Deionized water (H2O) was prepared in the laboratory.All these reagents were analytical pure and used without further treatment.
2.2 Synthesis of hollow SrCO3 pompons
The Synthesis of hollow SrCO3pompons is shown in Fig.1.The details are presented as follows:firstly, SrCl2• 6H2O (2.67 g) and PVP (0.4 g) were dissolved in deionized water (100 mL) and stir magnetically for 10 minutes to obtain a solution of SrCl2and PVP, called solution A.NH4HCO3(1.58 g)and PVP (0.4 g) were dissolved in deionized water(200 ml) and stir magnetically for 10 minutes to obtain a solution of SrCl2and PVP, called solution B.SDBS(3.48 g) were dissolved in deionized water (100 mL)and stir magnetically for 10 minutes to obtain SDBS solution, called solution C.Then, the solution A was slowly added to the solution C using a peristaltic pump with a rotating speed of 20 rpm, and a uniform mixed solution was obtained by ultrasound.The mixture into a three-necked flask and use a 20 rpm peristaltic pump to slowly drop solution B into the three-necked flask,stirred at a speed of 250 r·min-1in a 50 ℃ water bath for 12 hours, and naturally cooled to room temperature to get the mixed solution of SrCO3.Finally, the precipitate after centrifugation was washed three times with deionized water.The precipitate was dried at 50℃ for 6 h, and then calcined in air at 350 ℃ for 4 h to obtain white SrCO3powder.

Fig.1 Synthesis principle and pathway of hollow SrCO3 pompons
2.3 Characterization
The surface morphology of SrCO3samples was observed with EDS energy spectrum detector using JSM-6700F type field emission scanning electron microscope (SEM) of Japan Electronics Company, and the working voltage was 15 kV.The apparent morphology and particle size of SrCO3samples were observed under different conditions.The structure, morphology and dispersion of SrCO3samples were observed by FEI’s F20 transmission electron microscope (TEM).The crystal phase composition of SrCO3samples was determined by D8Advance X-ray diffraction (XRD).Infrared spectra of SrCO3samples were determined by FTIR using VERTEX-80 Fourier transform infrared spectrometer.Thermal stability of SrCO3samples were tested by STA449F5 thermogravimeter analysis and differential scanning calorimetry (TGA-DSC).The elements of SrCO3samples were tested using the Talos energy spectrometer (EDX) attached to FEI’s F20 transmission electron microscope.The specific surface area and pore diameter distribution of SrCO3samples were tested by ASAP2460 nitrogen adsorption-desorption isotherms from Mike, USA.
3 Results and discussion
3.1 Morphology analysis
Fig.2 shows the SEM diagram and local magnification diagram of the synthesized samples under the optimal synthesis conditions and the overall morphology of SrCO3samples can be seen.Figs.2(a-d)show the morphology of synthesized SrCO3samples with PVP and SDBS as composite soft film plate is pompons.The SrCO3pompons has uniform particle size and good dispersibility.According to the scale in the figure, the particle size of SrCO3pompons is mainly concentrates around 2 μm.And the sphericity of SrCO3pompons is relatively complete, which is assembled by the aggregation of flake SrCO3nanoparticles.It can be seen from Figs.2(e)-2(f) that a SrCO3pompons is embedded in another damaged SrCO3pompons,so SrCO3pompons may have hollow structure.Furthermore, Figs.2(g)-2(h) show the damaged SrCO3pompons has a hollow structure.This shows that the hollow SrCO3pompons was successfully synthesized for the first time.

Fig.2 SEM images and local enlargement of SrCO3 samples
3.2 Structure analysis
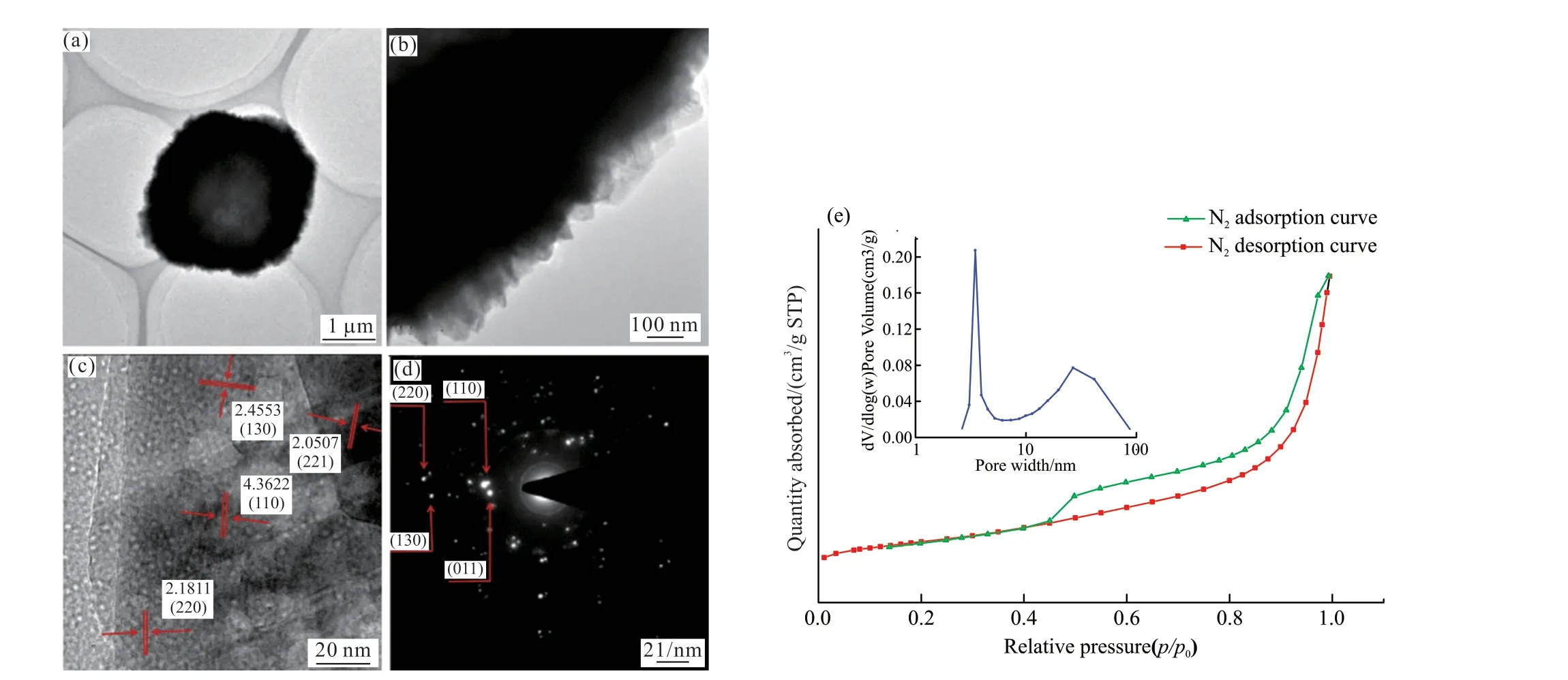
Fig.3 (a-d) TEM images of SrCO3 samples and (e) Nitrogen adsorption-desorption isotherms and pore diameter distribution plot of SrCO3 samples
Figs.3(a)-3(d) show the TEM images of the synthesized samples at the optimal conditions, the morphology and structure of the SrCO3pompons more clearly.It can be clearly seen from Fig.3(a) that the synthesized SrCO3pompons has a hollow structure.According to the local magnification figure (Fig.3(b)and Fig.3(c)), the shell of the hollow SrCO3pompons is composed of flake SrCO3nanoparticles.According to the scale in the figure, flake SrCO3nanoparticles have a width of 30 - 100 nm.The shell thickness of the hollow SrCO3pompons is about 0.6 - 1 μm.The lattice fringe (Fig.3(c)) shows an interplanar spacing of approximately 0.25, 0.21, 0.44, and 0.22 nm, which can be indexed to the (130), (221), (110), and (220) plane of the SrCO3phase.The diffraction ring can be seen through the electron diffraction image (Fig.3(d)), but it is not obvious.This is because the fewer particles in the field of view, the less clear the polycrystalline ring.The results show that the hollow SrCO3pompons have good crystallinity.
Fig.3(e) shows the nitrogen adsorption-desorption isotherms and pore size distribution for SrCO3samples.The synthesized SrCO3yielded a type IV isotherm with a capillary condensation step and an H3-type hysteresis loop is observed.In the low pressure section (P/P0is 0-0.45), the adsorption- desorption isotherms of SrCO3is rose slowly and almost identical.In the middle pressure section (P/P0is 0.45 - 0.9), the adsorption capacity gradually increases with the increase of relative pressure and the isotherm of adsorption and desorption was separation occurs.During the adsorption process, there is a capillary condensation phenomenon.The results show that the SrCO3pompons has mesoporous structure.In the high pressure section (P/P0is 0.9 - 1.0), the adsorption capacity increases sharply and reaches adsorption saturation at a certain relative pressure, which is due to the occurrence of multilayer adsorption, indicating that the SrCO3pompons also has a macroporous structure.The hysteresis loop belongs to the Hysteretic loop of H3-type, which indicates that the mesoporous of SrCO3pompons forming a slit formed by the accumulation of flake particles, which is consistent with the analysis in SEM (Fig.2(c)).The pore diameter had two concentrated distributions, one of which was about 2 - 6 nm.This is mainly due to the fact that the prepared SrCO3is assembled by flake nanometer particles and there are gaps between the particles to form a mesoporous structure.The other location is about 20 - 50 nm, which is caused by the cavity formed of the hollow SrCO3pompons.The calculated specific surface area is 20.79 m2/g by BET method.The BET specific surface area of prepared high-purity SrCO3by the traditional liquid-liquid and conventional gasliquid synthesis process is 1.8-3.0 m2/g and 10-15 m2/g.Compared with them, the specific surface area of the hollow SrCO3pompons is significantly increased.This show that the strontium carbonate pompons has a hollow structure, which increases the specific surface area of strontium carbonate and makes it have a large specific surface area.
3.3 Phase analysis
Fig.4 shows the energy spectrum analyses of the synthesized samples were analyzed by EDX.The surface of the synthesized samples contains only three elements: Sr, C, and O.Fig.5(a) shows the crystalline phase of SrCO3samples were analyzed by XRD.It can be seen from the figure that the samples show multiple diffraction peaks between 20° - 80°.According to the JCPDS standard card, the peak exit position and the relative strength of each peak in the figure are consistent with the SrCO3standard card PDF#99-0099.The peaks appeared at 20.34°, 25.19°, 25.84°,29.65°, 36.57°, 41.36°, 44.13°, 47.76°, 49.98°, 57.29°,63.98°, and 73.82° were attributed to {110}, {111},{021}, {002}, {130}, {220}, {221}, {132}, {113},{311}, {330}, and {313} reflections of the spinel phase of SrCO3.The SrCO3samples is a typical aragonite structure crystal of the orthorhombic system.No hybrid peaks appear in the figure, indicating that SrCO3is of high purity.The diffraction peak is strong and sharp,which indicates that the as-prepared hollow SrCO3pompons has good crystallinity.

Fig.4 EDX image of SrCO3 samples

Fig.5 (a) XRD pattern and (b) Infrared spectra of SrCO3 samples

Fig.6 (a) TGA-DTG curves and (b) TGA-DSC curves of SrCO3 samples
Fig.5(b) shows the FTIR spectra of SrCO3samples treated in the wavenumber range of 500 -4 000 cm-1.The observed bands at 858.18, 1 484.94,and 1 772.29 cm-1are corresponding to the characteristic absorption peaks of SrCO3, which proves that the sample is SrCO3powder.The strong absorption peaks at 705.83 and 3439.41 cm-1are characteristic peaks of O-H.The test results of EDX, XRD, and FTIR are consistent, indicating that the hollow SrCO3pompons has the advantages of high purity and good crystallinity.
3.4 Thermal analysis
In Fig.6(a), the TG curve shows the decomposition of SrCO3samples material starts at 770.1 ℃, while the DTG curve only has an obvious weight loss peak at 1 007.2 ℃.In Fig.6(b), DSC curve is concave and SrCO3samples has obvious heat absorption peak at 681.6 ℃, which is due to the decomposition of SrCO3samples and heat absorption.In the thermogravimetric analysis, the higher the thermal decomposition temperature of the material, the better the thermal stability of the material.Based on the above results,the hollow SrCO3pompons has high purity and good thermal stability.
3.5 Discussion on formation mechanism
Fig.1 shows that synthesis principle and pathway of hollow SrCO3pompons.PVP exists in aqueous solution in the form of long chain, quasi-circular,reticular, etc.In the experiment, when PVP is mixed with SrCl2solution, PVP is combined with Sr2+as a chelator.When the concentration of SDBS is much higher than the critical micelle concentration of SDBS in aqueous solution, SDBS existed in the form of micelles in the solution and spherical micelle clusters were formed between micelles through clustering.The strong electrostatic interaction between the polar sulfonic acid group HSO3-of SDBS and Sr2+causes Sr2+to gather around the clustered bundle.Meanwhile, Sr2+attracts a large number of long chain PVP molecules.PVP molecules are wrapped or embedded in the spherical SDBS micelles to form a composite soft template, which plays a template role in the precipitation process of SrCO3.When the mixed solution of NH4HCO3/PVP was dripped into the mixed solution of SrCl2/SDBS/PVP, CO32-combined with Sr2+enriched around the clustered bundle to form amorphous SrCO3.HSO3-is parallel to the plane where CO32-is located.Through stereochemical complementation, SrCO3nucleation along the plane can be induced and the formation of SrCO3crystal can be promoted.Steric hindrance effect of long chain molecule of PVP plays a stable and dispersive role in the formation of SrCO3.In the meantime,bridging action of PVP molecules also makes dispersed SrCO3particles close to each other, which provides the possibility for orderly assembly, forming flake SrCO3particles and making the spherical structure more rounded.Simultaneously, the stereochemical conformation of the similar circular PVP has a high energy, and the oxygen atom in group C=O can attract Sr2+in the solution from all directions, thus forming the spherical SrCO3particle.After centrifugation, washing,drying and calcination, SDBS and PVP are completely removed to form hollow SrCO3pompons.
4 Conclusions
In summary, a new hollow strontium carbonate pompons was successfully synthesized.The controllable preparation of hollow strontium carbonate pompons was realized by using environmental friendly and high repeatability composite soft template method.The particle size and specific surface area of the hollow strontium carbonate pompons are around 2 μm and 20.79 m2/g.The hollow strontium carbonate pompons itself has the characteristics of uniform particle size,good dispersion and large specific surface area.Overall, the successful synthesis of hollow strontium carbonate pompons has certain value for the control study of particle morphology and structure of strontium carbonate.
Conflict of interest
All authors declare that there are no competing interests.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Enhanced Electrochemical Performances of Ni Doped Cr8O21 Cathode Materials for Lithium-ion Batteries
- Design on the Prestressed Concrete Frame Beam-column
- Synthesis and Flocculation of Polyacrylamide with Low Water Absorption for Non-dispersible Underwater Concrete
- Experimental Behavior of Recycled Aggregate Concrete Filled Steel Tubular Columns
- Impact-abrasive Wear Behavior of ZTA and NbC Reinforced Fe60 Matrix Composites
- Preparation of Co/CoOx Derived from a Lowtemperature Etching of ZIF-67 for Oxygen Reduction and Oxygen Evolution Catalytic Reaction
