Preparation of Spherical Tungsten Particles Assisted by Hydrothermal Method
2024-01-03GUOJiawangWENXiaoqiangWUYingXUJianbingZHOUJieying
GUO Jiawang, WEN Xiaoqiang, WU Ying, XU Jianbing, ZHOU Jieying
(Ganzhou Nonferrous Metallurgy Research Institute Co., Ltd, Ganzhou 341000, China)
Abstract: We presented a strategy to prepare spherical tungsten powder by the combination of hydrothermal method and H2 reduction process.In hydrothermal process, the micelle of tetraethylammonium bromide (TEAB) act as spherical templates for the deposition of tungsten oxide, whereas the excessive TEAB inhibit the formation of spherical tungsten oxide due to the dense molecular layer of TEAB on the tungsten oxide particles.Citric acid (CA) can control the formation rate and structure of the tungsten oxide when its concentration is more than 0.2 mol/L, because of its ability to coordinate with tungsten atoms.The synergistic effect of TEAB and CA facilitates the formation of spherical tungsten oxide with nanorod crown.After being treated by H2 at 600 and 650 ℃, the tungsten oxide particles are reduced to tungsten particles, which maintain the spherical structure of tungsten oxide and have porous structure.
Key words: spherical tungsten particles; spherical tungsten oxide; hydrothermal method; citric acid;tetraethylammonium bromide
1 Introduction
Tungsten (W) is the most popular refractory material, because of its extraordinary properties, including high melting point, excellent thermal and electrical conductivities, high hardness, and corrosion resistance,etc[1,2].It has been applied widely in the fields of military industry, chemical industry, medical instruments micro-electronics, and nuclear industry[3-6].Usually,tungsten-based components were preparedviapowder metallurgy process.The properties of tungsten powder,such as constituents, morphology, and particle size,greatly determine the performance of tungsten-based components[7-9].Therefore, various tungsten powders have been developed for diverse tungsten-based parts.Among these, spherical tungsten powder has high flowability, low friction coefficient, high packing density,and high tap density[1,9].These features make spherical tungsten powder highly preferable raw material for metal powder injection molding (MIM), 3D printing,and thermal spraying.Hence, spherical tungsten powder has been increasingly demanded recently, due to the advancement of MIM and 3D printing[10-12].
Generally, spherical tungsten powder is prepared by plasma spheroidization process.In this process,the polyhedral W particles are axially injected into the plasma with ultrahigh temperature, subsequently the part or the whole of W particles are melted immediately, and then they are solidified to spherical W particles owing to being rapid cooled after leaving the plasma region[13,14].Although plasma spheroidization could be used to prepare the powder with high purity, high density, and high sphericity.Because of the wide particle size distribution and aggregation of as-received tungsten powder, it is hard to obtain narrow particle size distribution spherical tungsten powder, especially the size below 10 µm.The excessive evaporation of the tungsten particles in plasma often causes formation of extremely fine spherical tungsten particles that are undesirable.Except those, the wide application of plasma spheroidization process is also limited by the high cost in equipment and operation.There are several inexpensive methods to prepare spherical W particles, such as tungsten-oxido-reduction, localized preferential oxidation, and alkaline washing[15,16].However, in these processes, only the edges and corners having high surface energy can be wiping off, hence it is almost impossible to prepare spherical tungsten powder with good sphericity and high spheroidization ratio.
Hydrothermal method is recognized as a controllable and low-cost method for the preparation of micro/nano particles[17].In hydrothermal method, the constituent, structure and size of micro/nano particles could be adjusted by controlling the thermodynamic process and the dynamic process of the nucleation and growth of the particles[18].It is demonstrated that the tungsten oxide particles with various morphologies could be prepared by controlling the tungsten precursor, capping agent, temperature,etc.in hydrothermal process[19-23].WO3microparticles with the size of 3-10 μm were synthesized by Yao Jiannianet althrough hydrothermal treatment of WO3sol in the presence of rubidium sulfate (Rb2SO4) and oxalic acid[24].Su Xintaiet alprepared 800-1 000 nm WO3sub-micro spherical particles by thiourea-assisted hydrothermal process[26].Wang Leiet alpresented a pH-controlled strategy for synthesizing hierarchical WO3with microspheres, microdisks,nanorod bundles and nanorods[27].
In this work, we prepared spherical tungsten microparticles by the combination of hydrothermal method and hydrogen reduction process.In hydrothermal process, we selected the micelle of quaternary ammonium salts as templates to induce the formation of spherical tungsten oxide.Meanwhile, dicarboxylic acid was chosen as capping agent to control the reaction rate and growth direction of the tungsten oxide.Then, the obtained spherical tungsten oxide particles were reduced by H2atmosphere.The resultants, tungsten particles,maintained the spherical structure of tungsten oxide and possessed porous structure because of the generation of H2O gas in the reaction process.
2 Experimental
2.1 Materials and characterization
Sodium tungstate dihydrate (Na2WO4•2H2O, AR)was purchased from Aladdin Chemistry Co., Ltd.Hydrogen chloride (HCl, 37%) was obtained from Xilong Scientific Co., Ltd.Citric acid monohydrate (CA, AR)was purchased from Hunan Huihong Reagent Co., Ltd.Tetramethylammonium bromide (TMAB, 99%), tetraethylammonium bromide (TEAB, 99%), and tetrahexylammonium bromide (THAB, 99%) were purchased from Beijing Innochem.Hydrogen gas (H2, 99.9%)was supplied by Ganzhou Huamao Tungsten Materials Co., Ltd.Scanning electron microscope (SEM) analyses were performed on a TESCAN MIRA3 LMH.Xray diffraction (XRD) analyses were carried out using PANalytical X’ Pert Powder.
2.2 Synthesis of spherical tungsten oxide
In the typical experiment, 16.8 g of citric acid was added to 380 mL of 0.10 mol/L Na2WO4•2H2O aqueous solution.Next, the pH of the solution was adjusted to 1.0 with 3.00 mol/L HCl aqueous solution.After 30 min of stirring, 16.00 mL of 0.14 mol/L TEAB was added to the above mixture with stirring for 30 min.After that, 80 mL of the solution was transferred to a 100 mL autoclave, sealed and maintained at 170 ℃ for 4 h.Then, the precipitates were filtered and washed with deionized water, and subsequently dried at 80 ℃for 12 h to obtain the pure spherical tungsten oxide.The spherical tungsten trioxide was obtained by calcining the spherical tungsten oxide at 400 ℃ for 1 h in air atmosphere.
2.3 Preparation of spherical tungsten microparticles
One gram of spherical tungsten trioxide particles was filled into a quartz boat and then put into an alumina tube.Then the alumina tube was placed into the constant temperature zone of a furnace (OTF-1200X-S,Hefei Kejing Materials Technology Co., Ltd).After the air in the alumina tube was driven out by nitrogen, the furnace was heated up to 550-650 ℃ at a heating rate of 5 ℃/min and held at that temperature for 120 min.Then, the tube was cooled down to room temperature.Dynamic atmosphere of hydrogen gas was used in the experiments, using a gas flow of 400 mL/min.
3 Results and discussion
3.1 Controlled synthesis of spherical tungsten oxide
The scanning electron microscope (SEM) images of tungsten oxide prepared with tetraethylammonium bromide (TEAB) at different concentrations are shown in Fig.1.TEAB molecules can self-assemble into spherical micelle with positive charges.The polytungstate anions tend to adsorb on the spherical micelle,which resulted in the spherical tungsten oxide after being heated at 170 ℃.Therefore, the morphology of the products gradually transforms from flower-like structure to a solid sphere with a nanorod crown with the increase in TEAB concentration (Fig.1).Additionally,the diameters of spherical tungsten oxide decrease with the increasing concentration of TEAB (Figs.1(a)-1(d))because of the increasing number of nuclei.However,excess TEAB micelle lead to the formation of a dense layer on the polytungstate ion and nuclei of tungsten oxide, which inhibits the formation of spherical tungsten oxide.Hence, spherical tungsten oxide particles begin to reduce in amount when the concentration of TEAB is at 28.00 mmol/L (Fig.1(e)), and change completely to irregular particles when the concentration of TEAB raises up to 56.00 mmol/L (Fig.1(f)).
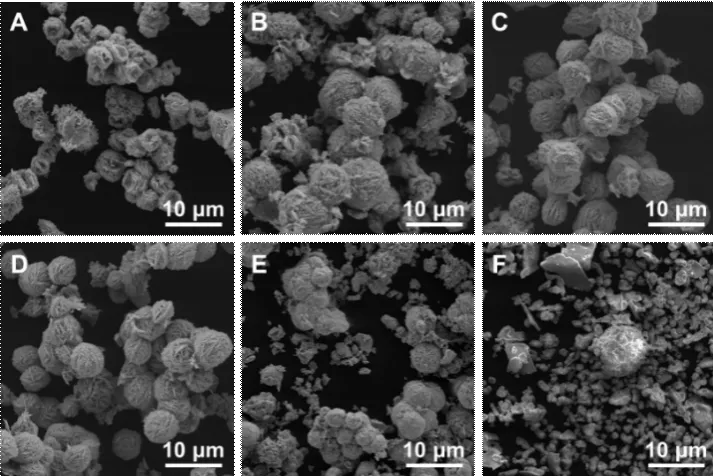
Fig.1 SEM images of products prepared with TEAB at various concentrations: (a) 0 mmol/L, (b) 3.50 mmol/L, (c) 7.00 mmol/L, (d) 14.00 mmol/L, (e) 28.00 mmol/L , and (f) 56.00 mmol/L

Fig.2 SEM images of products prepared by using the quaternary ammoniums with different alkyl chain lengths: (a, d) TMAB,(b, e) TEAB, and (c, f) THAB.The concentrations of the quaternary ammoniums were 7.00 mmol/L
We studied the effects of the alkyl chain length of quaternary ammoniums on tungsten oxide morphology.The spherical micelle of tetramethylammonium(TMAB) and tetrahexylammonium (THAB) can also be the templates for the formation of spherical tungsten oxide (Figs.2(a)-2(c)).There are irregular and low conductive microparticles in the product prepared with THAB, which might result from the irregular aggregation of tungstate anions before the reagents were heated.The sizes of the spherical tungsten oxide prepared with TMAB, TEAB and THAB are 8.3±1.5, 7.38±1.0,and 6.1±1.2 μm, respectively (Figs.2(a)-2(c)), which shows a slight decrease when the alkyl chain length increases.The sizes of nanorods on the surface of spherical tungsten oxide are 268±78, 141±39, and 130±42 nm when TMAB, TEAB, and THAB was used respectively(Figs.2(d)-2(f)), which shows that the sizes of nanorods reduce as the alkyl chain length increases.This results from the fact that the denser molecular layer of TEAB and THAB inhibits the growth of the nanorods on the surface spherical tungsten oxide because of the alkyl chain of TEAB or THAB is longer than that of TMAB.

Fig.3 SEM images at different scales for products prepared with citric acid at various concentrations: (a, e) 0 mol/L, (b, f) 0.10 mol/L, (c, g) 0.20 mol/L, and (d, h) 0.40 mol/L
As bidentate complexing agent, citric acid (CA)is able to coordinate with tungsten atoms through the central part (HO-C-COOH) of the molecules[28-30].At low pH, dinuclear tungstocitrate complexes are formed,with the formula [W2O3(OH)3(CtH)2]3-, where Ct is the completely deprotonated citrate[29].This indicates that CA can effectively control the morphology and size of tungsten oxide.As shown in Fig.3, tungsten oxides were synthesized with citric acid at different concentrations.The production of tungsten oxide decreases with the increasing concentration of citric acid and there are no precipitates and colloid when the concentration is more than 0.80 mol/L.This can be explained by the fact that the strong coordination between tungstate and citric acid inhibits the formation of tungstate oxide.In the absence of citric acid, the tungsten oxide generates rapidly and aggregates irregularly in order to reduce the interface energy (Figs.3(a)-3(e)).With inadequate citric acid, tungsten oxide is observed in flake-shaped aggregations (Figs.3(b)-3(f)).While the concentration of citric acid was more than 0.20 mol/L, the spherical tungsten oxide with a nanorod crown appears (Figs.3(c),3(d), 3(g), and 3(h)).Additionally, the diameters of spherical tungsten oxide and nanorod increase with the increasing concentration of citric acid (Figs.3(c)-3(d))because of the decrease in the reaction rate.
To investigate the growth process of the spherical tungsten oxide, we conducted experiments with different reaction durations.Initially, after 15 min of reaction,there are spherical tungsten oxide particles which have the mean diameter of 1.82±0.4 μm and are constructed by circle plates (Fig.4(a)).The mean thickness of the plates is 24 ± 6 nm.Subsequently, the tungsten oxide deposits on the spherical tungsten particles and lead to the increase in diameter and fills up the gaps between the plates (Figs.4(b)-4(c)).The nanorods begin to grow from the surface of the spherical tungsten particles after 45 min (Fig.4(d)).And the diameters of nanorod and spherical tungsten oxide particles continue growing (Figs.4(e)-4(f)).Based on the above results, the formation process of spherical tungsten oxide can be explained as follows: dinuclear tungstocitrate complexes with negative charges tend to adsorb on the surface of spherical micelle of TEAB with positive charges through the electrostatic interaction.The micelle act as templates and lead to the formation of the spherical tungsten oxide particles.The nanoplates, construction units of the spherical tungsten oxide, result from the directive effects of citric acid on the morphology.It was demonstrated that the nanorods grew on the nanoplates because of the increase in concentration of citric acid with the decomposition of dinuclear tungstocitrate complexes[31].Owing to the space confinement between the nanoplates on spherical tungsten oxide, the nanorods grow from the edges of the nanoplates and continue to grow until the exhaustion of tungstocitrate complexes.
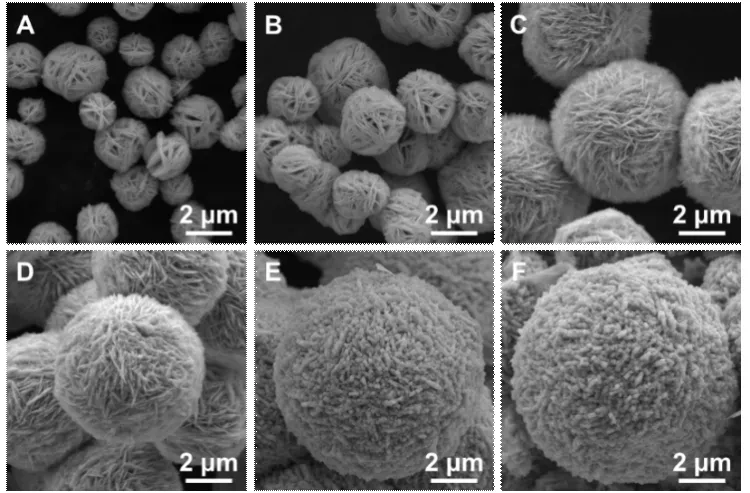
Fig.4 SEM images of tungsten oxide taken at different durations under 170 ℃: (a) 15 min, (b) 30 min, (c) 45 min, (d) 60 min,(e) 120 min, and (f) 480 min
3.2 Calcining of spherical tungsten oxide
The X-ray powder diffraction data of the spherical tungsten oxide particles were collected at room temperature at the scan speed of 5 °/min.As shown in the bottom picture of Fig.5, the characteristic peaks at 14.1°, 18.1°, 23.1°, 27.1°, 28.1°, 36.6°, 49.8°, and 55.8° can be indexed to the orthorhombic tungsten oxide hydrate (WO3•0.33H2O).The rest characteristic peaks at 23.1°, 23.6°, 24.3°, 26.6°, 33.5°, 34.1°,41.9°, 44.5°, 47.1°, 48.3°, and 53.6° correspond to the peaks of monoclinic tungsten trioxide (WO3).In order to reduce the effect of water in the reduction process,we calcined the spherical tungsten oxide with mixed phases.The WO3•0.33H2O phase completely transforms to monoclinic WO3phase after calcination for 1 h at 450 ℃.The WO3microparticles maintain the spherical structure of the tungsten oxide with mixed phases (Figs.6(a)-6(b)).The nanorods on the surface of the tungsten oxide change to nanoparticles which connect with each other because of dehydration (Figs.6(d)-6(d)).
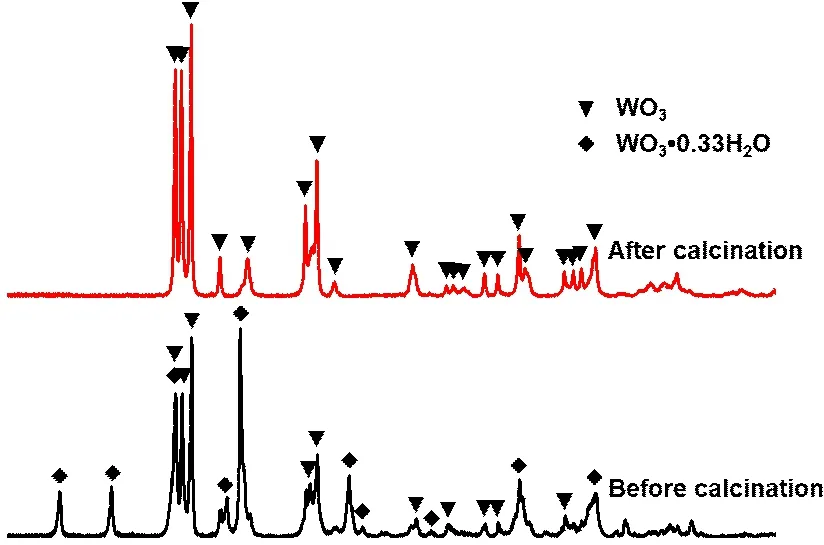
Fig.5 XRD patterns of spherical tungsten oxide before and after being calcined at 450 ℃

Fig.6 SEM images of spherical tungsten oxide (a, c) before and (b, d)after being calcined
3.3 Reduction of tungsten oxide
The reductions of tungsten oxide at different temperatures were carried out in H2gas atmosphere at the flow rate of 400 mL/min and their phase components after reduction are analyzed by XRD (Fig.7).There are characteristic peaks of tungsten dioxide (WO2),α-tungsten (α-W), andβ-tungsten (β-W) after being treated at 550 ℃.The tungsten oxide particles are completely reduced to tungsten metals that consist of α-W andβ-W phases when temperature was raised up to 600 ℃ and those that consist of onlyα-W phase at the temperature of 650 ℃.The similar results have been observed by other researchers[32].The crystallite sizes are determined using the Scherrer’s equation:D= 0.9λ/βcosθ, whereDis the crystallite size (nm), λ the wavelength of the X-ray radiation,θthe Bragg’s angle, andβthe full width at half maximum (FWHM) of the peak at 2θ.The crystallite sizes of spherical tungsten powder treated at 600 and 650 ℃ are 27.8 and 36.7 nm, respectively.
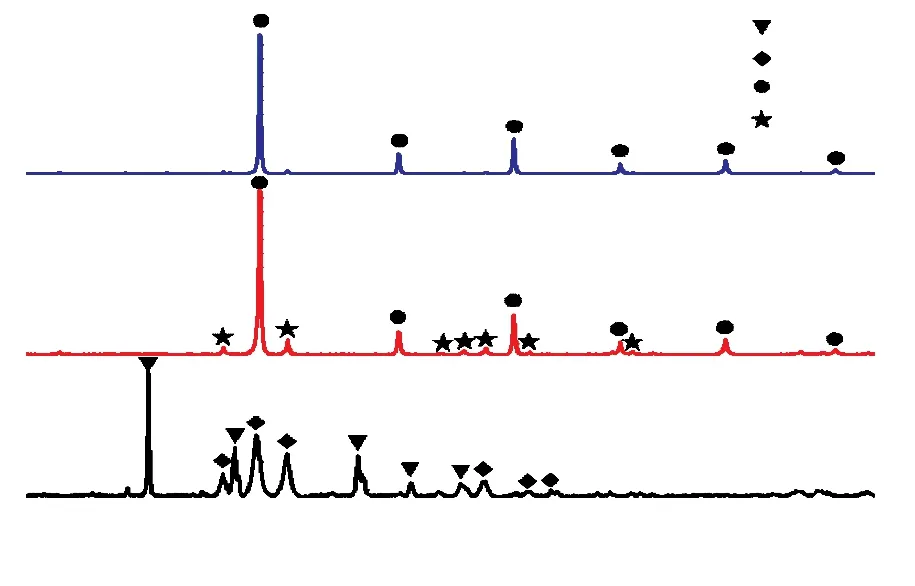
Fig.7 XRD patterns of the powders reduced by H2 gas at different temperatures
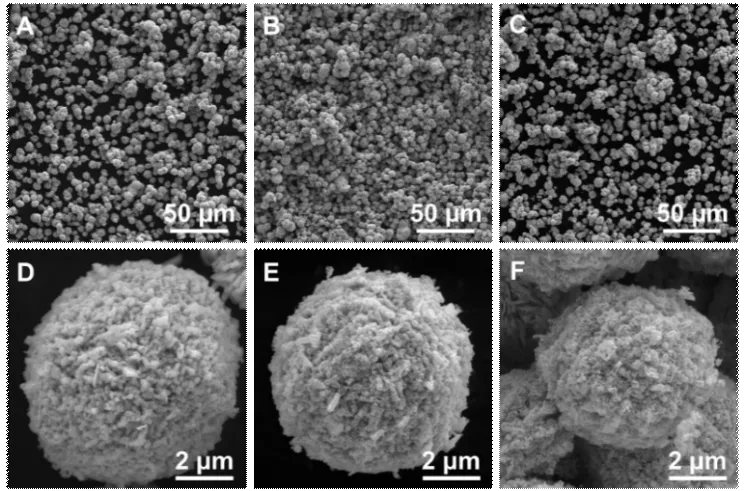
Fig.8 SEM images of the powders reduced by H2 gas at different temperatures: (a, d) 550℃, (b, e) 600 ℃, and (c, f) 650 ℃
The particles treated at 550-650 ℃ in dynamic H2gas maintain the spherical structure of the WO3particles and are assembled from sub-particles (Figs.8(a)-8(c)).The morphology of the sub-particles treated at 550℃ (Fig.8(d)) is similar to that of the ones after calcination at 450 ℃ (Fig.6(d)) because of the insuffi-cient reduction of tungsten oxide.By comparison, the sub-particles are porous after complete reduction at 600℃ owing to the release of H2O molecules generated by chemical reaction between H2and O atoms of WO3(Fig.8(e)).The porous structure of spherical tungsten metals obtained by reduction at 650 ℃ is more obvious than that of other samples because of the fast generation of H2O and stronger mobility of tungsten atoms(Fig.8(f)).
4 Conclusions
In summary, we have developed a method to synthesize the spherical tungsten oxide microparticles with nanorod crown through hydrothermal method in the presence of tetraethyl ammonium bromide (TEAB)and citric acid (CA).The spherical micelle of TEAB act as templates for the growth of spherical tungsten oxide because of the electrostatic interaction between the micelle with positive charges and tungstate anions.As capping agent to control the hydrolysis of tungstate ions, CA plays an important role in the formation of spherical structure.As CA concentration changes in the reaction process, nanoplates forms first and nanorods grow from the edges of nanoplates because of the space confinement of the spherical tungsten oxide particles.The spherical tungsten particles with porous structure can be obtained after the reduction of the spherical WO3particles at 600 and 650 ℃.
Generally, the plasma spheroidization process requires ultra-high temperature (>10 000 ℃), ultrapure argon gas and tungsten powder.These mean the needs of high energy consumption, high-cost equipment and expensive raw materials.In contrast, the hydrothermal assisted method in this work, can prepare spherical tungsten particles at low temperature and cheaper raw materials with cheaper equipment.Hence the spherical tungsten powder preparation assisted by hydrothermal method is a low-cost and short process.We believe that the low-cost spherical tungsten powders have great application potential in the fields of thermal spraying,metal powder injection molding (MIM), and 3D printing.
Conflict of interest
All authors declare that there are no competing interests.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Enhanced Electrochemical Performances of Ni Doped Cr8O21 Cathode Materials for Lithium-ion Batteries
- Design on the Prestressed Concrete Frame Beam-column
- Synthesis and Flocculation of Polyacrylamide with Low Water Absorption for Non-dispersible Underwater Concrete
- Experimental Behavior of Recycled Aggregate Concrete Filled Steel Tubular Columns
- Impact-abrasive Wear Behavior of ZTA and NbC Reinforced Fe60 Matrix Composites
- Synthesis and Characterization of Hollow Strontium Carbonate Pompons by Composite Soft Template Method
