Hot Corrosion Resistance of TB8 Titanium Alloy after ECAP and Heat Treatment
2024-01-03LIShuaidiXUXiaojingBAIXiangCAOBin
LI Shuaidi, XU Xiaojing, BAI Xiang, CAO Bin
(Institute of Advanced Manufacturing and Modern Equipment Technology, Jiangsu University, Zhenjiang 212013, China)
Abstract: The metastable β titanium alloy TB8 (Ti-12.76Mo-2.13Nb-2.73A1-0.16Si) was used as the original material, and the secondary processing method combining equal channel angular pressing (ECAP)and heat treatment was adopted.With the help of optical microscope (OM), scanning electron microscope(SEM) and X-ray diffractometer (XRD), the corrosion behavior of TB8 titanium alloy after different secondary processing (800 ℃/850 ℃ solid solution-520℃ aging, ECAP-800 ℃/850 ℃ solid solution-520 ℃ aging, and 800 ℃/850 ℃ solid solution-ECAP-520 ℃ aging) was studied.The experimental results show that the hot corrosion products of the six samples are similar, mainly Na2Si2O5, MoS2, TiCl2, Ti(SO4)2, and TiS.Due to the grains of the TB8 titanium alloy treated by 850 ℃ solid solution-ECAP-520 ℃ aging are obviously refined,the surface structure is the most smooth and dense, forming a continuous Al2O3 protective film, and the surface defects are the least after corrosion.Its corrosion layer thickness is the lowest (102.3 μm), only 36.5%-81.4% of that of other secondary processing titanium alloys.In addition, the corrosion kinetics curves of the six materials all follow parabolic laws, and the minimum corrosion weight gain of the samples after 850 ℃ solution-ECAP-520 ℃ aging treatment is 0.7507 mg·mm-2, showing better hot corrosion resistance.
Key words: TB8; ECAP; heat treatment; hot corrosion resistance
1 Introduction
Titanium alloys are widely used in aerospace,automotive, military industry and other fields due to their high strength, low density, oxidation resistance,corrosion resistance and other good comprehensive properties[1-4].However, due to the presence of substances such as Na2SO4and NaCl in the atmosphere,they will react with titanium alloy at high temperature environments, which greatly reduces the performance of the materials[5-7].Metastableβtitanium alloys can meet high standards in mechanical properties and maintain relatively good performance at high temperatures.Compared with otherβtitanium alloys, their cost is relatively low, and they have great potential as a substitute for super-strength steel materials[8,9].Therefore, the metastableβtitanium alloy TB8 (Ti-12.76Mo-2.13Nb-2.73Al-0.16Si) was used as the raw material in this study.
Studies have shown that the subsequent secondary processing of the metastableβtitanium alloy,such as strong plastic deformation, heat treatment, can effectively improve the comprehensive properties of the original titanium alloy, and enable it to work stably as a structural part under complex conditions of high temperature and high pressure[10,11].Chuvil'deevet al[12]studied the effect of severe plastic deformation caused by rotary forging on the corrosion resistance of Ti-2.5Al-2.6Zr titanium alloy, and found that the fine grain structure produced by forging can effectively increase the resistance of titanium alloy to electrochemical corrosion.Chuvil'deevet al[13]studied the effect of equal channel angular pressing (ECAP) on the microstructure, mechanical properties and corrosion resistance of Ti-5Al-2V titanium alloy.It is found that ECAP technique can help to form ultrafine crystal structure in titanium alloy Ti-5Al-2V.Superfine grained alloys have high thermal stability, which can ensure good corrosion resistance while improving strength and ductility.Compared with traditional metal forming processes (extrusion, forging, rolling,etc.), ECAP has many important process advantages, which can produce larger and uniform Shear deformation at relatively low pressure and load, and can refine the grains, effectively improve the high temperature resistance of the materials[14-16].In addition, studies have found that the formation of dense oxide protective film on the surface of the titanium alloy can effectively improve its corrosion performance, and heat treatment is helpful to the corrosion resistance of titanium alloy samples[17-19].Ettefaghet al[20]studied the effect of heat treatment after annealing on the corrosion resistance of additive manufacturing parts by comparing post-annealing heat treatment and cold-rolled titanium alloy samples.It is found that the presence of non-equilibrium phase in Ti-6Al-4V alloy microstructure can be effectively improved by the appropriate post-heat treatment process of 800 ℃-2 h,thus improving the hot corrosion resistance of the alloy.Therefore, the experiment adopts the secondary processing technology combining plastic deformation and heat treatment.
At present, research on the high-temperature corrosion resistance of titanium alloys has been explored, but the research scope is relatively narrow,and there is not much research on the high-temperature corrosion resistance ofβtitanium alloys.Based on the above research situation, this paper takes TB8 titanium alloy as the research object to study the high-temperature corrosion behavior of TB8 titanium alloy after solution-aging, ECAP-solution-aging and solution-ECAP-aging treatments.In this experiment,the cyclic corrosion experiment method was used to conduct hot corrosion treatment at 850 ℃ for TB8 titanium alloy whose microstructure and properties were regulated after secondary treatment under different conditions.In addition, the corrosion resistance of TB8 titanium alloy at high temperature and its hot corrosion mechanism were studied by analyzing the products after hot corrosion, observing the surface and section morphology after hot corrosion and drawing the corrosion kinetics curve.
2 Experimental
2.1 Materials and equipments
The forged TB8 titanium alloy (Ti-12.76Mo-2.13Nb-2.73Al-0.16Si) prepared by the Northwest Institute for Non-Ferrous Metal Research was used as the raw materials.Hot corrosion behavior of TB8 titanium alloy was studied after solution-aging, ECAP-solution-aging and solution-ECAP-aging.The main instruments and equipment used in the experiment are shown in Table 1.

Table 1 Experimental equipments
2.2 ECAP and heat treatment

Fig.1 ECAP die: (a) The 2D parameter drawing; (b) Actual photo
In this experiment, a single pass of ECAP was used for plastic deformation processing, a 100 T vertical hydraulic press (YB32-100) was used to provide pressure for the ECAP processing of titanium alloys.The ECAP mold used is shown in Fig.1, where (a) and(b) are the two-dimensional parameter diagram and the physical diagram of the die, respectively.Before extrusion, the material was cut into a cylinder with a diameter of 17 mm by wire cutting.In order to reduce the friction coefficient between the titanium alloy and the die, the titanium alloy samples were wrapped in red copper with an outer diameter of 20 mm and an inner diameter of 17 mm.Before ECAP deformation processing, in order to make the alloy microstructure distribution uniform and obtain better machinability,the samples wrapped in copper sleeve were pretreated at 400 ℃ × 2 h.In order to prevent the samples from cooling down sharply during the extrusion process, a heating plate was used to heat the ECAP mold for 5 h to keep the mold temperature near 300 ℃.After the extrusion was completed, the mold was removed for cleaning.In addition, the box-type resistance furnace(SX2-4-10) was used to preheat the TB8 titanium alloy at 400 ℃ × 2 h, solid solution at 800 ℃/850 ℃ × 4 h and aging treatment at 520 ℃ × 10 h.
2.3 Hot corrosion procedure
Firstly, the samples after ECAP and heat treatment were cut into 7 mm × 7 mm × 5 mm by wire cutting.Then silicon carbide sandpapers of 100#, 400#, 800#,1000#, 1500# and water abrasive sandpapers of 2000#,2500# were used for grinding and polishing.Before the experiment, samples will be cleaned in an ultrasonic cleaner (KQ3200DE) for 30 minutes to obtain a clean surface.The hot corrosion resistance test was carried out in the box-type resistance furnace (KF1400), with corrosion temperature at 850℃ for 40 h, and the mixed solution of 75% NaCl + 25% Na2SO4(wt%) was used as the corrosive medium.The samples were placed on the heated iron plate, and the corrosion solution was coated on the surface of the samples until the salt film with a concentration of 3 ± 0.5 mg/cm2was generated.Then they were weighed together with the crucible, and the initial weight was recorded as m0.Finally, the furnace temperature was heated to 850 ℃, and the crucible containing the sample was put into the heating furnace,and started timing.During the corrosion process, the samples were taken out at fixed time points (1, 3, 5, 7,10, 15, 20, 25, 30, 35, and 40 h).After air cooling, the crucible containing the samples was measured on an optical balance (Ohaus Discovery) with an accuracy of 0.01 mg by weight increasing method, and the weight was recorded asmi.After weighing, put the crucible with the sample back into the furnace for the next cycle and repeat the above experiment until the end of the test.In the experiment, boiling water and anhydrous ethanol were used for ultrasonic cleaning of the samples after corrosion, so as to prevent the residual corrosion salts on the surface from affecting the subsequent observation.The mass increment of the sample is calculated by the formula ∆m=mi–m0and the corrosion kinetics curve is drawn.Then the corresponding analysis equipment is used to analyze the phase composition and microstructure morphology of the samples.In order to ensure the accuracy and reliability of the experiment,three parallel samples were used in each group.
2.4 Phase and microstructure analysis
In this experiment, a 4XC-MS optical microscope(OM) was used for the metallographic analysis of the samples.The cut samples were corroded for 5 s with Kroll corrosion solution (4.2% HF + 12.5% HNO3+83.3% H2O (vol%)) before the microstructure observation.Then the samples were cleaned and blow-dried for metallographic observation.The surface phase composition of the samples was analyzed by XRD (Model:D8-ADVANCE) with Cu-Ka radiation in range of 2θ= 30°-120° and a scanning speed of 5 °/min.Then the MDI-jade software was used to analyze the obtained data, and the diffraction peak in XRD was compared with PDF card in the software, so as to obtain the surface phase composition.In addition, a scanning electron microscope (SEM) (JSM-IT300) equipped with an energy dispersive spectroscopy (EDS) was mainly used to analyze the surface, cross-sectional structure and related composition of the titanium alloy.
3 Results and discussion
3.1 Metallographic structure analysis
The surface metallographic structure of TB8 titanium alloy after different secondary treatments is shown in Fig.2.After 800 ℃/850 ℃ solid solution-520℃ aging treatment, secondary α phase (αs) precipitates on the surface of titanium alloy.The surface morphology in Fig.2(b) is branch-like distribution, and there are black areas on the surface that are not recrystallized.The titanium alloy without ECAP treatment has larger crystal grains and lower surface densification.Titanium alloy will be broken and extruded after ECAP, but after solution treatment, the grains grow into large equiaxed shapes, and part of the grain boundaries disappear due to growth factors, as shown in Fig.2(c).In Fig.2(d),after the titanium alloy is treated at ECAP-850 ℃ solid solution-520 ℃ aging, there are still obvious broken grains and new grains that nucleate and grow during the extrusion process.The crystal grains are smaller and more numerous, and the surface density is higher.As can be seen from Figs.2(e, f), under the condition of 800 ℃/850 ℃ solid solution-ECAP-520 ℃ aging, the precipitation ofαsphase of titanium alloy can effectively inhibit the growth ofβphase, and ensure the stability of grain shape and size after solid solution-ECAP,which promotes the grain refinement of titanium alloy.In general, the surface grain boundaries of 800 ℃ solid solution titanium alloy are more obvious than those of 850 ℃ solid solution titanium alloy.However, the combination between titanium alloy grains with solution at 850 ℃ is better, and the grain boundary is not very obvious, indicating that the higher the solution temperature is, the more energy released after EACP-aging treatment, resulting in better adhesion between grains and higher surface density.The dense surface helps to improve its hot corrosion resistance.

Fig.2 OM images of TB8 titanium alloy after different secondary treatments: (a) 800 ℃ solution-520 ℃ aging; (b) 850 ℃solution-520 ℃ aging; (c) ECAP-800 ℃ solution-520 ℃aging; (d) ECAP-850 ℃ solution-520℃ aging; (e) 800℃solution-ECAP-520 ℃ aging; (f) 850 ℃ solution-ECAP-520℃ aging
3.2 Phase compositions
Fig.3 shows the surface XRD patterns of the secondary processing titanium alloy corroded at 850 ℃ for 40 h.From the corrosion products, it can be seen that in the high temperature corrosive medium at 850 ℃, not only corrosion reaction, but also oxidation reaction occurs, so the corrosion layer on the surface of the material is a composite layer composed of corrosion products and oxidation products.The oxidation products of the corrosion layer in the samples are mainly rutile TiO2(ICDD PDF 46-1238), Al2O3(ICDD PDF 35-0121),SiO2(ICDD PDF 47-1144), and Nb2O5(ICDD PDF 19-0862).As the corrosive medium contains elements such as Na, S and Cl, at high temperature, Na2Si2O5(ICDD PDF 29-1261), MoS2(ICDD PDF 17-0744), TiS (ICDD PDF 09-0289), TiCl2(ICDD PDF 10-0315) and Ti(-SO4)2(ICDD PDF 18-1604) and other compounds were generated.There is no Ti(SO4)2phase in 800 ℃/850 ℃solution-520 ℃ aging, mainly because Ti(SO4)2phase is covered by a large number of oxides generated on the surface, and Ti(SO4)2phase is decomposed at high temperature.

Fig.3 XRD patterns of titanium alloy by secondary processing after corrosion at 850 ℃ for 40 h
3.3 Surface morphology
Fig.4 shows the surface morphology of the TB8 titanium alloy after different secondary processing after being corroded at a high temperature of 850 ℃ for 40 h.As can be seen from Fig.4(a), after the titanium alloy treated with 800 ℃ solution-520 ℃ aging, there are many defects in the surface morphology, such as cracks, spalling and pores.These defects provide channels for corrosive elements and oxygen elements to enter the alloy, making the corrosion layer on the surface unable to effectively adhere to the substrate surface.In Fig.4(a-1), it can be clearly seen that the sample surface has obvious warpage, which cannot effectively protect the titanium alloy, resulting in more serious surface morphology corrosion.The surface morphology of Fig.4(b) does not have large areas of peeling, but there are defects such as bumps, cracks and pores.In the partial enlarged view of Fig.4(b-1), there are granular and lamellar morphological structures.Combined with EDS analysis, it can be seen that the granular form is mainly TiO2and the lamellar form is mainly Al2O3,and the area covered by granular is located in the depth of cracks and the surface is relatively loose, while the lamellar shape is mainly located on the denser surface,which prevents the corrosion atoms from entering the interior of the alloy.Therefore, Na and Cl elements can be detected on the surface.According to the XRD phase analysis of the sample surface, they are Na2Si2O5and TiCl2.

Fig.4 SEM surface morphologies of TB8 alloy by different secondary treatment after corrosion at 850 ℃ for 40 h: (a):800 ℃ solution-520 ℃ aging; (b): 850 ℃ solution-520℃aging; (c): ECAP- 800 ℃ solution-520 ℃ aging; (d): ECAP-850 ℃ solution-520 ℃ aging; (e): 800 ℃ solution-ECAP-520 ℃ aging; (f): 850 ℃ solution- ECAP-520 ℃ aging; (a-1),(b-1), (c-1), (d-1), (e-1), and (f-1) are the corresponding local enlarged graphs
Figs.4(c, d) show the surface morphology of the TB8 titanium alloy treated by ECAP-800 ℃/850 ℃solution-520 ℃ aging.Compared with the titanium alloy treated only by solid-solution-aging treatment, the defects of the surface corrosion morphology are significantly reduced.In Fig.4(c), there are a few bumps and cracks on the local surface.It can be seen from the magnified image of Fig.4(c-1) that the surface structure is mainly composed of needle-like, growing prisms and cylinders.Combined with EDS analysis, it can be seen that the growth of prismatic is mainly TiO2, the cylindrical structure is mainly a mixture of Al2O3and SiO2,and the needle-like structure is mainly Al2O3.Due to the dense covered needle-like area, the entry of corrosive elements is blocked, which leads to the detection of Na, S, Cl and other elements on the surface.According to XRD, it can be inferred that it contains several compounds such as Na2Si2O5, MoS2, TiCl2, Ti(SO4)2,and TiS.In Fig.4(d), there are no obvious cracks and only a small amount of bumps, and the surface morphology has a cylindrical and granular mixture mainly composed of TiO2and Al2O3, and a lamellar structure mainly composed of Al2O3.
Figs.4(e,f) show the titanium alloy after 800℃/850℃ solution-ECAP-520℃ aging treatment,its surface morphology is more flat than that of the previous four samples.As shown in Fig.4(e), no obvious defects were found on the surface of titanium alloy treated at 800℃ solution-ECAP-520℃ aging, but the corrosion layer on the surface was relatively loose and mainly composed of agglomerated, short and fine needles and prisms.In the corresponding point analysis Table 2, it can be seen that the three types of structures all contain Na and Cl elements, and it is inferred that there are Na2Si2O5and TiCl2compounds on the surface.The agglomerate and short needle-like composition of the composite products are dominated by Al2O3, while the prismatic composition is dominated by TiO2.The surface of Fig.4(f) has fewer cracks and a small amount of pores, but the overall structure is relatively dense.The structure in its partial enlarged view is evenly distributed.In addition, the components are mainly Al and O elements, indicating that there are more Al2O3on the surface, which can form protective films.
3.4 Analysis of corrosion layer cross-section structure
Fig.5 shows the cross-sectional morphology and its corresponding line scan diagram of secondary processed TB8 titanium alloy after hot corrosion.There are no obvious defects in the section morphology of the six samples, and the thickness of the corrosion layer reached 280.3, 190.5, 175.4, 135.6, 125.7, and102.3 μm, respectively.In general, the corrosion layer section of titanium alloy treated at 800 ℃/850 ℃solution-520 ℃ aging has many pits, while there are pores on the surface of the titanium alloy treated at 800℃ solution-520 ℃ aging, resulting in the formation of corrosion channels inside the material, which makes it impossible to effectively inhibit the penetration of corrosive atoms.After ECAP-800 ℃/850 ℃ solution-520℃ aging treatment, the corrosion layer of titanium alloy has a few pits, which are irregularly distributed.However, the corrosion layer of titanium alloy treated by 800 ℃/850 ℃ solution-ECAP-520 ℃ aging is relatively flat and compact, without pits and other defects,and has a good cross-section morphology.

Table 2 EDS analysis of typical surface microstructure of TB8 alloy by different secondary treatment after corrosion at 850 ℃ for 40 h
In the line scan of the cross-section, the change of the corrosion S element is more obvious, indicating that the S element has different degrees of infiltration in the six samples.The change trend of S element is positively correlated with the change trend of the Ti element and the Mo element, indicating that the S element mainly exists in the corrosion layer in the form of MoS2and TiS.In the line scan corresponding to Figs.5(e, f), the Al element has a large peak value on the surface of the corrosion layer, indicating that Al element is enriched near the surface, and the content of Al2O3increases, making the surface of the titanium alloy treated with 800 ℃/850 ℃ solution-ECAP-520 ℃aging can form an effective and continuous protective film, thereby improving the alloy's resistance to hot corrosion.
3.5 Corrosion kinetics curves
Fig.6 shows the corrosion kinetics curves of the TB8 titanium alloy after different secondary processing.It can be seen that the curves of the six samples are all parabolic.The corrosion weight gain of the titanium alloy treated with 800 ℃/850 ℃ solution-520 ℃ aging reached 1.2517 and 1.1069 mg·mm-2, respectively.The corrosion weight gains of titanium alloys after ECAP-800 ℃/850 ℃ solid-solution-520 ℃ aging and 800℃/850℃ solution-ECAP-520 ℃ aging are 0.9976,0.8975, 0.8682, and 0.7507 mg·mm-2, respectively.The corrosion weight gain is less than that of the titanium alloys without ECAP treatment, indicating that the ECAP treatment can improve the hot corrosion resistance of titanium alloys.The color of the macro illustration gradually changed from dark gray to bright yellow, and the corrosion layer showed different degrees of cracking and warping.The surface of the sample treated with 800 ℃ solution-520 ℃ aging showed a large area of shedding, then the surface defects of the subsequent samples gradually decreases, and the surface becomes more and more dense, indicating that the hot corrosion resistance is gradually enhanced.
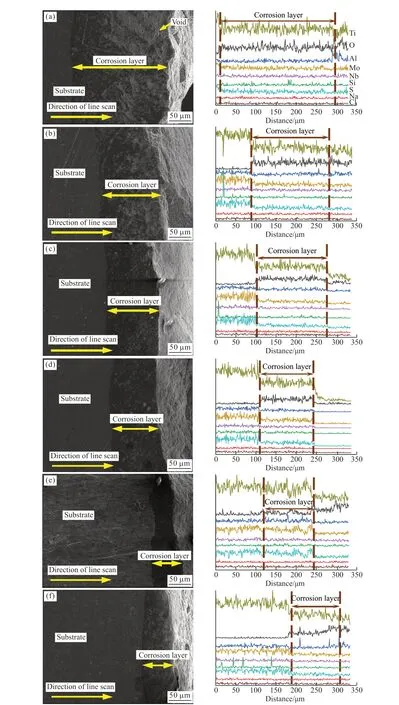
Fig.5 Cross-section morphologies and corresponding line scan of TB8 alloy by different secondary treatments after corrosion at 850 ℃ for 40 h: (a) 800 ℃ solution-520 ℃ aging; (b) 850 ℃ solution-520 ℃ aging; (c) ECAP-800 ℃ solution-520 ℃ aging; (d) ECAP-850 ℃solution-520 ℃ aging; (e) 800 ℃ solution-ECAP-520 ℃ aging; (f) 850 ℃ solution-ECAP-520 ℃ aging
In general, inflection points appear at 1, 25, and 35 h, respectively in the corrosion kinetics curves.Before 1 h, the new metal in contact with the corrosive molten salt reacts with a high corrosion rate, resulting in a large corrosion increment.From 1-25 h, the upward trend moderated slightly but the rising rate was still large, mainly because after the new metal on the surface reacted with corrosive elements, large warping cracks formed on the surface, which provided channels for the entry of corrosive elements.As the corrosion time increases, the corrosion rate decreases.When the corrosion time reaches 35 h, the thickness of the corrosion layer increases to the extent that the corrosion elements cannot effectively penetrate into the metal and react with the metal atoms, so that the corrosion rate is greatly reduced, resulting in an inflection point at 35 h,which indicates that the corrosion process has entered a stable stage, until 40 h, the reaction between the metal element and the corroded atom is basically complete.Among them, the corrosion increase of ECAP-850 ℃solution-520 ℃ aging before 10 h is the smallest, indicating that the corrosion resistance is excellent in the initial stage of corrosion, but with the extension of the corrosion time, the corrosion rate is still maintained to 25 h, without a downward trend, resulting in a higher corrosion increase than the samples after solution-ECAP-aging.The titanium alloy treated with 850 ℃ solution-ECAP-520 ℃ aging maintained a low oxide increment, followed the parabolic rule for most of the corrosion time, and showed better hot resistance corrosion.
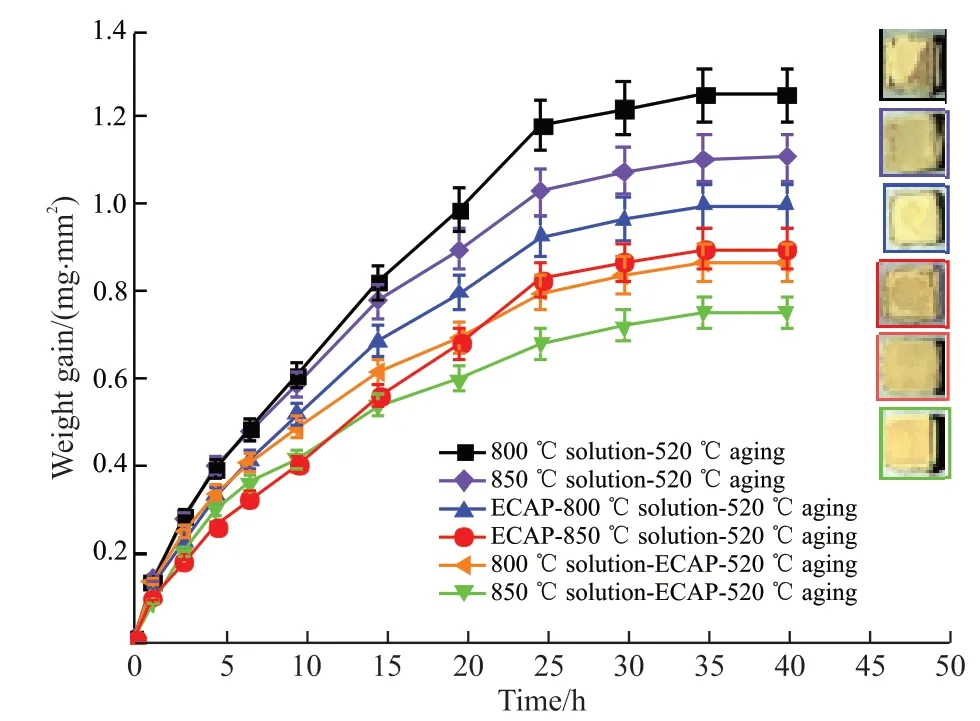
Fig.6 Corrosion kinetics curve and macroscopic surface illustration of TB8 alloy after corrosion by secondary processing at 850℃ for 40 h
3.6 Hot corrosion mechanism
In the process of corrosion, along with the process of accelerated oxidation, the oxidation products produced are similar to those of the oxidation experiment at the same temperature, mainly including TiO2,Al2O3, SiO2, and Nb2O5, but the corrosion products of Na2Si2O5, MoS2, TiCl2, Ti(SO4)2, and TiS are produced.The melting point of Na2SO4is 884℃, with the addition of NaCl, the melting point of the mixed salt can reach about 650℃.In this experiment, the mixed molten salt is coated on the surface of the alloy at 850℃,so that it can contact the surface of titanium alloy, at the same time the following reactions occur:
Titanium alloy reacts with Cl element decomposed by NaCl to generate volatile oxychloride.As the content of generated oxychloride increases, the gas inside the corrosion layer will accumulate and increase the pressure, resulting in the formation of shedding,pores and cracks.The titanium alloy discharges oxychlorides, and also provides an infiltration path for corroded atoms.The Ti in the titanium alloy reacts with the generated chlorine gas to generate volatile chlorides and loose TiO2, which reduces the density of the titanium alloy surface.
In the process of hot corrosion, the concentration of SO3and Na2O decomposed by sulfate at high temperature and the partial pressure of surface oxygen have great influence on the corrosion layer.During the corrosion process, when oxide is continuously generated on the surface covered with sulfate, the partial pressure of Na2SO4directly attached to the corrosion layer decreases, and the partial pressure of SO3generated by decomposition increases continuously.In the process of maintaining a relative balance, the following reaction will occur in SO3,
When S element passes through the corrosion layer and reacts with the metal elements inside the alloy to form sulfides, the original element composition inside the alloy will be destroyed, resulting in accelerated corrosion.It can be seen from the line scan diagram that the curves of S element in the corrosion layer have obvious changes, indicating that the S element penetrates into the alloy to different degrees during the corrosion process.
With the consumption of a large amount of S element in sulfate, the content of Na2O will continue to increase, and promote the activity of O2-ions, so that the oxides in the corrosion layer can be alkaline dissolved in the molten salt, thus reducing the surface density.A large number of corrosive atoms continuously enter the surface of the alloy through these gaps and holes, increasing the corrosion rate, forming the surface morphology of titanium alloy treated by 800 ℃/850℃ solution-520 ℃ aging.Meanwhile, the generated Si2O52-reacts with Na+ions in the molten salt to form Na2Si2O5.
4 Conclusions
a) The corrosion layer of the six samples is mainly composed of oxidation and corrosion products, among which the oxidation products are mainly TiO2, Al2O3,SiO2, and Nb2O5, while the corrosion products are mainly Na2Si2O5, MoS2, TiCl2, Ti(SO4)2, and TiS.The surface of the titanium alloy treated with 800 ℃/850℃ solution-ECAP-520 ℃ aging has no obvious defects and is flatter.Among them, the surface of the titanium alloy treated by 850 ℃ solution-ECAP-520 ℃ aging is the densest.
b) The corrosion layer thickness of the six materials is 280.3, 190.5, 175.4, 135.6, 125.7, and 102.3 μm, respectively.It can be seen from the line scan that the peak value of Al element appears near the surface of the corrosion layer, indicating that the Al content on the surface increases and the Al element is enriched,resulting in the formation of protective Al2O3film on the surface, which effectively inhibits the infiltration of corrosive atoms and improves the hot corrosion resistance of the titanium alloy treated by 850 ℃ solution-ECAP-520 ℃ aging.
c) The corrosion kinetic curves of the six materials all follow the parabolic law.The corrosion weight gains were 1.2517, 1.1069, 0.9976, 0.8975, 0.8682,and 0.7507 mg·mm-2, respectively, of which 850 ℃solution-ECAP-520 ℃ aging has the smallest corrosion weight gain, showing better hot corrosion resistance.
Conflict of interest
All authors declare that there are no competing interests.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Enhanced Electrochemical Performances of Ni Doped Cr8O21 Cathode Materials for Lithium-ion Batteries
- Design on the Prestressed Concrete Frame Beam-column
- Synthesis and Flocculation of Polyacrylamide with Low Water Absorption for Non-dispersible Underwater Concrete
- Experimental Behavior of Recycled Aggregate Concrete Filled Steel Tubular Columns
- Impact-abrasive Wear Behavior of ZTA and NbC Reinforced Fe60 Matrix Composites
- Synthesis and Characterization of Hollow Strontium Carbonate Pompons by Composite Soft Template Method
