Preparation of Co/CoOx Derived from a Lowtemperature Etching of ZIF-67 for Oxygen Reduction and Oxygen Evolution Catalytic Reaction
2024-01-03TANShifengTUWenmaoPANHongfeiZHANGHaining
TAN Shifeng, TU Wenmao,2*, PAN Hongfei,3* , ZHANG Haining,3
(1. State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan 430070,China; 2. Hubei Key Laboratory of Fuel Cells, Wuhan University of Technology, Wuhan 430070, China; 3. Foshan Xianhu Laboratory of the Advanced Energy Science and Technology Guangdong Laboratory, Foshan 528200, China)
Abstract: Catalysts consisting of Zeolite imidazolyl ester skeleton-67 (ZIF-67) and graphene oxide(GO) were fabricated through a solvothermal method, followed by etching ZIF-67 with oxygen-rich functional groups on GO in a reduction atmosphere at 400 ℃.During this process, an open type of cobalt metal center was formed by the partial vaporization and oxidation of ZIF-67, further reducing to Co and partially combining with oxygen species to amorphous CoOx.Benefiting from the rich functional N, and metal/oxides active centers derived from the calcination process, the synthesized Co/CoOx@NSG-400 showed a low OER overpotential of 10 mA·cm-2 at 298 mV, and an ORR half-wave potential of 0.8 V, which demonstrated its excellent bifunctional catalytic activity.Such a controllable calcination strategy with high yields could be expected to pave the way for synthesizing low-cost and efficient bifunctional electrocatalysts.
Key words: oxygen evolution reaction; oxygen reduction reaction; bifunctional electrocatalyst; ZIF-67
1 Introduction
As the intensification of global crisis originated from the shortage of energy and pollution of traditional fossil energy[1], clean, high-performance energy storage and conversion devices matched with new energy sources, such as fuel cells[2]and metal-air batteries[3],have received widespread attention.However, the adverse kinetics of electrode reaction and the resulting large overpotential during the operation of these energy conversion devices still challenges their largescale applications.Developing cost-effective oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) electrocatalysts with high performance is a practical approach to address the above issues[4].Heretofore, Pt-based precious metals are still recognized as the most effective electrocatalysts for ORR[5,6], while Ir-based and Ru-based precious metals are most appropriately applied in OER[7,8].However,high cost, scarcity, single activity, and poor stability limit the practical application of these platinum group precious metals (PGM)[9].Fortunately, some nonnoble metal catalysts have been developed, which is the potential to supersede PGM catalysts due to their low cost, satisfactory stability, and large reserves.It has been widely authenticated that heteroatom (such as N, S, P) doped porous carbon and its transitionmetal oxide graft derivatives show ideal OER/ORR catalytic activities[10,11].For example, N-Doped Graphitic nanofiber/Co3O4nanoparticle composites were reported to possess a low half-wave potential of 0.8 V with excellent stability[12].Enhanced chemical bonding between metal-containing materials and heteroatom-doped carbon carriers has been reported to greatly facilitate interfacial electron transfer in hybrid electrocatalysts[13,14].
Zeolite imidazole skeleton-67 (ZIF-67) is connected by the coordination bonds of Co2+metal ions and imidazole ligand (C4H6N2), characterized by long-range order and high crystallinity[15].Due to the high specific surface area, adjustable pore size and topological structure, diverse metal nodes, chemical stability and surface function, ZIF-based materials are considered ideal precursor candidates for ORR/OER[16].The general treatment method is high temperature annealing (≥ 600 ℃).However, the Co@N-C structure derived in such a method is unsatisfactory because hightemperature annealing could cause a sharp decrease in porosity, low yield (generally less than 30%) and over-reduction of active metal centers.In addition, the samples derived from the high-temperature annealing of ZIF in a simple inert atmosphere typically have poor OER properties.Hence, a second oxidation step is usually necessary for synthesizing carbon-supported metal/metal oxides with better bifunctional activity[16],which is complicated and uncontrollable.To address such issues with a simple method, dispersing metal/metal oxides on conductive carbon nanostructures such as graphene oxide (GO) could be an effective way to enhance the transport of reactants and products and the conductivity of catalysts[17,18].For example, Zhang et al.synthesized Co/Co3O4@carbon fiber by a twostep annealing method.The obtained catalyst exhibits excellent ORR and water-splitting performance[19].Another example introduced the construction of Co/Co3O4with N-doped carbon by a template method[20].It demonstrated a high half-wave potential of 0.84 V, and an overpotential of 390 mV at 10 mA·cm-2.However,their catalysts suffered from massive loss of precursors and aggregation of metal oxides.
In this work, we reported a series of carbonsupported amorphous cobalt-based metal oxide composites formed by controlled temperature (300-500℃) etching of ZIF-67/GO.Furthermore, the sulfides generated by the slight decomposition of DMSO during the solvothermal process were uniformly incorporated into the catalyst material.The nitrogen-rich functional groups on the surface came from ZIF materials.Based on retaining most of the pore structure of the precursor material, the synthesized Co/CoOx@NSG-400 with a yield rate over 60% showed good bifunctional catalytic activity for OER/ORR.
2 Experimental
2.1 Materials
Graphite powder was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd., (≥99.99%).Sulfuric acid and phosphoric acid were obtained from Sinopharm Chemical Reagent Co., Ltd(AR, ≥ 98%).2-methylimidazole and cobalt nitrate tetrahydrate were purchased from Aladdin Industries(AR, ≥ 98%).2 Methyl sulfoxide (DMSO) was obtained from Shanghai Macklin Biochemical Co.,Ltd, (AR, 99%).Pt/C catalyst (20wt%, Pt).and RuO2(98%, metal basis) were acquired from Shanxi Kaida Chemical Co., Ltd.Deionized water (18.25 MΩ·cm-1)was self-made.
2.2 Synthesis of ZIF-67/GO
3 g graphite powder and 1.5 g NaNO3were added to the mixed acid solution (80 ml concentrated sulfuric acid and 20 mL phosphoric acid) in the flask.Control the temperature of the system at about 4 ℃, then added 9 g KMnO4,and stirred for 2 h to make the mixture react fully.Then raise the temperature to 35 ℃ for an hour, then raise the temperature of the system to 95 ℃,after 20 minutes of reaction, filtered the mixed solution while it was hot, and finally wash repeatedly with deionized water until pH is close to 7, and then freezedried.
90 mg GO and 8 mmol 2-methylimidazole were added to 60 mL DMSO solution and dispersed by ultrasonic for 2 hours.Cobalt acetate tetrahydrate of 4 mmol was dissolved in 10 mL DMSO solution,absorbed by an eyedropper, and dropped into the mixed solution.The reaction temperature was 140 ℃ and the reaction time was 24 hours.After centrifugation, the mixture was washed many times with the mixture of ethanol and deionized water, and the sample was frozen quickly.The dried sample is named ZIF-67/GO.
2.3 Synthesis of samples annealed at different temperatures
The precursor ZIF-67/GO was placed in a porcelain boat and annealed in a mixed atmosphere (5%H2and 95% Ar) for 2 h at different temperatures (300,400, and 500 ℃), respectively.The obtained black powder was named ZIF-67/RGO-300, Co/CoOx@NSG-400, and Co/CoOx@NSG-500, respectively.
2.4 Characterization
The morphology of the samples was observed by scanning electron microscope (FE-SEM, Zeiss Ultra Plus, Zeiss, Germany).The crystal characteristics of the powder were analyzed by D8 advanced X-ray diffractometer.X-ray photoelectron spectroscopy (XPS)analysis was carried out on the ESCALAB250XiX X-ray photoelectron spectrometer (Thermo Fisher Science), and the excitation source was Al source.
2.5 Electrochemical measurement
The ink was prepared by mixing catalyst (5 mg), Nafion solution (10 μL, 5wt%), 250 μ L ethanol and 240 μL deionized water solution and dispersed uniformly by ultrasonic for at least 30 minutes.Then,the ink was dripped onto the glassy carbon electrode and the catalyst loading was controlled at 0.3 mg·cm-2.OER and ORR tests were carried out in a threeelectrode system, using CHI 660E electrochemical workstation (CHI instrument, Chenhua).ORR test used a Pt wire electrode as the counter electrode, Ag/AgCl electrode as the reference electrode, and the potential was corrected to reversible hydrogen potential (RHE)using the equation:
The LSV curves at different rotational speeds were tested, and the data were processed with the Koutecky-Levich equation to calculate the electron transfer number (n) of the catalyst.The corresponding K-L equation is as follows[21]:
where,JandJKare the measurements of current density and dynamic current density, respectively;ωthe rotational speed;nthe number of transferred electrons;Fthe Faraday constant (96485 C·mol-1);C0the saturated concentration of O2in the electrolyte(1.21·10-6cm-3);D0the diffusion coefficient of O2(1.9 C ·10-5cm-2s-1);μthe kinetic viscosity of the electrolyte (0.01 cm2·s-1);Kthe rate constant of electron transfer.
Similar to the ORR test, OER used graphite rods as counter electrodes, and Hg/HgO as reference electrodes, the following equation was used to convert the potential into RHE,
The electrochemical impedance test voltage is the corresponding potential at 10 mA·cm-2, the amplitude is 5 mV, the low-frequency region is 0.01 Hz, and the high-frequency region is 100 000 Hz.
3 Result and discussion
The synthesis process of ZIF-67/GO and its derivatives is schematically illustrated in Fig.1.Due to its temperature sensitivity, all calcination processes were chosen in a relatively low-temperature range (below 500 ℃).As shown in Fig.1, a simple solvothermal method achieved growth of ZIF-67 on GO, followed by a series of annealed processes.When the annealed temperature was 300 ℃, fewer oxygen species were released from GO, and ZIF maintains its integrity.When the annealing temperature increased,GO released more oxygen species, and ZIF was further etched to expose more metal active centers.The ZIF structure was maintained at this time.Some metal centers were combined with oxygen to form Co3O4, and some were reduced to Co.
Firstly, the crystal phases of the precursors and the corresponding annealed samples at different temperatures were studied by powder X-ray diffraction(PXRD).As the results are shown in Fig.2(a),the prepared ZIF-67/GO has prominent crystal characteristics and corresponds to the simulated ZIF-67.The sample annealed at 300 ℃ retains most of the skeleton and crystal structure of ZIF-67, similar to the diffraction pattern of ZIF-67/RGO-300.However, there is no obvious crystal form in the samples calcined at 400 to 500 ℃, and the PXRD data show a sizeable bulging peak, indicating that most of the products exist in amorphous form.It is worth noting that the sample annealed at 400 ℃ has a small peak at 44.2°,corresponding to the (111) crystal plane of Co.It can be inferred that with the further increase in temperature,more metal centers are exposed, and some are reduced to Co in a weak reduction atmosphere.
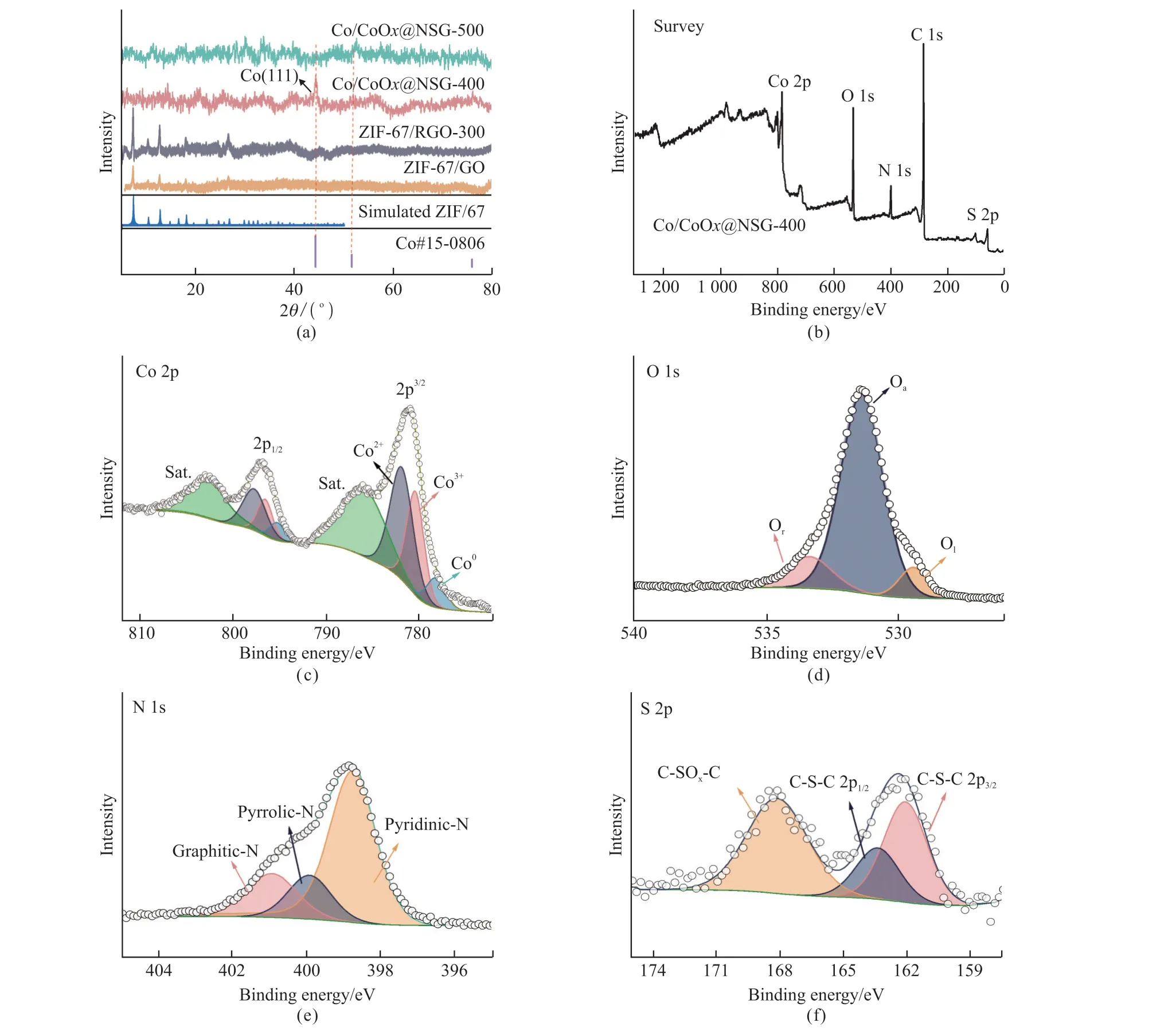
Fig.2 (a) PXRD patterns of samples calcined at different temperatures (350-500 ℃) and XPS analysis of Co/CoOx@NSG-400; (b) XPS survey; (c) high-resolution XPS spectra results of Co 2p; (d) O 1s, (e)N 1s, and (f) S 2p
The chemical properties and related atomic level information of these derivatives were analyzed by X-ray photoelectron spectroscopy.As displayed in Fig.2(b), XPS surveys show five major peaks at 284.8,400.2, 532.3, 781.2, and 163.2 eV, corresponding to C 1s, Co 2p, N 1s, O 1s, and S 2p, respectively.In addition, the Co 2p3/2spectrum can be decomposed into Co (778.1 eV), Co2+(781.6 eV), and Co3+(780.3 eV[22], which suggested that the central part of the metal cobalt is oxidized to a higher valence state, and some were reduced to Co.This is also supported by the O 1s spectrum (Fig.2(d)).O1s can be deconvoluted into three peaks, in which C-O (533.2 eV) and C=O (531.3 eV) belong to the remained oxygen functional groups on the surface of RGO, and lattice oxygen (529.5 eV)belongs to a small number of crystalline Co oxides[23].In addition, pyridinic-N, pyrrole-N, and graphitic-N convolution of N 1s peak can be observed from Fig.2(e).These rich nitrogen functional groups are often considered to be one of the sources of catalytic activity.Moreover, the high-resolution S 2p peak can be convoluted into three peaks centered on 164,165, and 169 eV (Fig.2(f)), respectively.The 165 eV peaks correspond to the S 2p3/2and S 2p1/2positions of thiophene-S (-C-S-), while the 169 eV peaks belong to the oxidized S group[24].Thiophene species exist at the edges and defect sites of carbon materials, thus improving the performance of ORR.These small amounts of S were derived from the sulfides produced by the thermal decomposition of DMSO during the solvothermal process, which were decomposed and uniformly doped into the graphene carrier during the calcination process.

Fig.3 SEM images of calcined samples at different temperatures: (a)ZIF-67/GO, (b)ZIF-67/RGO-300, (c)Co/Co3O4@NSG-400,and (d) Co/Co3O4@NSG-500
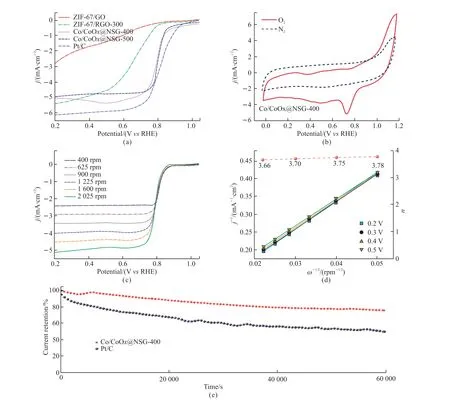
Fig.4 ORR characteristics and comparative data of the samples: (a) LSV curves at 10 mV·s-1 and 1 600 rpm; (b) CV curves (at 50 mV·s-1)of Co/CoOx@NSG-400 under saturated N2 and O2; (c) LSV curves under different rotation speeds for Co/CoOx@NSG-400; (d) The accordingly fitted K-L curves, and (e) i-t plots for Co/CoOx@NSG-400 and Pt/C
The morphology and microstructure of the formed samples were investigated by scanning electron microscopy (SEM), as shown in Fig.4.It can be seen that the ZIF-67 with the cubic structure is uniformly loaded on GO with a size of 100 - 200 nm (Fig.4(a)),and the cube structure can be basically maintained after annealing at a relatively low temperature (≤ 400℃) according to Figs.4(b)-4(c).However, as shown in Fig.4(d), the original cube structure disappears when the annealing temperature reached 500 ℃ due to the severe etching of ZIF caused by the release of more oxygen species from GO.
To obtain the optimized annealing temperature for the designed materials, the electrocatalytic performance of the synthesized samples was evaluated by using a three-electrode system in O2-saturated 0.1 mol·L-1KOH, and the according results are shown in Fig.4(a).The linear sweep voltammetry (LSV) curve also showed significant activity changes among the samples calcined at different temperatures.The increase in activity can be attributed to the enhancement of the CoOxphase and the new-formed Co phase, which is consistent with PXRD data (Fig.2(a)).It can be seen in Fig.4(a) that the half-wave potential of Co/CoOx@NSG-400 reaches 0.8 V, which is close to the value of Pt/C (0.82 V) and Co/CoOx@NSG-500(0.81 V).In addition, as shown in Fig.4(b), the CV curve of Co/CoOx@NSG-400 under oxygen-saturated electrolyte has an oxygen reduction peak at 0.8 V (vs RHE), but not under nitrogen saturation, which is one of the main active characteristics of ORR.Co/Cobased amorphous oxide nanoparticles expose more edges and defects/vacancies, forming a more uneven surface, which facilitates the acquisition of effective oxygen adsorption and catalysis.In addition, metal or metal oxides in carbon can further increase the density of active centers by enhancing the electrostatic gravitational force of molecular oxygen.Another important kinetic parameter of ORR (n, electron transfer number) is represented by the parallel lines of Koutecky-Levich at different scanning rates, as shown in Fig.4(c).It ranges from 3.66 to 3.78 (Fig.4(d)), close to the main four electron pathways of Pt/C (the number of electrons transferred by O2reduction).Compared with the two-electron pathway, this four-electron reaction pathway is widely considered beneficial to the effective ORR activity.Apart from the electrochemical performance above, long-term stability is still a critical index for the practical application of catalysts.As shown in Fig.4(e), the chronoamperometric test (the response of the current at a fixed potential relative to time) showed relatively enhanced stability with a current response activity retention of more than 80%,which is superior to that of Pt/C (50.5% maintained after a 60 000-soperation).
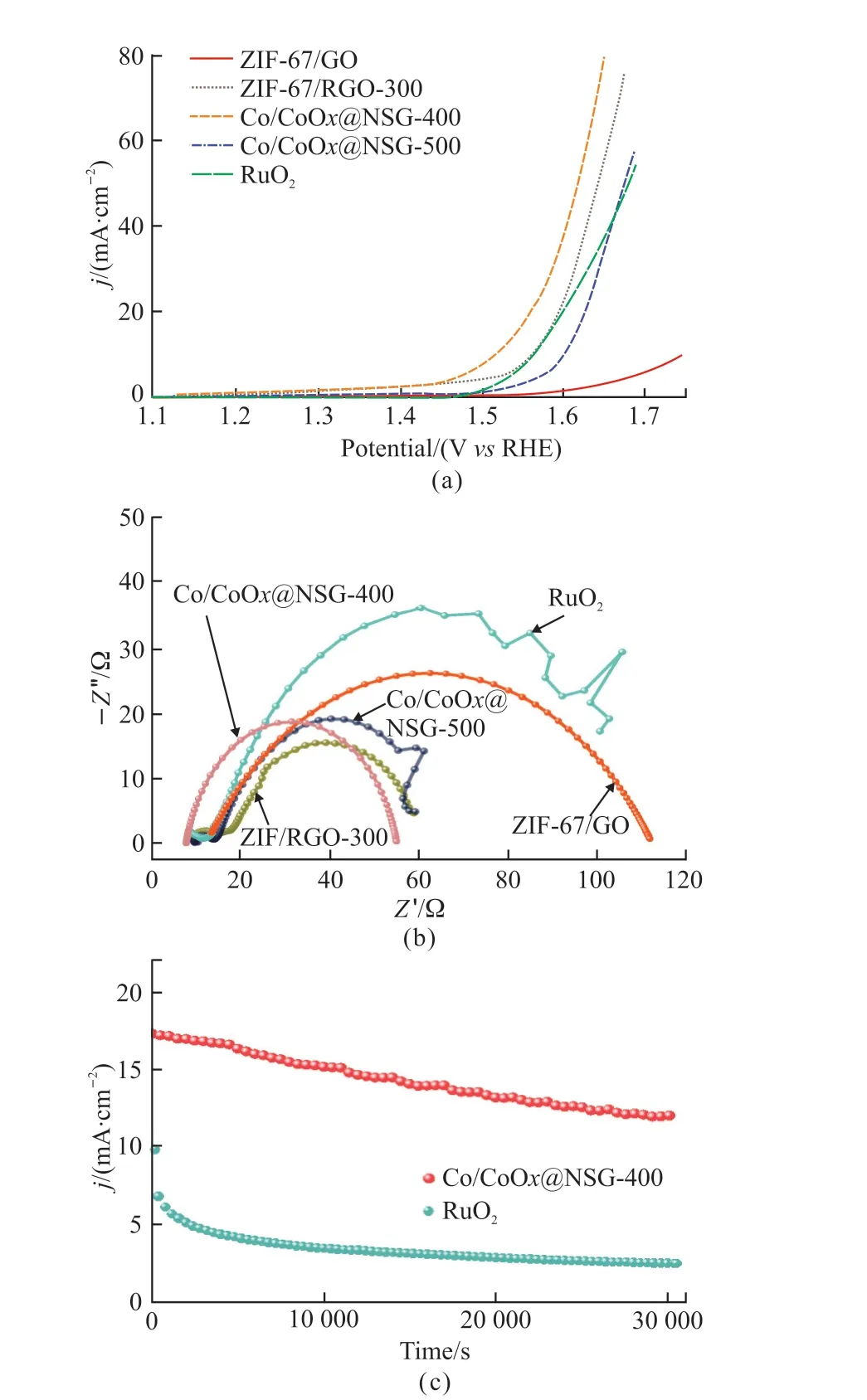
Fig.5 Comparison of OER activity characteristics of different samples in 1 mol·L-1 KOH: (a) LSV curves at 5 mV·s-1; (b)electrochemical impedance Nyquist diagrams and (c) i-t plots for Co/CoOx@NSG-400 and RuO2
The OER catalytic performance of the prepared ZIF-67/GO-derived composites in 1 mol·L-1KOH electrolytes in a standard three-electrode system was also evaluated to explore the prospect of multi-function application.The current densities of the samples calcined at different temperatures under 10 mA·cm-2were measured by linear sweep voltammetry (LSV).Compared with the precursor ZIF-67/GO, it should be mentioned that the overpotential of all calcined samples decreased to a certain extent (Fig.5(a)).Moreover,Co/CoOx@NSG-400 shows the lowest overpotential of 298 mV in all annealed samples, which is even lower than that of commercial RuO2(330 mV).The improvement of the catalytic activity of calcined samples to OER can be attributed to the escape of oxygen-containing functional groups of GO during the calcination process, which reduced GO to RGO and was beneficial to electron transport in the OER process.In addition, the mismatch of the imidazole ring during calcination also exposed more metal active centers and subsequently combined with oxygen released by GO to form amorphous Co-based oxides under mild temperature conditions.Based on the determined structure, further theoretical calculations suggest that this structure promotes O-O bond coupling compared to crystalline cobalt oxide leads to enhanced water oxidation activity[25].It is worth noting that ZIF-67/RGO-300 also shows a low overpotential of 336 mV.According to the results of PXRD (Fig.2(a)), ZIF-67/RGO-300 still retains most of the crystal characteristics and framework of ZIF-67, which is beneficial to the catalytic process of OER.However, when the calcined frame collapses, the overpotential of Co/CoOx@NSG-500 is 370 mV.Electrochemical impedance spectra were recorded to investigate the charge transfer further and reveal the interfacial behavior of the electrocatalysts under OER conditions, and the corresponding Nyquist diagram is drawn in Fig.5(b).Compared with ZIF-67/GO, all calcined samples reveal significantly lower charge transfer resistance (Rct) that benefits electron transport, indicating that their kinetics increase in the OER process.Moreover, Co/CoOx@NSG-400 exhibits the smallest Rct and the best highspeed charge transfer in all samples.In addition, after 30 000-s of stability test, the current of Co/CoOx@NSG-400 drops to 70.5 % of its initial value, while that of RuO2is only 25% of its original value, which shows that Co/CoOx@NSG-400 has better OER stability(Fig.5(c)).
4 Conclusions
Consequently, carbon-supported cobalt-based oxide derivatives were prepared by low-temperature annealing, demonstrating their enhanced OER and ORR electrocatalytic activities.All annealed samples showed better performance in the above electrochemical measurements compared to ZIF-67/GO, which can be attributed to the oxygen etching of ZIF to expose more metal centers and reduction of GO substrates.Notably, the obtained Co/CoOx@NSG-400 showed minor charge transfer resistance,enabling fast electron transfer.Besides, the amorphous Co/Co-based oxide nanoparticles derived from the sample can exhibit more exposed edges and defects/vacancies, forming a more inhomogeneous surface,which is favorable for obtaining effective oxygen adsorption and catalysis.In addition, the homogeneous N, S doping on carbon substrate can enhance the active inhomogeneous surface and electron transfer.More importantly, such a low-temperature synthesis strategy can help preserve the ZIF framework, improve catalyst yields, and regulate the growth and surface oxidation of metal particles, making it promising and efficient electrocatalysis in OER and ORR.
Conflict of interest
All authors declare that there are no competing interests.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Enhanced Electrochemical Performances of Ni Doped Cr8O21 Cathode Materials for Lithium-ion Batteries
- Design on the Prestressed Concrete Frame Beam-column
- Synthesis and Flocculation of Polyacrylamide with Low Water Absorption for Non-dispersible Underwater Concrete
- Experimental Behavior of Recycled Aggregate Concrete Filled Steel Tubular Columns
- Impact-abrasive Wear Behavior of ZTA and NbC Reinforced Fe60 Matrix Composites
- Synthesis and Characterization of Hollow Strontium Carbonate Pompons by Composite Soft Template Method
