Transformation of Coal Gangue to Sodalite and Faujasite Using Alkali-hydrothermal Method
2024-01-03WANGChengFENGKaiWANGLipengYUQianruDUFulingLIYanYUKaining
WANG Cheng, FENG Kai, WANG Lipeng, YU Qianru, DU Fuling, LI Yan, YU Kaining
(1. Hebei Center for Ecological and Environmental Geology Research, Hebei GEO University, Shijiazhuang 050031, China; 2. School of Material Science and Engineering, Shaanxi Key Laboratory of Green Preparation and Functionalization for Inorganic Materials, Shaanxi University of Science and Technology, Xi’an 710021, China; 3. School of Gemmology and Materials Science, Hebei GEO University, Shijiazhuang 050031, China)
Abstract: The coal gangue as the only source of silicon and aluminum was employed to synthesize sodalite and faujasite using hydrothermal method, which directly treated the mixture of pre-treated coal gangue and NaOH solution under hydrothermal environment.X-ray powder diffraction analysis (XRD),thermogravimetry analysis (TG) and differential thermogravimetry analysis (DTG), scanning electron microscopy (SEM), high resolution transmission electron microscopy (HRTEM), N2 adsorption-desorption technique, X-ray photoelectron spectroscopy (XPS), etc.were used to characterize the samples.Cd2+ ion was used to evaluate the heavy metal ions removal performance of the samples.The experimental results show that the coal gangue, which consists of quartz, calcium feldspar, potassium feldspar and kaolinite, can transform to sodalite and faujasite under alkali-hydrothermal condition at 150 and 180 ℃, respectively.The as-prepared sodalite and faujasite can effectively remove the simulated Cd2+ ion wastewater and actual industrial wastewater containing As3+, Cd2+, and Cr3+ ions, and the good heavy metal ion removal performance of the zeolites is mainly attributed to their low Si/Al ratio and high Na+ content.This alkali-hydrothermal method appears to be a simple and efficient method for transformation of coal gangue to high purity zeolites.
Key words: coal gangue; alkali-hydrothermal method; sodalite; faujasite
1 Introduction
Coal gangue is a solid waste from the coal mining and washing processes, and accounts for approximately 10%-15% of the total coal production.The cumulative amount of coal gangue in China has risen to more than 5 billion tons and is still rising at an annual rate of 300-350 million tons[1-4].Untreated coal gangues not only occupy a large amount of land, but also bring about a series of environmental problems, such as regional environmental pollution, collapse, debris flow and other geological disasters, land degradation, crop reduction,ecological system and landscape damage, and these problems greatly harm the ecological environment and human health[4].Therefore, it is urgent to study the treatment, disposal and comprehensive utilization of coal gangue.
The main chemical components of coal gangues are SiO2and Al2O3, which are expected to become the main raw materials of synthetic zeolite.In recent years,researchers tried to prepare zeolites from coal gangue using hydrothermal method.For example, Qianet al[5]mixed the coal gangue with NaOH and heated at 400℃ for 2 h, and the melted mass was dissolved in water and then hydrothermal treated, and the zeolite Na-A was finally obtained.Jinet al[6]used coal gangue and sodium meta-aluminate as raw materials to synthesize zeolite Na-A using hydrothermal method.Buet al[7]mixed coal gangue with Al2O3and NaOH, and heat treated at 400 ℃ for 2 h.The obtained alkaline product was ground again, then added to the NaOH solution,and aged for 12 h.The slurry was then hydrothermally treated and zeolite Y was finally obtained.Hanet al[8]firstly mixed coal gangue with KOH solution, and preprocessed using ultrasound for 1 h.TMAdaOH was secondly added into the mixture, and pretreatment was continued using ultrasound for 1 h.Thirdly, fumed silica was added into the mixture, and pretreatment was continued using ultrasound for 1 h.Finally, the gel was hydrothermally treated and the SSZ-13 was synthesized.Although previous studies have proved that the coal gangue could transform to zeolite using hydrothermal method, additional silicon or aluminum resources and two or more steps were generally adopted during the hydrothermal synthesis process.However, to the best of our knowledge, very limited studies have adopted the coal gangue as the only source of silicon and aluminum for syntheses of zeolites using one-step alkali-hydrothermal method.
In this study, the coal gangue from Yulin City,Shaanxi Provence, China was used as the only source of silicon and aluminum for syntheses of sodalite and faujasite using one-step alkali-hydrothermal method.XRD, TG-DTG, SEM, HRTEM, N2adsorption-desorption technique, and XPS,etc.were used to characterize the samples.Cd2+model wastewater and two actual industrial wastewaters containing As3+, Cd2+, and Cr3+ions were adopted to evaluate the heavy metal ion removal performance.
2 Experimental
2.1 Materials
The coal gangue ore was obtained from Yulin City, Shaanxi province, China.The ore was manually ground in a corundum mortar and then separated by dry sieving using a 74 μm aperture sieve.The main chemical composition of the ore was consisted of SiO2(69.84wt%), Al2O3(16.64wt%), K2O (3.46wt%), Na2O(2.18wt%), Fe2O3(1.93wt%), SO3(1.08wt%), MgO(0.72wt%), TiO2(0.523wt%), CaO (0.345wt%), and P2O5(0.108wt%),etc.according to the X-ray fluorescence spectroscopic analysis.The total content of SiO2and Al2O3was 86.48wt% and the SiO2/Al2O3mole ratio of the ore was 7.14 (Si/Al mole ratio = 3.57).CdCl2(AR, 99%) was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd.Two industrial wastewaters containing As3+, Cd2+, Cr3+ions (donated as I1 and I2) were obtained from Henan Jiaozuo Petrochemical Plant.The concentrations of As3+, Cd2+, Cr3+ions in the I1 wastewater were 0.17, 0.09, and 0.15 mg/L, while these ions in the I2 wastewater were 0.12, 0.09,and 0.17 mg/L, respectively.
2.2 Synthesis of zeolites
The hydrothermal method used for the synthesis of zeolites is shown as follows: The fine coal gangue solids were heat treated at 850 ℃ for 2 h to remove the organic substances.5 g of the heat-treated coal gangue powders were mixed with 50 mL 6 mol/L sodium hydroxide solution (The Na/Si mole ratios was about 15).The mixture was sealed in a 100 mL high-pressure autoclave and placed in an oven at certain temperature(150 and 180 ℃) for 24 h.The powders were separated from the suspension by the centrifuge and repeatedly washed with deionized water until the pH value of suspension was close to neutral, and finally dried at 60 ℃for 24 h.The hydrothermal products synthesized at 150 and 180 ℃ were denoted as Z1 and Z2, respectively.CdCl2(AR, 99%) was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd.for heavy metal ion removal experiments.
2.3 Characterization of the samples
XRD analyses were conducted on a diffractometer(AXS D8-Focus, Germany) with Cu Kα radiation and a graphite monochromator under 40 kV and 40 mA,respectively.TG-DTG analyses were conducted on a thermogravimetric analyzer (TGA Q500, USA) from room temperature to 1 000 ℃ at a heating rate of 10 K/min.SEM analyses were conducted on a scanning electron microscope (FEI Verios 460, USA) operating at an accelerating voltage of 15.0 kV.HRTEM analyses were conducted on a high-resolution transmission electron microscope (FEI Tecnai G2 F20 S-TWIN, USA) with an accelerating voltage of 160 kV.N2adsorption-desorption analyses were performed on an automated physisorption analyzer (Gemini VII2390, USA) to determine the specific surface area and pore structure of the sample.All samples were degassed at 350 ℃ under vacuum for 6 h before the measurements were conducted.XPS analyses were conducted on a vacuum generator AXIS SUPRA spectrometer (Kratos Company, England) with an Al/Mg dual anode X-ray source used for the recording of the X-ray photoelectron spectra.The pass energies of the survey spectra and high-resolution spectra were 160 and 20 eV, respectively.
2.4 Heavy metal ion removal experiments
The heavy metal ions removal experiments were conducted using 50 mL polyethylene bottles in a Rotary mixer (MX-RL-E, SCILOGEX, USA) with a rotational speed of 80 rpm.2 g/L of samples was added to the heavy metal ions solution.The mixture was stirred at room temperature (22.5 ℃) for a given time and then centrifuged to separate the solids from the supernatant.The experiments were conducted in triplicate: data represented the average of three experiments and error bars indicated the standard deviation.
The adsorption capacity of heavy metal ionsqand the ion removal efficiencies of heavy metal ionsRare calculated as:
where,Ct(mg/L) andC0(mg/L) are the final and initial concentrations of heavy metal ions,Vthe volume of water, mL, andWthe weight of the samples, mg.
3 Results and discussion
3.1 Composition and structure of coal gangue
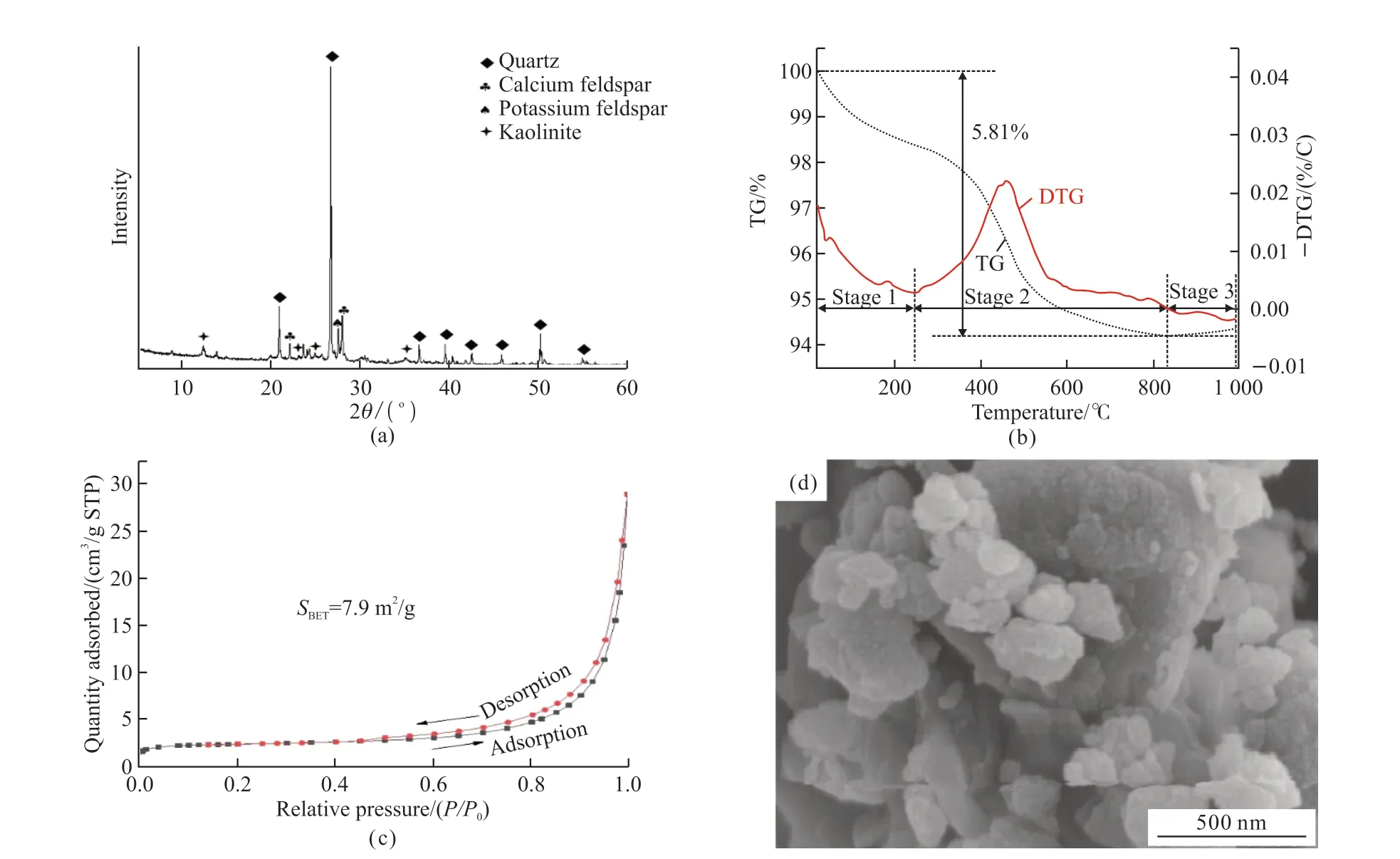
Fig.1 (a) XRD pattern, (b) TG-DTG curves, (c) N2 adsorption-desorption isotherms, and (d) SEM image of coal gangue
Fig.1 shows the XRD, TG-DTG curves, N2adsorption-desorption isotherms, SEM images of coal gangue.The XRD patterns in Fig.1(a) show that the main mineral phases of the coal gangue are quartz,calcium feldspar, potassium feldspar and kaolinite.The TG curves in Fig.1(b) show that the mass loss of the coal gangue is 5.81%.The DTG curve can be divided into three stages according to the mass loss: < 245.7℃, 245.7-834.2 ℃, and 834.2-1 000 ℃.The first stage is mainly attributed to the vaporization of water molecules and the release of volatiles, the second stage is probably due to the dehydroxylation of inorganic substance,eg, feldspar and kaolinite, and the combustion of volatiles and fixed carbon, and the third stage is probably attributed to the recrystallization of kaolinite and other minerals[1,9].
The N2adsorption/desorption isotherm curves in Fig.1(c) can be indexed as the type Ⅳ isotherm with H3 hysteresis loop, suggesting the presence of platelike mesopores in the coal gangue.The mesopores are attributed to the feldspar and kaolinite minerals in the coal gangue.The SEM image in Fig.1(d) shows that the coal gangue particles are dozens of nanometers to several microns with irregular morphology.
3.2 Composition and structure of zeolites
The XRD patterns in Fig.2(a) show that the main crystal phases of the Z1 and Z2 samples are sodalite and faujasite, respectively.Almost no characteristic peaks belonging to coal gangue can be found in the two samples, indicating the highly efficient transformation of coal gangue to zeolites with high purity.
Fig.2(b) shows the N2adsorption/desorption isotherm curves of the samples.The slight increases of N2adsorption under lowP/P0(0.005) and type Ⅳ isotherms with H3 hysteresis loops indicate the presence of plate-like mesopores with few of micropores in the two samples.The specific surface areas of the Z1 and Z2 samples are 29.7 and 8.7 g/m2, which are higher than that of the raw coal gangue.
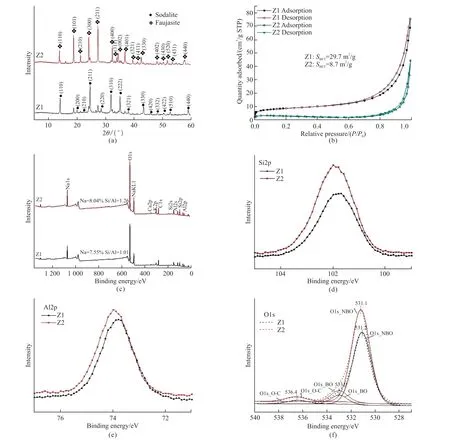
Fig.2 XRD patterns (a), N2 adsorption-desorption isotherms (b), XPS survey spectra (c), and XPS high-resolution spectra of Si (d), Al (e), O (f)of the Z1 and Z2 samples
The XPS survey spectra of the samples in Fig.2(c)show that the surface of the samples contains Si, Al,O, C, Na, Ca, and K elements.The Na contents on the surface of Z1 and Z2 samples are 7.55% and 8.04%,and the Si/Al ratio of the Z1 and Z2 samples are 1.01 and 1.26, respectively.The XPS high-resolution spectra in Figs.2(d)-2(f) show that the binding energies of Si,Al, and O of the two samples are slightly different with each other, which is owing to the difference of crystal structure of the two samples.The O 1s spectra for each of the two samples in Fig.2(f) show three distinct peaks,of which those at 536.4 (Z1 sample) and 536.4 eV (Z2 sample) are ascribed to O in the O-C bond, 533.0 (Z1 sample) and 533.0 eV (Z2 sample) are ascribed to O in the bridging Si-O-Si bond (BO), and 531.1 (Z1 sample)and 531.2 eV (Z2 sample) are due to O in the M-O-Si moieties (M = Al, Na, K, and Ca), which are referred to as non-bridging oxygen atoms (NBO)[10].Based on the areas of the three distinct peaks, it is indicated that the surfaces of the two samples contain high amount of non-bridging oxygen atoms.
Fig.3 shows the HRTEM images of the samples.The lattice fringes of the Z1 and Z2 samples are 0.204 and 0.416 nm, which are consistent with the spacing of the sodalite (330) plane and faujasite (210) plane,respectively.The particle sizes of the four samples are a few to a dozen nanometers.This HRTEM results further confirm the transformations of coal gangue to zeolites.
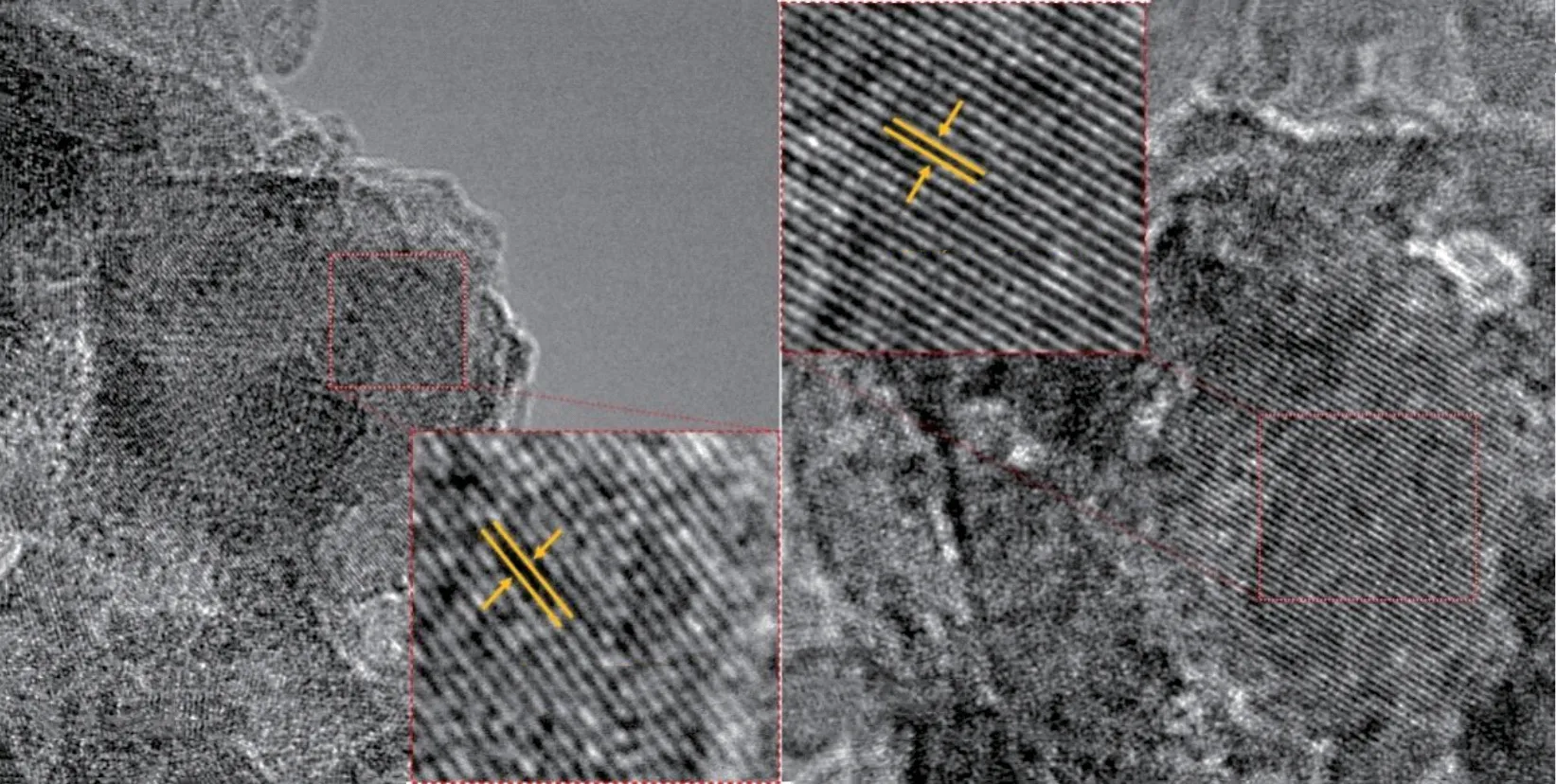
Fig.3 HRTEM images of the Z1 (a) and Z2 (b) samples
3.3 Heavy metal ions removal performances of the samples
Fig.4(a) shows that the Cd2+adsorption capacities of the Z1 and Z2 samples are 40.9, and 36.4 mg/g, while the corresponding Cd2+removal efficiencies are 41.2%, and 55.6%, respectively.The Z1 sample exhibits relatively higher Cd2+removal performance as compared with Z2 sample, which is attributed to its relatively high specific surface area, low Si/Al ratio, and high Na+content.
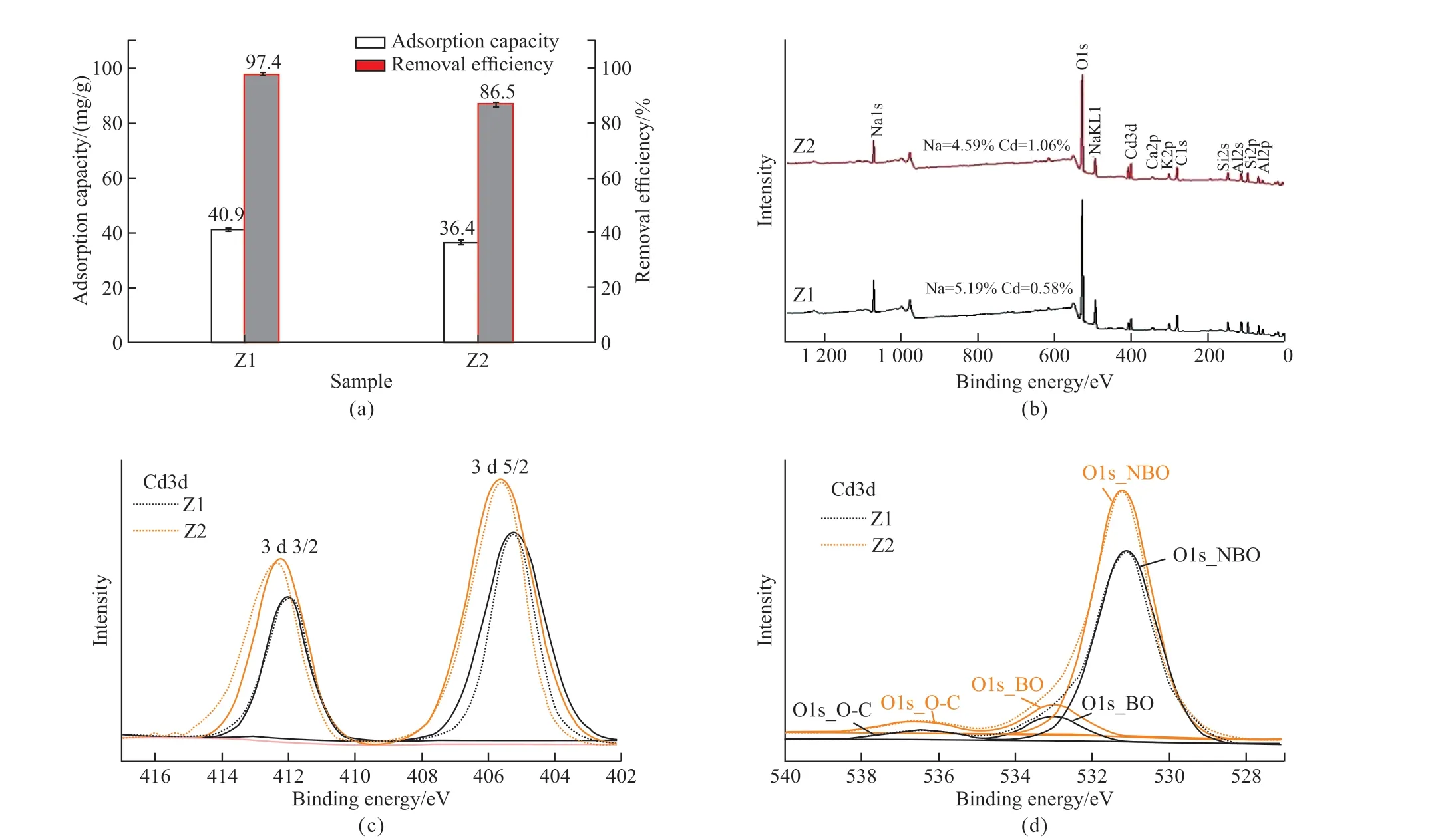
Fig.4 Cd2+ adsorption capacities and removal efficiencies (a), XPS survey spectra (b), XPS high-resolution spectra of Cd (c) and O (d) of the Z1 and Z2 samples after adsorption of the Cd2+ ion
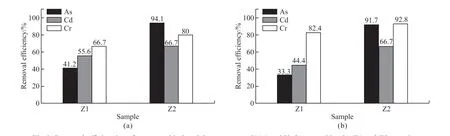
Fig.5 Removal efficiencies of two actual industrial wastewaters I1(a) and I2 (b) treated by the Z1 and Z2 samples
The XPS survey spectra in Fig.4(b) and high-resolution spectra of Cd 3 d of the Z1 and Z2 samples after adsorption of the Cd2+ion show that the surfaces of the two samples contain certain amount of Cd2+, which confirms the adsorption of Cd2+on the two samples.In addition, the Na+contents in the Z1 and Z2 samples are obviously lower than the corresponding samples before adsorption of Cd2+, which suggests that the removal of Cd2+from the water is mainly due to the ion exchange of Cd2+with Na+on the samples.The XPS high-resolution spectra of O1s of the two samples show that the binding energies of the O 1s assigned to BO and NBO shift to higher binding energies compared with those of the raw samples, and this is probably attributed to the interaction of zeolites with Cd2+ions.
In order to further investigate the heavy metal ion removal performance of the Z1 and Z2 samples, two actual industrial wastewaters containing As3+, Cd2+, and Cr3+ions were adopted and the removal efficiencies of these ions are shown in Fig.5.It shows in Fig.5(a)that the As3+, Cd2+, Cr3+removal efficiencies of the I1 wastewater treated by the Z1 and Z2 samples are 41.2%, 55.6%, 66.7%, and 94.1%, 66.7%, 80.0%, respectively.The As3+, Cd2+, Cr3+removal efficiencies of the I2 wastewater treated by the Z1 and Z2 samples are 33.3%, 44.4%, 82.4%, and 91.7%, 66.7%, 92.8%, respectively (Fig.5(b)).These results indicate that the Z1 and Z2 samples exhibit good heavy metal ion removal performance, they can simultaneously and effectively remove multiple heavy metal ions from actual industrial wastewaters.
4 Conclusions
Coal gangue with mineral composition of quartz,calcium feldspar, potassium feldspar and kaolinite, and SiO2/Al2O3mole ratio of 7.14 was adopted as the only source of silicon and aluminum for synthesizing of zeolites using one-step alkali-hydrothermal method.The results showed that the coal gangue could transform to sodalite and faujasite with high purities under alkali-hydrothermal conditions at 150 and 180 ℃, respectively.The specific surface areas of the sodalite and faujasite samples are 29.7 and 8.7 g/m2, and the Si/Al ratio of the two samples were 1.01 and 1.26, respectively.The Cd2+ion adsorption capacities of the as-prepared sodalite and faujasite were 40.9 and 36.4 mg/g, respectively.The sodalite and faujasite could simultaneously and effectively remove multiple heavy metal ions,i e,As3+, Cd2+, Cr3+from actual industrial wastewaters.
Conflict of interest
All authors declare that there are no competing interests.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Enhanced Electrochemical Performances of Ni Doped Cr8O21 Cathode Materials for Lithium-ion Batteries
- Design on the Prestressed Concrete Frame Beam-column
- Synthesis and Flocculation of Polyacrylamide with Low Water Absorption for Non-dispersible Underwater Concrete
- Experimental Behavior of Recycled Aggregate Concrete Filled Steel Tubular Columns
- Impact-abrasive Wear Behavior of ZTA and NbC Reinforced Fe60 Matrix Composites
- Synthesis and Characterization of Hollow Strontium Carbonate Pompons by Composite Soft Template Method
