Effect of Hydrated Calcium Aluminate Cement on the Chloride Immobilization of Portland Cement Paste
2024-01-03ZhoulingTANHongboLIUXiaohaiCHENPianWANGYifanLIANGWenjie
LÜ Zhouling, TAN Hongbo,*, LIU Xiaohai, CHEN Pian, WANG Yifan, LIANG Wenjie
(1. State Key Laboratory of Silicate Materials for Architectures, Wuhan University of Technology, Wuhan 430070, China; 2. School of Materials Science and Engineering, Zhengzhou University, Zhengzhou 450001, China)
Abstract: To improve the efficiency and stability of chloride immobilization of portland cement paste,hydrated calcium aluminate cement (HCAC) prepared by wet grinding of CAC was added into portland cement paste as an additive.The immobilized chloride ratio (ICR) was evaluated, and the mechanism of chloride immobilization was researched by XRD, DTG, NMR, and MIP tests.The analysis results demonstrated that HCAC could improve the chloride immobilization capacity of portland cement paste.The mechanism was attributed to the following aspects: chemical binding capacity was enhanced via producing more Kuzel's salt;physical adsorption capacity was reduced by decreasing the C-S-H gel; migration resistance was enhanced through refining the pore structure.
Key words: hydrated calcium aluminate cement; portland cement paste; chloride immobilization;kuzel’s salt; pore structure
1 Introduction
With the rapid growth of marine engineering construction in coastal countries, reinforced concrete has been widely used as its main structural material.At present, the raw materials for marine engineering concrete mainly use river sand transported from inland.The use of inland river sand not only had the problems of long transportation distance and high transportation cost but also faced the issues of over-exploitation and resource depletion[1-3].On the contrary, using coastal sea sand resources had the advantages of abundant production and low transportation cost in remote island engineering projects[4].And the use of coral sand resources had also gained the support of numerous researchers[5,6].Nevertheless, the chloride ions remained in sea sand or coral sand could accelerate the rusting reaction of steel reinforcement and then damage the concrete structure[7,8].Nowadays, researchers had proposed several prevention and treatment methods for the corrosion of steel reinforcement because of the high content of endogenous chloride ions in concrete.The methods mainly included active protection of reinforcement such as surface protection of rebar and application of stainless steel, rust inhibitor protection, and electrochemical protection[9-15].Among them, the free chloride immobilization technology achieved by the immobilization of cementitious materials in reinforced concrete had been accepted as one of the most effective means to reduce the risk of reinforcement corrosion[16-18].
Chloride immobilization of cementitious materials depended on three main means: chemical binding,physical adsorption, and migration resistance[19,20].Chemical binding was mainly achieved through the chemical reaction between hydration products of cementitious materials and free chloride ions by generating Friedel's salts (FS, 3CaO·Al2O3·CaCl2·10H2O)and Kuzel's salts (KS, 3CaO·Al2O3·0.5CaCl2·0.5CaSO4·10H2O)[21,22].Chemical binding was regarded as one of the most stable and effective ways to achieve chloride immobilization[23].Physical adsorption primarily relied on the C-S-H gel generated from hydration to immobilize free chloride ions via surface charge adsorption[24-27].Migration resistance prevented the migration of free chloride ions among cementitious pore channels by refining the pore structure of cementitious materials and reducing access between free chloride ions and steel bar[28].
Several mineral admixtures and chemical additives were beneficial to the chloride immobilization capacity[29-37].For example, fly ash (FA) as a common mineral admixture significantly enhanced the chloride chemical binding capacity through dissolving the aluminum phase and producing FS and KS during the pozzolanic reaction[30,33,35].The chemical additive triisopropanolamine (TIPA) could also improve the chloride chemical binding capacity by facilitating the process of aluminum phase dissolution through its chelating effect[32,34].The increase of the dissolved aluminum phase could significantly enhance the chloride immobilization capacity.Therefore, the active aluminum-rich phase in supplementary cementitious materials acted as an important role in immobilizing chloride in portland cement system.
CAC as an active aluminum-rich phase material was expected to enhance the chloride immobilization of portland cement.CAC had been widely used as an admixture in portland cement paste[38-43], and not only shortened the setting time but also improved the erosion resistance.However, high content of CAC admixture to portland cement paste was likely to cause the risk of flash-setting and late strength reduction.XUet al[43]proposed the setting time of CAC-PC system would rapidly decline after adding more than 6wt%CAC.Accordingly, the dosage of CAC as an admixture should be controlled in engineering applications.Jinet al[40]studied the regularity of chloride binding in ordinary portland cement paste mixed with CAC.It revealed that the CAC mixture doped with 5wt%-15wt% observably enhanced the chloride binding capacity.However, the mechanism behind the effect of CAC mixture on chloride binding was still not fully explained.To reduce the negative effects on the CAC-PC system from the blended CAC, the CAC with a lower dosage was adopted.And a wet grinding process for CAC was to improve the activity of aluminum phase.It could advance the hydration of CAC to promote the dissolution of aluminum phase by reducing the particle size[44].Then the hydrated calcium aluminate cement(HCAC) was obtained and might have an excellent promotion on the chloride binding capacity.
In this paper, the effects of HCAC on the chloride immobilization capacity of portland cement paste were investigated.The ratios of chloride immobilization of portland cement pastes were tested for different dosages of HCAC at different curing ages.The contents of FS and KS in connection with chemical binding capacity was characterized by X-ray diffraction (XRD),thermogravimetric analysis (DTG), and27Al nuclear magnetic resonance (27Al NMR).The C-S-H gel content was elucidated by29Si nuclear magnetic resonance(29Si NMR) to compare the chloride ion physical adsorption capacity.The pore structure was identified by MIP test and fractal dimension to characterize the chloride migration resistance of the hardened samples.The workability and mechanical properties were also tested.Finally, the mechanism of chloride immobilization of portland cement pastes with HCAC as an additive was discussed.
2 Experimental
2.1 Materials
The calcium aluminate cement (CAC, according to Chinese standard GB/T 19001-2008) was gained from Zhengzhou, Henan Province.And PI 42.5 portland cement (PC, according to the Chinese standard GB 8076-2008 standard) was obtained from China Building Materials Academy.Table 1 lists the chemical compositions of CAC and PC, and the contents of chloride and alkali meet the standard requirements.
2.2 Preparation and characterization of HCAC
HCAC, as the hydration product of CAC, was produced by wet grinding.Firstly, CAC and water were added to the ball grinding jar with a ratio of 1:9.Then,2.5 mm diameter agate balls were placed in the ball grinding jar with the ratio of ball-to-powder weight 4:1.Finally, after the ball grinding jar was sealed, it was operated on the grinding mill at 30 r/min for 24 h.The wet grinding process was carried out in a constant temperature standard curing room, and the ambient temperature was set as 25 ℃.

Table 1 Chemical compositions of materials/wt%
Fig.1 gives the SEM images of CAC and HCAC.A field emission scanning electron microscope (FESEM, Zeiss Ultra Plus, produced by Zeiss, Germany)could give the images.By comparing the two images of the materials before and after wet grinding, it could reveal that the morphology of the material changed observably by wet grinding.It could be found that more fine particles appeared in the HCAC than in the CAC.

Fig.1 SEM images of CAC (a) and HCAC (b)

Fig.2 Particle size distributions
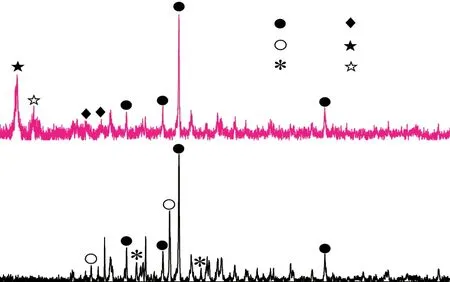
Fig.3 XRD patterns of CAC and HCAC
The particle size distributions of CAC and HCAC are presented in Fig.2.The patterns were offered by a laser particle size analyzer.It could be observed that theD(50) (median particle diameter) of HCAC was 4.27 μm, while theD(50) of CAC was 20.71 μm.The data confirmed that the wet grinding method would remarkably reduce the particle size and narrow the particle size distribution range.
The XRD patterns of CAC and HCAC are presented in Fig.3.And the phase compositions of CAC and HCAC were characterized by XRD.From the XRD pattern of HCAC, it should be noted that the CA(CaAl2O4, PDF#70-0134) and CA2(CaAl4O7, PDF#89-3851) peaks almost disappeared compared to the XRD pattern of CAC.And the hydration phrases of HCAC were mainly C2AH8(Ca2Al2O5·8H2O, PDF#11-0205),C4AH13(Ca4Al2O7·13H2O, PDF#02-0077) and AH3(Al(OH)3, PDF#70-2038).The results indicated that the hydration reaction of CAC occurred during wet grinding.Meanwhile, a large amount of hydrated calcium aluminate was generated.As the main component of CAC, the CA mainly occurred the following hydration reaction at 15-30 ℃.
2.3 Samples preparation
In this study, HCAC was added directly to portland cement paste as an additive.The water-binder ratio for pure slurry was selected as 0.38.During the cement mixing process, HCAC was added with 0wt%,1wt%, 2wt%, and 4wt% dosages of the binders.NaCl(1.11wt%) was introduced to offer chloride ions so that the chloride concentration in the mixing water was 0.5 mol/L.At this point, polycarboxylate superplasticizer(PCE) was dripped in the paste to ensure the same fluidity[45,46].After the cement paste was stirred evenly, it was put into 40 mm×40 mm×40 mm molds.Then the samples were placed in the environment of 95% relative humidity and 20±2 ℃ until the specified ages.
After the compressive strength was tested, the remained samples could be crushed and collected.Then the samples were submerged with pure alcohol and then sealed for 3 d to terminate the hydration.And then the dried samples were sealed for the testing of immobilized chloride ratio (ICR) and microscopic properties.Among the samples, the pieces for MIP test were 3-5 mm diameter, the powders for ICR test were smaller than 0.15 mm diameter and the powders less than 0.045 mm diameter were used for other micro tests.
2.4 Testing methods
2.4.1 Immobilized chloride ratio (ICR)
According to the Chinese standard (SL 352-2006),ICR could be calculated by Eq.(1):
where,Ctis the total chloride content, %;Cfthe free chloride ion content in the paste, %.
In terms of the total water loss of 25wt% of the hardened paste after curing, alcohol immersion and drying,Ctwas calculated by the mass of the initial addition of NaCl and deionized water.TheCfwas measured and calculated by titration tests.Firstly, 10 g test sample was placed into a conical flask with 100 mL deionized water, sealed and shaken, and placed at 25℃ for 24 h.Then, the suspension was filtered to obtain the filtrate, and 20 mL filtrate was used for titration.Finally, several drops of dilute sulfuric acid were added to neutralize to pH=7 with an appropriate amount of phenolphthalein as indicator, and the free chloride ion content in the paste (Cf) was tested by the Mohr method[30,47].It employed 0.5 g K2CrO4solution (5wt%) as an indicator and 20 mL AgNO3solution (0.02 mol/L)for titration.The end point of the titration was when the brick-red precipitate Ag2CrO4appears.After each sample was tested twice, the value of Cfcould be calculated by Eq.(2) and the average was considered as the target value.
where, C is the AgNO3concentration, mol/L;V3the consumption volume of AgNO3solution, mL;Gthe sample weight, g;V2the volume of the filtrate used for titration,mL;V1the water volume for soaking sample, mL.
2.4.2 X-ray diffractometer (XRD)
The phases of the samples were revealed by an X-ray diffractometer of the D8 Discover type (manufactured by Bruker, Germany).Cu (Kα) target was chosen as the target material.The current was 40 mA and the voltage was 40 kV.The test rate was set as 2 °/min while the step size was set as 0.02°.
2.4.3 Thermo-gravimetric analysis (TGA)
TGA was commonly used to analyze the thermal stability and components of materials.In this study, the samples were analyzed by STA449F3 thermal analyzer from NETZSCH, Germany, with the N2environment,the heating rate of 10 ℃/min and the temperature range of 50-1 000 ℃.
2.4.429Si NMR
The chemical states of silicon atoms in samples with and without HCAC added at different ages were analyzed by29Si NMR (Bruker Advance III 400 spectrometer).In this experiment, the working frequency was 79.5 Hz, and the relaxation time was 5 s.
The reaction degrees of the samples (Ac) after the deconvolution of the29Si NMR pattern was calculated by Eq.(3)[48].And the computed result for main chain length (MCL) of C-S-H gels could be obtained by Eq.(4).
where, the fitting signal intensities ofQ0,Q1, andQ2are expressed asI(Q0),I(Q1), andI(Q2).Q0represents the Si-O tetrahedron in unhydrated cement minerals;Q1(chain-end groups) andQ2(middle-chain groups) are indicated as the Si-O tetrahedron in hydration products.
2.4.527Al NMR
27Al NMR used Bruker Advance III400 spectrometer, rotational frequency of 10 kHz, relaxation time of 2 s.The distribution of aluminum phase could be clearly characterized by27Al NMR spectra[48,49].
2.4.6 Pore structure
The porosity of sample was tested by mercury intrusion porosimetry (MIP, produced by Kangta Instruments, USA) with a maximum pressure of 210 MPa and the angle of contact for the mercury and the sample set at 140°[50].
Fractal dimension is a fantastic parameter to characterize the pore space structure of cementitious materials.The fractal dimension was acquired from MIP data using Zhang's model, which was calculated from Eq.(5) and Eq.(6)[51-53]:
where,Wis the surface energy of the pore;Pithe pressure at stepi;Vithe intruded volume of stepi;rnthe pore radius at stepn;Vnthe intruded mercury volume of stepn;Dsthe surface fractal dimension.
2.4.7 Setting time
According to the standard ASTMC191, the setting time of cement pastes including the initial setting time and final setting time, was determined using a Vicat apparatus at 20 ℃[54].The setting time was measured from the moment of adding water.The experimental was conducted three times, and the result was the average value.
2.4.8 Compressive strength
The mechanical properties of the samples were tested according to the Chinese standard GB/T 17671-1999.And the compressive strength of the samples would be tested at the specified curing age.The loading rate of the tests was set as 2.4 kN/s.The results were taken as the average of three measurements of the samples.The average of the three measurements of the samples was considered as the result.
3 Results and discussion
3.1 Immobilized chloride ratio
Fig.4 presents the ICR values of the cement samples with various dosages of HCAC at different curing ages.The relative ratios of ICR of each sample were labeled by defining ICR of the control group sample as 100% at each age.It could be observed that the ICRs increased gradually with the increase of the additive HCAC at 7, 28, and 60 d.In comparison with the control group HCAC0 (0wt% HCAC), the ICRs of HCAC1, HCAC2, and HCAC4 increased by 8%, 11%,and 13% at 7 d, 3%, 7%, and 15% at 28 d, and 4%, 6%,and 11% at 60 d, respectively.The results indicate that HCAC as an additive could markedly increase the ICR of portland cement paste.
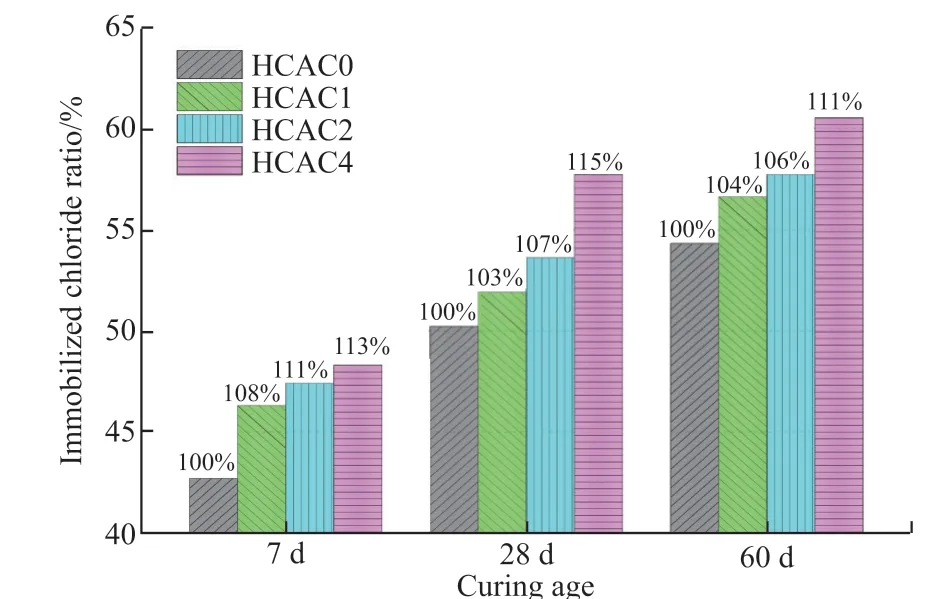
Fig.4 ICRs of the samples at different ages
The increase of ICR in portland cement paste might be relevant to three different types of behaviors on chloride immobilization.The first was chemical binding, which was related to Friedel's salt and Kuzel's salt of the system.The second was physical adsorption,which was generally relevant to the C-S-H gels.The last was migration resistance in connection with the pore space structure of the cement system.Therefore,the following tests mainly focused on the three ways to explore the reasons for the improved chloride immobilization capacity.
3.2 Chemical binding
3.2.1 XRD
Fig.5 displays the phase compositions of the samples with different dosages of HCAC at various ages.And the phases including Ca(OH)2, Friedel's salt and ettringite (AFt) could be clearly observed, as presented in Fig.5(a), Fig.5(c), and Fig.5(e).More detailed diffractive peaks from 8.5° to 11.5° could be displayed in Fig.5(b), Fig.5(d), and Fig.5(f).And the diffractive peaks mainly include AFt, AFm, Kuzel's salt (KS), and Friedel's salt (FS).
It could be observed in Fig.5(b), Fig.5(d), and Fig.5(f) that the KS peak intensities of the samples were significantly increased after adding 4wt% HCAC at all curing ages, especially at 7 d.However, the FS peak intensity of HCAC4 barely changed in comparison with HCAC0.In addition, it was noteworthy that the AFm peak intensity of HCAC4 changed differently compared to HCAC0 at different curing ages.HCAC4 had a stronger intensity of the AFm peak than HCAC0 at 7 d.The AFm peak intensity of HCAC4 remained unchanged at 28 d but decreased at 60 d compared to that of HCAC0.In contrast, the AFt peak intensity of HCAC4 rarely changed at 7 d, and decreased slightly at 28 and 60 d compared to that of HCAC0.
Based on the above results, Kuzel’s salt increased with the addition of HCAC in portland cement paste.It could also be proved that HCAC could help the PCCAC system generate a large amount of AFm at the early age.The increased AFm in HCAC4 might be formed by the reaction of C2AH8in HCAC with CH(Ca(OH)2) and gypsum in portland cement paste.And the AFm would combine with chloride ions to form a large amount of KS as the hydration gradually proceeded.The increased KS would be the main reason for enhancing the chloride immobilized capacity of the PCHCAC system.
Consequently, one conclusion could be drawn that HCAC could be conducive to increasing more AFm and KS of portland cement paste at the early period.And the increased KS would make more chloride ions immobilized.
3.2.2 DTG
The DTG curves of the hardened portland cement pastes with the dosage of 0wt% or 4wt% HCAC at 7, 28, and 60 d are presented in Fig.6.It could be observed that each sample revealed some peaks with similar temperature positions at different ages.The peak near 100 °C ascribed to C-S-H gel and AFt.It was unable to prove the C-S-H gel and AFt on account of the similar decomposition temperatures from the DTG curves.And the thermal decomposition peaks for C-S-H gel and AFt would overlap into a peak at 50-250℃[55,56].The peaks for FS and KS could be observed at the temperature from 250 to 380 ℃[57].The water loss peak of Ca(OH)2was displayed at 380-500 ℃[58].The peaks presented at 550-750 ℃ were attributed to the decomposition of CaCO3[59].
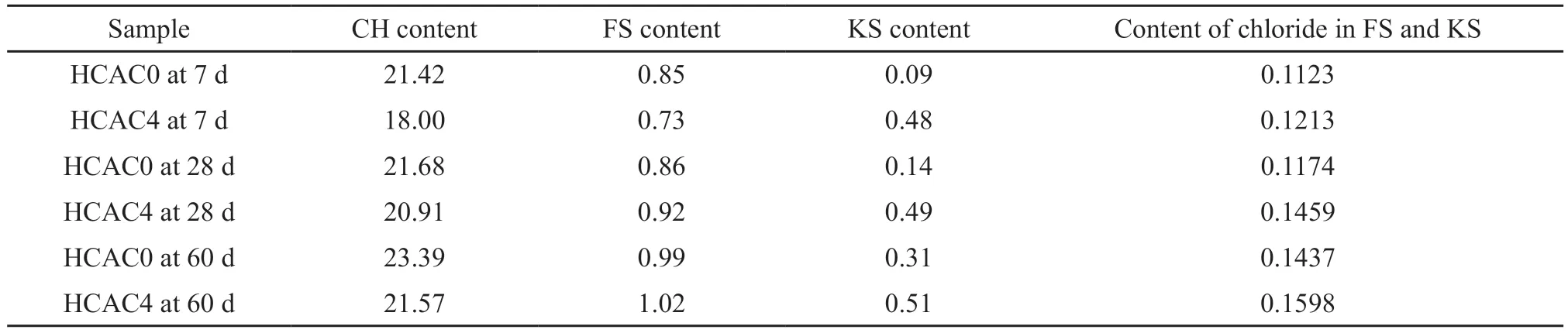
Table 2 The contents of CH, FS, KS, and bound chloride ion/wt%
It could be noted from Fig.6 that the peak intensities of FS and KS changed with the addition of HCAC.It indicated that HCAC increased the FS and KS of the PC-HCAC system at 7, 28, and 60 d.The production of FS and KS might be associated with the enhancement of the chemical binding capacity of the PC-HCAC system.And it presented the same result as the XRD analysis.
To compare the variation of FS and KS content accurately, reference intensity ratio (RIR) analysis was adopted.The XRD patterns in Fig.5 were refined by the Rietveld method.The relative weight ratio of CH and FS/KS could be obtained by semi-quantitative analysis.The weight loss ratios at different temperature ranges could be calculated by TG data.And the CH content in the sample could be calculated from the water weight loss of Ca(OH)2and CaCO3.Then the contents of FS and KS could be obtained according to CH content.The content of bound chloride ion could be gained from chemical formula of FS and KS.And the results for contents of CH, FS, KS and bound chloride ion are shown in Table 2.
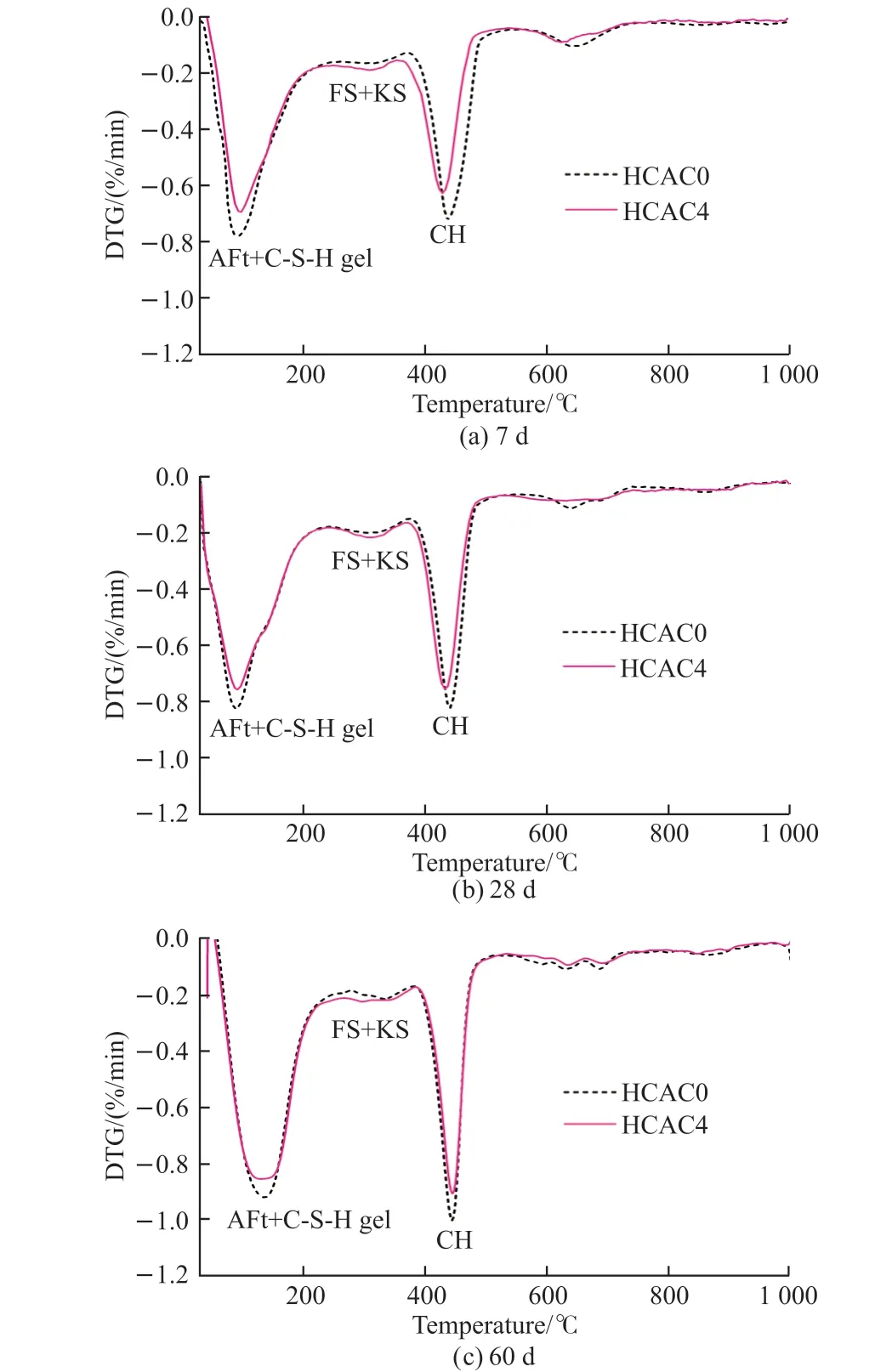
Fig.6 DTG curves of paste samples at different curing ages
It could be observed that the CH content decreased with the additive of HCAC in Table 2, which might be connected with the hydration reaction.At each age, the KS content of HCAC4 sample was significantly increased compared to HCAC0 sample.Furthermore, the amounts of chloride bound by FS and KS in HCAC4 at 7, 28, and 60 d were more than HCAC0,indicating an improvement of chemical binding capacity in HCAC4.
Based on the above discussion, HCAC could increase FS and KS of the PC-HCAC system at each age,which was beneficial to the improvement of chemical binding capacity.
3.2.327Al NMR
Different types of aluminum phases could be analyzed by27Al NMR.In hardened cement pastes, the aluminum phases were generally presented in two coordination types, including octahedral aluminum (AlVI) and tetrahedral aluminum (AlIV).Aluminum atoms located in the AFm-like phrase (chloroaluminates or AFm) at 11.5 ppm and the third aluminum hydrates (TAH) at 6.5 ppm belong to AlVI, while aluminum atoms located in the C-A-S-H gel near 70 ppm belong to AlIV[60,61].

Fig.7 Deconvoluted 27Al NMR spectra of raw cement materials and the hardened cement samples
Fig.7 reveals the27Al NMR spectra of the raw cement materials and the hardened cement paste samples at different ages.The aluminum spectra of portland cement and HCAC are given in Fig.7(a).The peaks of portland cement were close to 80 and 10 ppm while the main peak of HCAC was at 8.4 ppm.Figs.7(b)-7(d)show the deconvoluted27Al NMR spectra of HCAC0 and HCAC4 at 7, 28, and 60 d, respectively.the AlVIpeak positions of HCAC4 sample were 8.9 ppm at 7 d, 8.8 ppm at 28 d and 8.7 ppm, which were all shifted compared to 8.4 ppm of HCAC in Fig.7(a).It indicated that HCAC was involved in chemical reactions after adding to portland cement.The intensity of the AlVIpeak was significantly higher in the HCAC4 compared to the HCAC0 in Fig.7(b), and the same result is demonstrated in Fig.7(c) and Fig.7(d).The results illustrated that the addition of HCAC could influence the content of AFm phase in the cement paste at all ages.Combined with the XRD test results above, the result further confirmed that HCAC participated in the chemical reaction and promoted KS in the system.Furthermore, the AlIVpeak for HCAC4 rarely changed compared to HCAC0 at all ages, indicating that HCAC had little effect on the production of C-A-S-H gel.
In accordance with the above statements, it could be summarized HCAC could increase the KS content in portland cement paste by participating in chemical reactions.
3.3 Physical adsorption
The quantity of C-S-H gels is a dominant factor on the physical adsorption capacity of chloride ions.In this test,29Si NMR was to analyze the quantity of C-S-H gels formed in cement pastes.The29Si NMR spectra of the samples with 0wt% and 4wt% HCAC additives at each age were presented in Fig.8, where three apparent peaksQ0,Q1, andQ2peaks could be observed.TheQ0peak at a chemical shift of 71 ppm was associated with unhydrated portland cement, and the chemical shifts at 79 and 85 ppm correspond toQ1andQ2peaks, respectively, representing the production of C-S-H gels of portland cement hydration products.
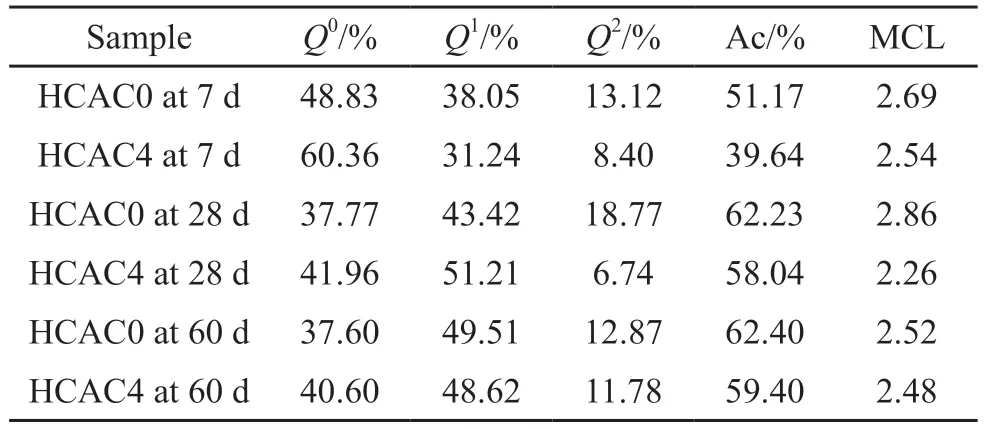
Table 3 Deconvolution results of the samples
The deconvolution results were collated by Fig.8 as shown in Table 3.And the intensity of theQ0peaks of HCAC4 was higher than HCAC0 at all ages.It could also be observed that at 7 d, the hydration degree of HCAC4 decreased significantly from 51.17% to 39.64% in contrast to HCAC0.The hydration degree of HCAC4 was reduced from 62.23% to 58.04% compared to HCAC0 at 28 d.At 60 d, the hydration degree of HCAC4 decreased from 62.40% to 59.40% as opposed to HCAC0.
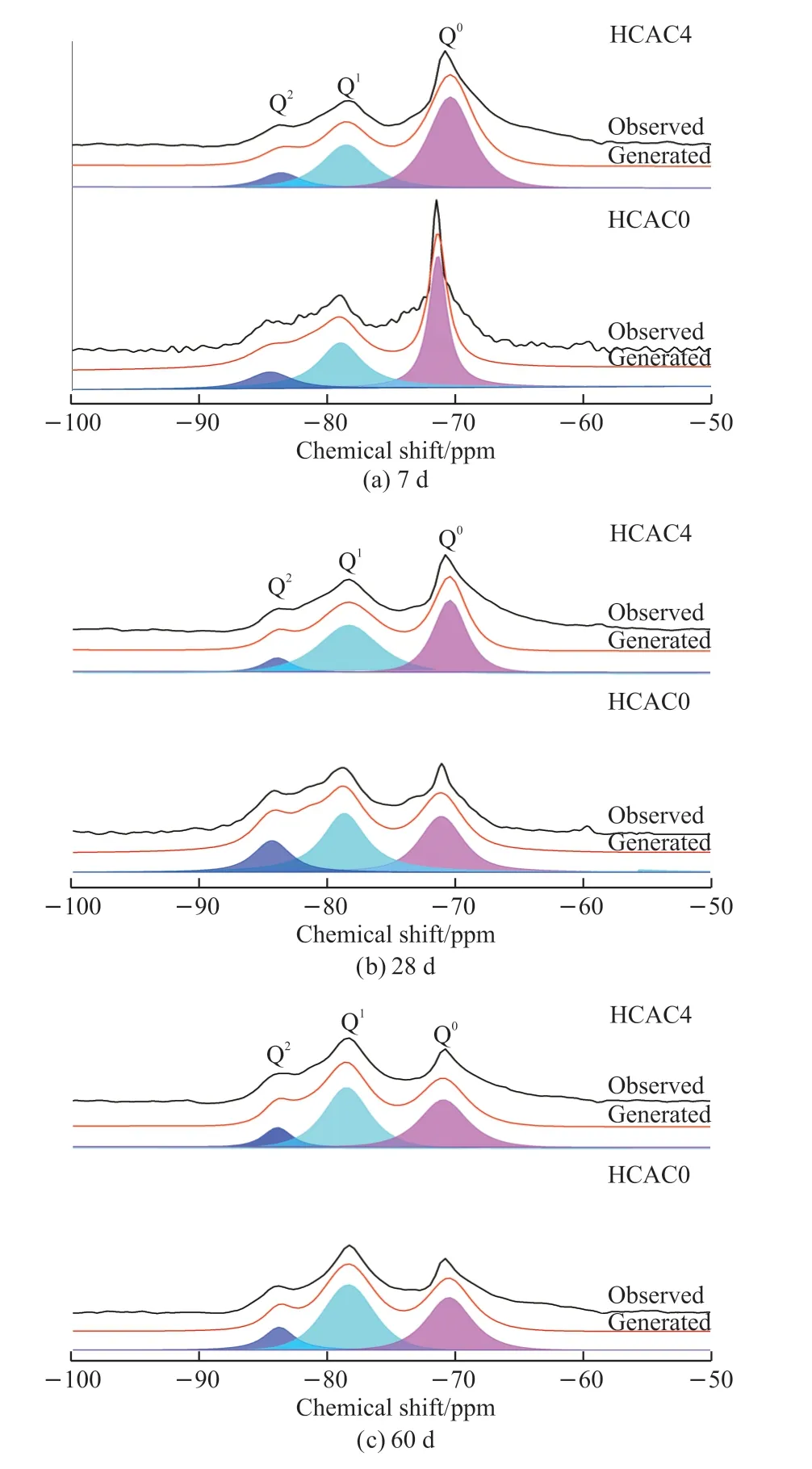
Fig.8 Deconvoluted 29Si NMR spectra of hardened samples
Accordingly, the additive of HCAC caused a decrease in the physical adsorption capacity, which was unfavorable to the chloride immobilization capacity of the PC-HCAC system.In addition, the MCL values of the HCAC4 sample decreased at each age compared to the control groups, which indicated that the additive of HCAC caused the shortening of the sample C-S-H gel main chain length.
Consequently, HCAC was negative on the physical adsorption capacity of chloride ions in portland cement paste.
3.4 Migration resistance
In reinforced concrete, the negative effect of chloride ion on steel bar was carried out by the reaction occurred on its surface[15].Concrete contained abundant pores which provided a fast migration channel for free chloride ions, so the pore structure became a vital factor affecting the migration of free chloride ions[62].The pore size distributions of HCAC0 and HCAC4 samples at 28 d were characterized by MIP tests.The treated results of MIP tests are displayed in Fig.9 and Table 4.

Fig.9 Pore size distribution of HCAC0 and HCAC4 samples cured for 28 d
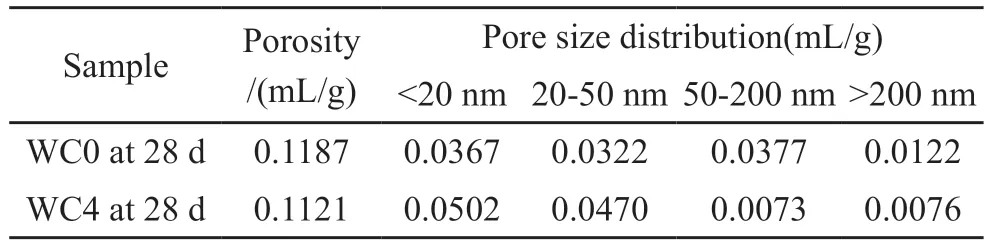
Table 4 Pore size distribution of samples
In Fig.9, the strong peak position of HCAC4 was more concentrated in a small pore diameter than HCAC0, indicating that the pore size of HCAC4 was finer than HCAC0.Meanwhile, the most probable pore size of the HCAC4 sample was 0.0344 μm at 28 d,which was finer than 0.0566 μm of the HCAC0 sample at 28 d.
Table 4 shows the total pore volume of the HCAC4 sample at 28 d was 0.1121 mL/g, which was smaller than 0.1187 mL/g of the HCAC0 sample at 28 d.The pore volume of more-harmful pores (> 200 nm) of the HCAC4 sample decreased to 0.0076 mL/g from 0.0122 mL/g of the HCAC0 sample at 28 d.And the pore volume of harmful pores (50-200 nm)decreased from 0.0377 mL/g of HCAC0 to 0.0073 mL/g of HCAC4 at 28 d.In contrast, the number of the harmless pores (< 20 nm) increased compared to the HCAC0 sample at 28 d.
The above results demonstrated that HCAC4 had more fine pores than HCAC0.It indicated that HCAC adding to portland cement paste could refine the deleterious coarse pores into safe fine pores and enhance the chloride ion migration resistance.
The heterogeneity and complexity of the pore structure of samples could be characterized by the fractal geometry principle.The pore structure surface fractal dimensions (Ds) could be calculated from the MIP test data[52,53], labeled in Fig.10.TheDs-i (Dsin the microscopic region) andDs-a (Dsin the macroscopic region) at 28 d for the HCAC0 and HCAC4 samples are displayed in Figs.10(a) and Fig.10(b).It displays that theDs-i of HCAC4 sample increased from 2.420 to 2.699 and theDs-a increased from 1.782 to 2.003 compared with HCAC0 at 28 d.

Fig.10 Logarithm plots of Wn/rn2 versus Vn(1/3)/rn for HCAC0 and HCAC4 samples at 28 d
It illustrates that HCAC could effectively increase the heterogeneity of the interparticle stacking state and the connection state among the hydration products in the samples.The degree of heterogeneity and disorder in the pore structure could be enhanced.And then the migration of chloride ions in the pore channels could be more arduous.
Based on the above research, HCAC refined and disordered the pore structure of samples, which would enhance the chloride ion resistance migration and thus contribute to the chloride immobilization.
3.5 Setting time
The results of the setting time are presented in Fig.11.The setting time of cement paste was gradually reduced as the HCAC dosage was increased from 0wt%to 4wt%.HCAC4 reduced the initial setting time by 47% compared to HCAC0.And the final setting time of HCAC4 was decreased by 43% than HCAC0.

Fig.11 Setting time of cement paste samples
HCAC could affect the workability of portland cement paste.Especially when the dosage was 4wt%,the initial setting time and final setting time were significantly shortened.It indicated that the addition of HCAC could affect the hydration reaction of portland cement.
3.6 Compressive strength
The compressive strength values of HCAC0,HCAC1, HCAC2, and HCAC4 at 7, 28, and 60 d were obtained by the tests.Fig.12 lists the experimental results, where the compressive strength values of HCAC0 at each age were set to 100% as reference values.Compared with HCAC0, the compressive strength of HCAC1 decreased by 8% at 7 d, 13% at 28 d, and 18% at 60 d.It revealed that the added HCAC affected the compressive strength.The compressive strength reduction of HCAC4 was 24% at 7 d, 24% at 28 d, and 30% at 60 d in comparison with HCAC0.
The addition of HCAC would reduce the strength of hardened portland cement paste, which might be associated with the effect of HCAC on hydration inhibition in portland cement paste.

Fig.12 The compressive strength of hardened paste samples
3.7 Mechanism
Based on the above results, HCAC enhanced the chloride capacity of portland cement paste.In the literature[63], the chloride immobilization could be attributed to a combinative effect of the following three approaches: chemical binding, physical adsorption, and migration resistance.Based on the above discussion, it could be specifically analyzed the mechanism focusing on the three ways.
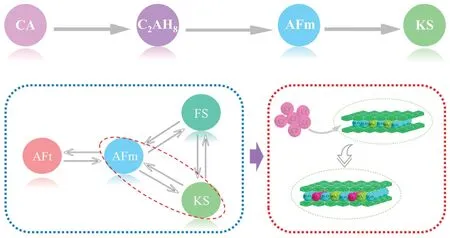
Fig.13 Mechanism of chemical binding in portland cement with the addition of HCAC
Chemical binding.HCAC played a positive role in chloride chemical binding, and the mechanism flow diagram could be summarized in Fig.13.CA was the main mineral phase of CAC.During the wet grinding,the CA in CAC could be fully hydrated and transform into C2AH8in HCAC.It generally had an appropriate amount of gypsum to regulate the working performance in the portland cement.And portland cement paste commonly carried calcium hydroxide on account of hydration.The C2AH8in HCAC would react with the gypsum and calcium hydroxide to generate a large amount of AFm after adding HCAC to portland cement paste.The AFm could combine with Cl-in portland cement paste to produce more KS, thus enhancing the chloride ion chemical binding capacity.In the literature[64,65], the transformation between AFt and AFm phases (AFm, KS, and FS) generally occurred in the chloride chemical binding, as presented in Fig.13.In this paper, the reaction from AFm to KS played the most significant role in the chemical binding.Furthermore, Gu et al had proved the production of AFt while CAH10/C2AH8from hydrates of high alumina cement reacted with gypsum in OPC at the initial age[42].Over time, the gypsum would gradually be consumed.When the sulfate concentration was relatively low, AFt was gradually converted to AFm.Therefore, the increase of AFm in HCAC4 might be caused by the phase transformation between AFt and AFm at the early hydration period.
Physical adsorption.HCAC decreased the C-S-H gel in portland cement paste and played an inhibitory role in terms of chloride ion physical adsorption capacity.Based on the relevant Refs.[39,43], the hydration was probably inhibited in the PC-HCAC system, which might be related to the increase of AFm phase.
Migration resistance.The particle size of HCAC obtained by wet grinding was significantly reduced.And the increased AFm phase in the PC-HCAC system would refine and disorder the pore space structure to enhance the ability of chloride resistance to migration.
4 Conclusions
HCAC as an additive could improve chloride immobilization capacity of portland cement paste and the increased production of KS was the major cause.HCAC refined the pore structure but decreased the C-S-H gel in portland cement samples.
Conflict of interest
All authors declare that there are no competing interests.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Flow Characteristics Analysis of TC18 Titanium Alloy during Hot Deformation Based on Phase Transformation
- Expansion Performance and Microstructure of High-performance Concrete using Differently Scaled MgO Agents and Mineral Powder
- Effect of Curing Age on Tensile Properties of Fly Ash Based Engineered Geopolymer Composites (FA-EGC) by Uniaxial Tensile Test and Ultrasonic Pulse Velocity Method
- A Fiber Optic Sensor for the Simultaneous Measurement of Dual-parameter Based on Hydrogelimmobilized Enzyme Complex
- Energy-dispersive X-ray Spectroscopy for the Quantitative Analysis of Pyrite Thin Specimens
- Bioprocess-inspired Actin Biomineralized Hematite Mesocrystals for Energy Storage
