Bioprocess-inspired Actin Biomineralized Hematite Mesocrystals for Energy Storage
2024-01-03XUWeiZHAOChaoXIEJingjingWANGRongjie
XU Wei, ZHAO Chao, XIE Jingjing, WANG Rongjie
(1. Tianjin Institute of Power Sources, Tianjin 300384, China; 2. CETC LAN TIAN Technology Co., Ltd., Tianjin 300384, China; 3. State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan 430070, China)
Abstract: Biomineralization is a biological process of synthesizing inorganic minerals within organisms.It has been found that intracellular proteins are involved in the room temperature synthesis process of anatase TiO2 in living mussels.Here, we used intracellular actin to synthesize hematite by biomineralization.Biomineralized hematite has a nano spindle structure with a particle size of approximately 150 nm.The microstructure indicates that the prepared hematite is a mesocrystals composed of ordered arrangement and assembly of primary nanoparticles.In addition, hematite mesocrystals exhibit good lithium storage performance as electrode materials for lithium batteries.The discharge specific capacity of the battery remained at 560.7 mAh·g-1 after 130 cycles at a current density of 200 mA·g-1.This work expands the synthesis methods of hematite by biomineralization, and provides a new strategy for preparing inorganic materials by intracellular proteins.
Key words: actin; hematite; biomineralization; mesocrystals; lithium battery
1 Introduction
Inspired by the formation process of natural materials, bioprocess-inspired fabrication of materials has been developed in recent years[1-4].Biomineralization is a typical biological process that has been widely studied[5,6].The inorganic minerals or inorganic-organic composite materials were synthesized by biomineralization within an organism[7].Living organism acted as a synthesis factory, and secreted a large amount of biomass[8].Biominerals with exquisite structures or unique functions were prepared through ion transport, self-assembly, and other methods within living organism[9,10].By learning how natural organisms guide the synthesis of biominerals, innovative methods can be developed for material fabrication[11-13].
In recent years, functional inorganic materials have been successfully synthesized in living organisms by using natural biological platforms[14,15].Chiet alused living mussels as a natural mineralization platform to synthesize SnO2/graphene oxide composite materials with improved lithium storage performance[16].Xieet alutilized living mussels to mineralize nitrogen doped anatase TiO2with hierarchical porous structure and higher photocatalytic activityin vivo[17].A large number of intracellular proteins, such as actin, have been found in mineralized products, which were involved in the synthesis of inorganic materials[17].However,the regulatory mechanism of intracellular proteins on the formation process of inorganic materials has not been studied yet.Therefore, in order to broaden our understanding of the field of biomineralization,it is necessary to explore the synthesis of inorganic materials by intracellular proteinsin vitro.
Hematite, as a natural mineral, has properties of such as light, electricity, and magnetism, and is widely used in fields of energy, environment, and biomedicine[18-21].Hematite is considered a promising electrode material for lithium-ion batteries due to its high theoretical capacity, extensive sources, and environmental friendliness[22,23].Existing research has synthesized hematite through various extracellular proteins[24,25].For example, Feiet alutilized silk fibroin prepared nanoporous α-Fe2O3mesocrystals with tunable hierarchical nanostructures[26].Heet alused collagen to synthesize α- Fe2O3mesocrystals by hydrothermal method at 160 ℃[27].However, research on the synthesis of hematite through intracellular proteins based biomineralization is limited.
In this work, inspired by the involvement of intracellular proteins in the synthesis of inorganic materials in living organisms, we developed a method for preparing α-Fe2O3mesocrystals by actin based biomineralization.The regulation of actin on the morphology of hematite and the phase transition process of α-Fe2O3were studied.The results indicate that the prepared hematite has a nano spindle structure with a particle size of approximately 150 nm.Besides,biomineralized α-Fe2O3is a mesocrystals composed of ordered arrangement and assembly of primary nanoparticles.Finally, the α-Fe2O3was applied to the negative electrode material of lithium batteries.The discharge specific capacity of the battery remained at 560.7 mAh·g-1after 130 cycles at a current density of 200 mA·g-1.This work expands the preparation method of hematite by biomineralization.
2 Experimental
2.2 Materials
Ferric chloride (98% pure, Alfa Aesar).Urea(>99.3% pure, Alfa Aesar).Deionized water was selfmade in the laboratory.Actin was extracted from fresh chicken breast meat using the Spudich and Watt method.
2.2 Biomineralization synthesis of α-Fe2O3
0.01 mol FeCl3and 0.01 mol urea were dissolved in 10.0 mL distilled water with vigorous stirring for 30 min to form a homogeneous solution.Then, different concentrations of actin were added to the solution and stirred for 30 min.The mixture solution was transferred into a Teflon-lined stainless-steel autoclave.The autoclave was heated to 150 ℃ and maintained for 24 h and then cooled to the room temperature.The final biomineralized product was isolated by centrifugation and washed with deionized water, then dried in a lyophilizer.
2.3 Characterization
X-ray diffraction (XRD) data were got on a PANalytical X’Pert Pro instrument with a Cu Kα line source.The microscopic morphology of products was observed by using a scanning electron microscope(SEM, Hitachi, Regulus-8230).The microstructure and crystal structure of products were characterized by using a high-resolution transmission electron microscope (HRTEM, Talos, F200S).
2.4 Lithium storage performance tests of α-Fe2O3
The α-Fe2O3was assembled into a CR2025-type coin cells.Lithium metal foil was used as the counter electrode and reference electrode.First,N-methyl-2-pyrrolidone (NMP, Aladdin, China) slurry of α-Fe2O3was mixed with Super P carbon black and polyvinylidene fluoride in a weight ratio of 7:2:1.After adequate grinding, the resultant slurry was coated on Cu foil and dried under vacuum at 120 ℃ for 12 hours.The mass loading of each electrode was about 1.2-1.8 mg.Coin cells were assembled in a glovebox filled with pure argon gas, and 1 M lithium hexafluorophosphate in ethylene carbonate (EC)/diethyl carbonate(DEC) (1:1v/v) was used as the electrolyte solution.Celgard polypropylene was used as the separator.Galvanostatic charge/discharge measurement was performed by a multichannel battery testing system(LAND CT2001A) with a voltage window of 0.01-3 V(vsLi+/Li) at various current densities.
3 Results and discussion
3.1 Characterization of actin biomineralization α-Fe2O3 mesocrystals
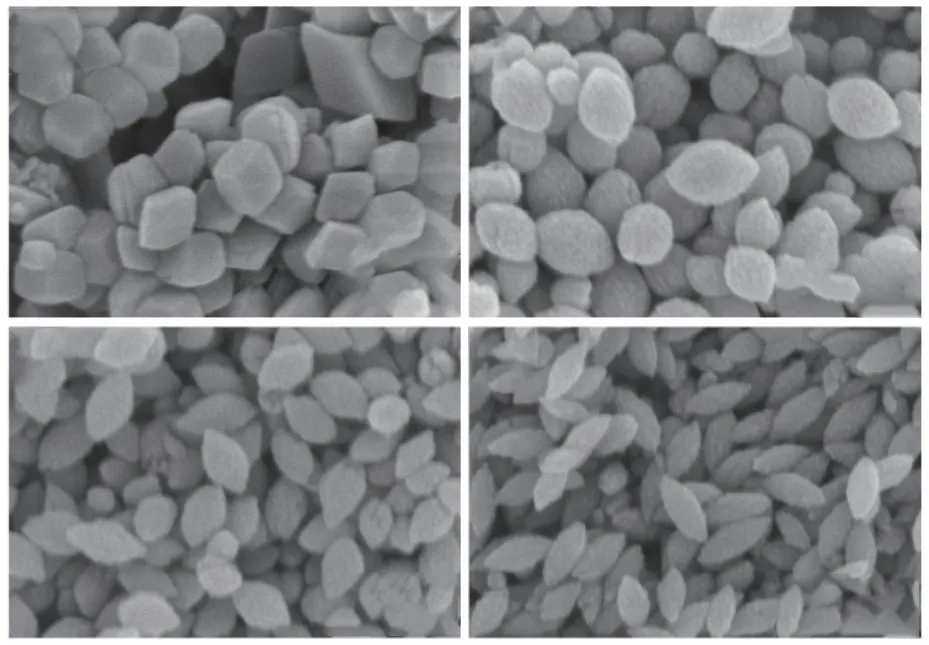
Fig.1 SEM images of biomineralized products with different concentrations of actin: (a) 0 μg/mL; (b) 300 μg/mL; (c) 600 μg/mL; (d) 900 μg/mL
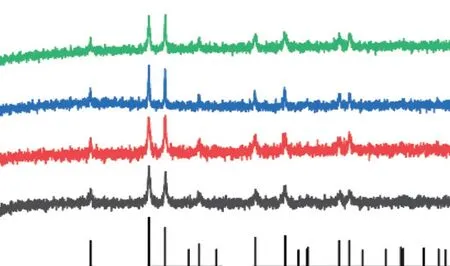
Fig.2 XRD patterns of biomineralized products with different concentrations of actin

Fig.3 SEM images of biomineralized products at different mineralization time: (a) 0.5 h; (b) 1 h; (c) 2 h; (d) 4 h; (e) 8 h; (f) 12 h
Ferric chloride was used as an iron source.Urea provided alkaline conditions during thermal decomposition, which can promote the hydrolysis of iron ions in the solution.Actin was extracted from fresh chicken breast meat and used to regulate the synthesis of hematite.Hematite was synthesized by hydrothermal method at 150 ℃.Microscopic morphology of mineralized products was observed by scanning electron microscopy.As shown in Fig.1(a),after 24 hours of mineralization, the product without actin has a rhombohedral structure.When adding actin at a concentration of 300 μg/mL, the mineralized products begin to transform into ellipsoidal particles at 200 nm, as shown in Fig.1(b).As the concentration of actin increases to 600 μg/mL, the elliptical particle size begins to decrease, as shown in Fig.1(c).When the concentration of actin increases to a concentration of 900 μg/mL, the prepared hematite has a nano spindle structure with a particle size of approximately 150 nm.The results show that as the concentration of actin increased, the mineralized hematite transformed from rhombohedral to spindle shaped structure, and the particle size gradually decreased.This can be attributed to the interaction between actin and hematite, which inhibits particle growth.As shown in Fig.2, the XRD patterns correspond to the standard PDF card (# 33-0664), indicating that the phase of mineralized products is single α-Fe2O3phase.
In addition, When the actin content was 900 μg/mL, the phase transition process of mineralized products was studied, as shown in Fig.3 and Fig.4.When the mineralization time is 0.5 h, the product is a singleβ-FeOOH with a nanorod structure, as shown in Fig.3(a).When the reaction time is extended to 1 h,the mineralized product is a mixture ofβ-FeOOH and α-Fe2O3, as shown in Fig.3(b).After 2 hours of reaction,the product is a single α-Fe2O3with an ellipsoidal structure, as shown in Fig.3(c).With the mineralization time prolongs, the grain gradually grows, and the particle size gradually increases, as shown in Figs.3(d)-3(e).The final mineralized product is a singleα-Fe2O3spindle with a particle size of approximately 150 nm,as shown in Fig.3(f).
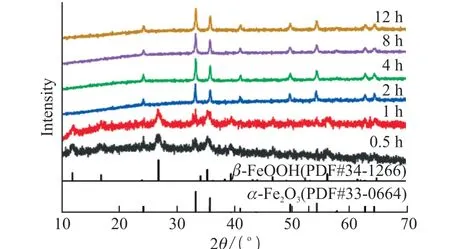
Fig.4 XRD patterns of biomineralized products at different mineralization times
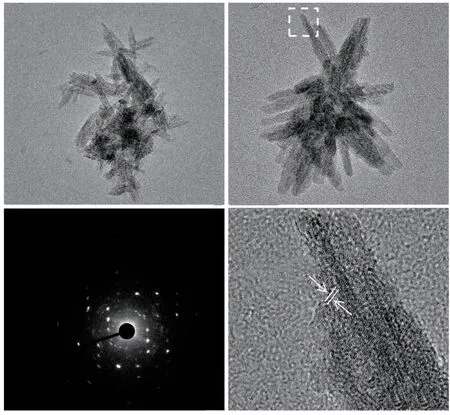
Fig.5 (a), (b) TEM images of products with a mineralization time of 0.5 h; (c) Electron diffraction pattern; (d) HRTEM image of products
The microstructure of the material was further observed using a transmission electron microscope.As shown in Fig.5(a), after mineralization for 0.5 h, the product is a nanorod with a length of 100 nm.Fig.5b shows that nanorods are stacked together to form particles.The electron diffraction pattern indicates the presence of polycrystalline diffraction in the particles,as shown in Fig.5(c).In addition, the diffraction spot indicates an ordered crystal structure within the particles.As shown in Fig.5(d), the (400) crystal plane ofβ-FeOOH nanorods can be observed in the HRTEM image.
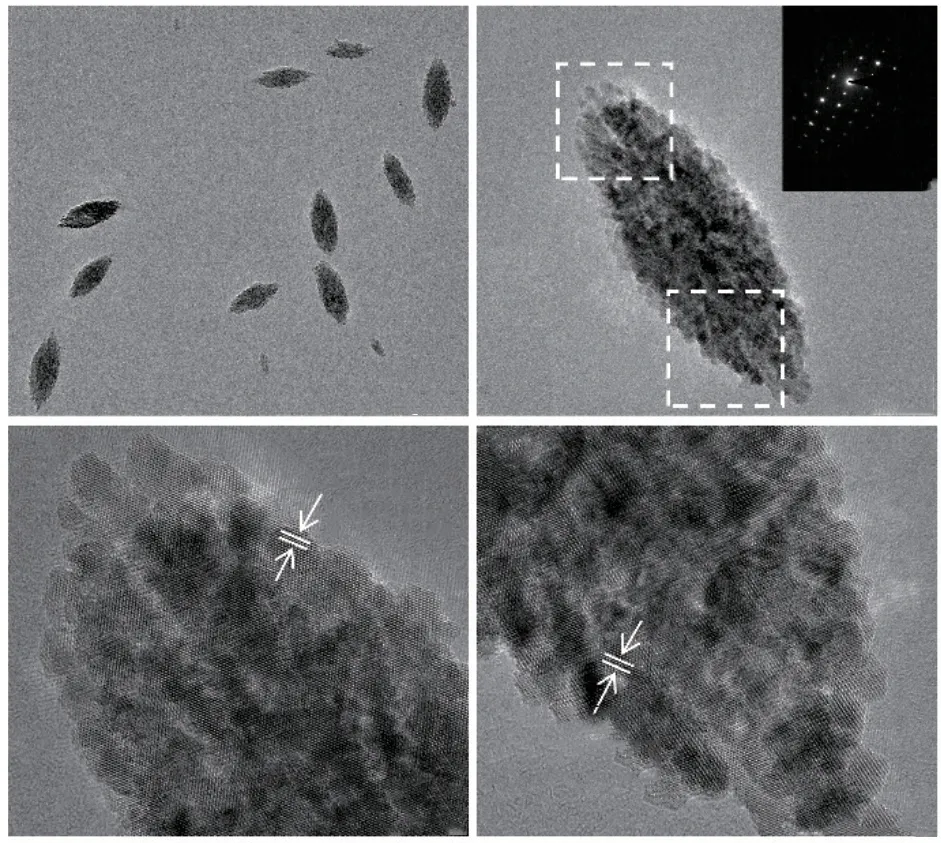
Fig.6 (a), (b) TEM images of products with a mineralization time of 24 h; (c), (d) HRTEM images of products
After 24 h of mineralization, the product is a nano spindle particle with a particle size of approximately 150 nm, as shown in Figs.6(a).Fig.6(b) shows that a singleα-Fe2O3particle is assembled from smaller primary nanoparticles.The electron diffraction in the illustration shows single crystal electron diffraction spots.The results indicate a highly ordered arrangement of primary nanoparticles within theα-Fe2O3crystal,which is a mesocrystal.Besides, the HRTEM images show the both ends microstructure of a singleα-Fe2O3crystal, as shown in Figs.6(c)-6(d).The results indicate that the crystal planes at different positions are (012)crystal planes ofα-Fe2O3.The crystal orientation is consistent.Mineralized hematite is a mesocrystal formed by ordered arrangement and assembly of smaller primary nanoparticles.
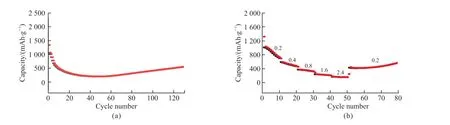
Fig.8 (a) Cycling performance at current density 200 mA·g-1; (b) Rate capability
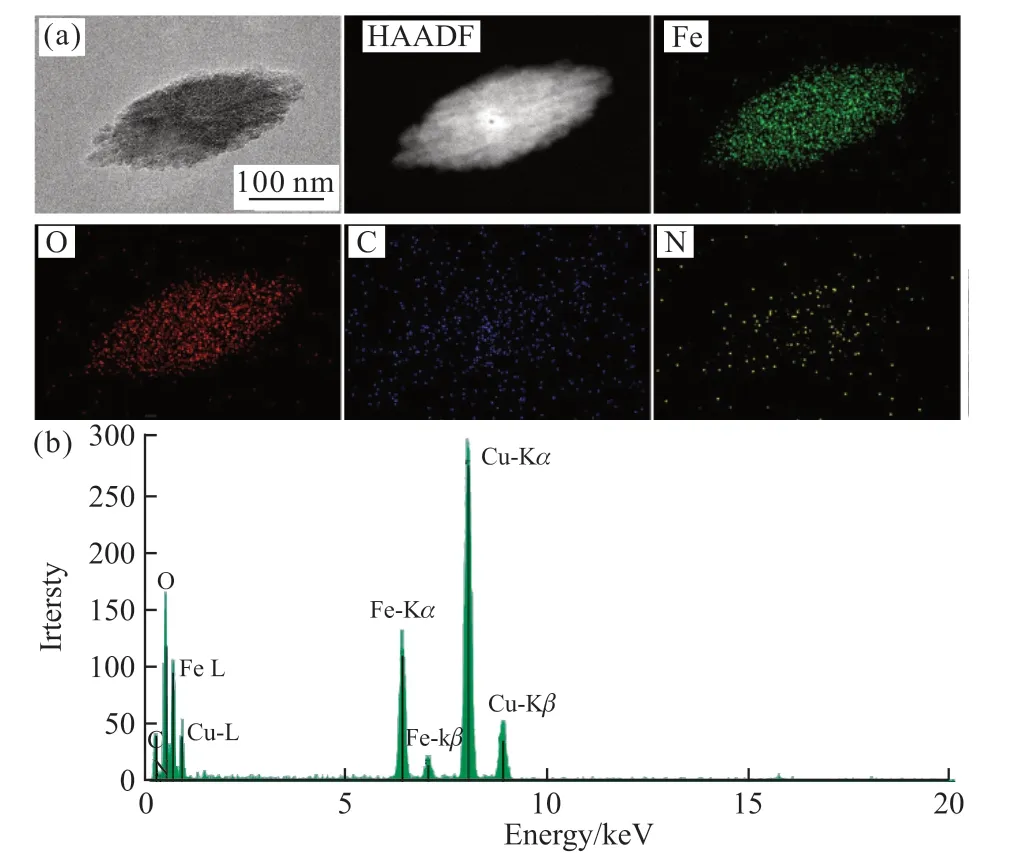
Fig.7 (a) TEM images and elemental maps of α-Fe2O3; (b) EDX elemental mappings of α-Fe2O3
Fig.7(a) shows the elemental distribution ofα-Fe2O3particles.Fig.7(b) shows the EDX elemental mappings ofα-Fe2O3.The results indicate that Fe,O, C, and N elements are uniformly distributed inα-Fe2O3.Actin was involved in the formation ofα-Fe2O3mesocrystals.This may be due to the attachment of actin to the surface of primaryα-Fe2O3nanoparticles during the mineralization process, reducing the surface energy and stabilizing small nanoparticles in solution.Afterwards, the primaryα-Fe2O3nanoparticles were orderly assembled and gradually grew, formingα-Fe2O3mesocrystals.
3.2 Electrochemical performance of artin biomineralized α-Fe2O3 mesocrystals
The mineralized hematite was assembled as negative electrode materials for lithium-ion battery.The cycling performance and rate capability were tested.Fig.8(a) shows the cycling performance of the electrode at a current density of 200 mA·g-1.The discharge specific capacity of the battery remained at 560.7 mAh·g-1after 130 cycles.Fig.8(b) shows the rate capability under different current densities.The results indicate that the α-Fe2O3electrode can maintain good specific capacity.When the current density returns to 200 mA ·g-1, the specific capacity recovers to 562.9 mAh·g-1.Biomineralizedα-Fe2O3exhibits good electrochemical reversibility.This may be due to the mesocrystal structure ofα-Fe2O3, which makes it have good lithium storage performance
4 Conclusions
In summary, we successfully synthesizedα-Fe2O3mesocrystal by actin base biomineralization.The microstructure ofα-Fe2O3can be regulated by changing the concentration of actin.When the concentration of actin is 900 μg/mL, theα-Fe2O3has a spindle structure with a particle size of approximately 150 nm.Biomineralizedα-Fe2O3mesocrystal is composed of ordered arrangement and assembly of primary nanoparticles.In addition, theα-Fe2O3exhibits good lithium storage performance as an electrode material for lithium battery.This method develops the preparation of hematite by biomineralization.This work provides a pathway for the biomineralization of inorganic materials by intracellular proteins.
Conflict of interest
All authors declare that there are no competing interests.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Flow Characteristics Analysis of TC18 Titanium Alloy during Hot Deformation Based on Phase Transformation
- Effect of Hydrated Calcium Aluminate Cement on the Chloride Immobilization of Portland Cement Paste
- Expansion Performance and Microstructure of High-performance Concrete using Differently Scaled MgO Agents and Mineral Powder
- Effect of Curing Age on Tensile Properties of Fly Ash Based Engineered Geopolymer Composites (FA-EGC) by Uniaxial Tensile Test and Ultrasonic Pulse Velocity Method
- A Fiber Optic Sensor for the Simultaneous Measurement of Dual-parameter Based on Hydrogelimmobilized Enzyme Complex
- Energy-dispersive X-ray Spectroscopy for the Quantitative Analysis of Pyrite Thin Specimens
