A Fiber Optic Sensor for the Simultaneous Measurement of Dual-parameter Based on Hydrogelimmobilized Enzyme Complex
2024-01-03TONGYilinZHANGYuHANXuecaiYUKanBAOJiaqi
TONG Yilin, ZHANG Yu, HAN Xuecai*, YU Kan, BAO Jiaqi
(1. Faculty of Information Science and Technology, Wenhua College, Wuhan 430074, China; 2. Hubei Institute of Measurement and Testing Technology, Wuhan 430223, China)
Abstract: A novel fiber optic sensor based on hydrogel-immobilized enzyme complex was developed for the simultaneous measurement of dual- parameter, the leap from a single parameter detecting fiber optic sensor to a fiber optic sensor that can continuously detect two kinds of parameters was achieved.By controlling the temperature from high to low, the function of fiber sulfide sensor and fiber DCP sensor can be realized, so as to realize the continuous detection of dual-parameter.The different variables affecting the sensor performance were evaluated and optimized.Under the optimal conditions, the response curves, linear detection ranges, detection limits and response times of the dual-parameter sensor for testing sulfide and DCP were obtained, respectively.The sensor displays high selectivity, good repeatability and stability, which have good potentials in analyzing sulfide and DCP concentration of practical water samples.
Key words: hydrogel-immobilized enzyme complex; dual- parameter; simultaneous measurement; fiber optic sensor
1 Introduction
Chlorinated phenols such as 2-chlorophenol(2-CP), 4-chlorophenol (4-CP) and 2,4-dichlorophenol(DCP) are widely used in the manufacturing of pharmaceuticals, resins and plastics, coal refineries,painting, pulp and paper, pesticides, herbicide and even from food processing industries[1-3].2,4-dichlorophenol(DCP) has drawn the attention since it is a precursor for the synthesis of carcinogenic endocrine,2,4-dichlorophenoxyacetic acid, which is the active ingredient of more than 1500 herbicides.The toxicity linked to DCP exposure has been evidenced to cause endocrine related cancers and chronic conditions such as chloracne and porphyria in humans[4,5].Phenolic wastewater usually contains sulfide, which is highly toxic and corrosive, and even endanger human life safety[6].Due to the toxic properties, DCP and sulfide are important indicators for the detection of wastewater discharge standards in various industries[7,8], and the detections of DCP and sulfide in water or soil have an important significance to human health and natural environment[9,10].
In current studies, the detections of DCP and sulfide are mostly independent, several methods have been used including the gas chromatography[11,12],flow injection analysis[13], HPLC[14], photocatalysis[15],electrode[16]and electrochemical sensor[17,18].However,these methods have many deficiencies, such as high cost, complicated sample preparation, difficult operation and impossibility of online detection[19,20].Recently, Huyan’s group reported that the two parameters can be detect simultaneously by flow injection analysis method (FIA)[24].Based on the spectrophotometry method and with the help of iFIA7 automatic flow injection analyzer, this method has improved the detection limit, accuracy and recovery rate.However, its complex operation, high cost and limited detection range limit its application.The fiber optic sensors have many advantages, such as resist disturbance, high selectivity, long distance sensing and low cost, especially can be used for on-line and real time detection[21,22].Therefore, the fiber optic sensors have a bright application prospect[23].
Temperature-sensitive intelligent hydrogels are a kind of hydrogels that can respond to temperature stimulation.When the ambient temperature changes,the volume of temperature-sensitive hydrogels can undergo reversible “swelling-contraction”[25,26], which makes hydrogels have a unique use in temperaturecontrolled molecular release[27,28].In this work we prepared and characterized hydrogel-immobilized enzyme complex consisting both temperature-sensitive hydrogel(PNIPAAm) and catalyst immobilized enzyme(laccase) of DCP, and designed and fabricated a twoparameter fiber optic sensor based on the hydrogelimmobilized enzyme complex.By changing the detection temperature, the catalytic behavior of the DCP and sulfide can be controlled, and the continuous detection of the two parameters to be tested can be realized at different temperatures.And the effects of different conditions, such as pH, catalyst concentration and temperature on the sensor performance were investigated.Under the optimal conditions, the response curves, linear detection ranges, detection limits and response time of the dual-parameter sensor for testing sulfide and DCP were obtained, respectively.The fiber optic sensor shows good repeatability,selectivity and stability, and the detection results of real samples by using this sensor indicate a promising application prospect.We proposed the fiber optic sensor of two parameters expanded the research field of detection technology, which can be further applied to the detection of some important chemical quantities and biomass in the environment and human body.
2 Experimental
2.1 Reagents and apparatus
SiO2was synthesized and purified by Stober method according to the Ref.[29].Laccase was purchased from Aladdin.Manganous sulfate (MnSO4), 3-aminopropyl trihexaoxilane(APTS), Glutaraldehyde, Disodium phosphate dodecahydrate(Na2HPO4Ÿ12H2O), Sodium dihydrogen phosphate (NaH2PO4), N-isopropyl acrylamide(NIPA),N,N’-methylene diacrylamide(BIS), Nitrogen(N2),Tetramethylethylenediamine (TEMD), Ammonium persulfate(APS) were obtained from Shanghai Chemical Reagent Company.Sulfide standard solution(No GSB07-2733-2022) was purchased from Standard Sample Institute, Ministry of Environmental Protection.All the reagents were of analytical grade and used without further purification.
A lock-in amplifier (SR830, Standford Research Systems, USA) was used for measuring the phase delay of the sensor head.The IR spectrum was taken onto a FT-IR Spectrometer (60SXB, USA).The morphological characterization was evaluated by JSM-5600 FESEM.
2.2 Preparation of hydrogel-immobilized enzyme complex
4 mL of freshly prepared 2% (V/V) APTS solution was added to the beaker and magnetically stirred for 20 min to fully hydrolyze.34 mg SiO2was added to the solution and continued to stir at 25 ℃ for 24 h, and the activated SiO2nanoparticles were repeatedly washed with distilled water to remove the unreacted APTS.The washed activated SiO2nanoparticles were redispersed in 4 mL PBS (0.1 M, pH 7.0).A certain amount of 2% (V/V) glutaraldehyde solution was added into the amino-functionalized SiO2nanoparticle sol before being magnetically stirred at 25 ℃ for 1.5 h.After the reaction, the activated SiO2nanoparticles were washed repeatedly with PBS (0.1 M, pH 7.0) for several times.After centrifugation, glutaraldehyde activated SiO2nanoparticles were obtained.The activated SiO2nanoparticles were redispersed in PBS (0.1 M, pH 7.0),and 2.0 mg laccase was added before being crosslinked for 12 h in a 4 ℃ constant temperature shaker.After centrifugation, the immobilized laccase was washed three times with PBS (0.1 M, pH 6.5) to remove the unfixed free laccase.The immobilized laccase was redispersed in PBS (0.1 M, pH 7.0) and placed at 4 ℃for standby.
200 mg NIPA and 10 mg BIS were dissolved in deionized water before being magnetically stirred, and N2was injected into the water solution for more than 30 minutes to remove the dissolved oxygen in the water.8 mg SiO2immobilized laccase and 20 μL TEMD were added to the above transparent mixed solution,and the solution was magnetically stirred for 1 min to make it disperse evenly.8 mg APS was added, after continuing the reaction for 24 h at 20 ℃, hydrogel-SiO2nanoparticles immobilized laccase was obtained.The complex was immersed in deionized water for more than 72 h, during this time the immersion solution was changed every 3-5 h to remove unreacted raw materials and other impurities.
2.3 Preparation and principle of dualparameter fiber optic sensor
The experimental device of dual-parameter fiber sensor is shown in Fig.1, including phase-locked amplifier, a LED with the excitation wavelength of 416 nm as the light source, sensor probe fixed with optical oxygen sensitive membrane, temperature control installation and a computer for data processing.The composite membrane was prepared according to our previously work[30].
The sensor is based on the oxygen consumption and fluorescence quenching to realize the simultaneous measurement of sulfide and DCP.Catalyzed by MnSO4and laccase, sulfide and DCP in the solution can be separately oxygenated, which deplete the oxygen in the solution.When raising the reaction temperature to make it higher than the lower critical solution temperature(LCST=32 ℃) of the hydrogel, the hydrogel is in a tight state, the laccase in the hydrogel is insulated with the DCP, and MnSO4in the solution catalyzes the oxidation of sulfide.When sulfide in solution after being completely oxidized, the reaction temperature is lowered below the LCST of the hydrogel, at which point the hydrogel bulges, the laccase in the hydrogel can contact DCP and catalyze oxidation of DCP.In this way, by controlling the temperature from high to low,the function of fiber sulfide sensor and fiber SCP sensor can be realized, so as to realize the continuous detection of dual-parameter.The change of oxygen concentration is detected by measuring fluorescence of Ru(bpy)3Cl2quenched by oxygen.Since a lock-in amplifier is used,the quchening can be described as
where,φ0andφare the phase delay change of the sensor in the absence and presence of the oxygen quencher, respectively;Ksvthe Stern-Volmer constant;and [Q] the oxygen concentration.Sinceφis very small(i e<5o) in the experiment, tanφ≈φ.By collecting the data of phase delayφin two oxidation processes, the concentrations of sulfide and DCP are achieved.
2.4 Measurements
The sensor detection system was used to detect sulfide and DCP at different temperatures as shown in Fig.1.The probe of the sensor was placed in a reaction cell containing hydrogel-SiO2nanoparticles immobilized laccase, MnSO4, sulfide, and DCP solutions.The reaction cell was sealed to prevent oxygen from entering the reaction cell and affecting the results of detection.A temperature control device was used to adjust the temperature of the reaction cell.The temperature of the reaction cell was adjusted to 40 ℃ (higher than the LCST of hydrogel) in the first place, the data of phase delayφwas recorded when the sensor’s probe signal was stable, so concentration of sulfide can be measured.Then the temperature of the reaction cell was reduced to 25 ℃ (lower than the LCST of hydrogel), the data of phase delayφ' was recorded once the sensor’s probe signal stabilized,which determined the concentration of DCP.Each concentration was measured three times.
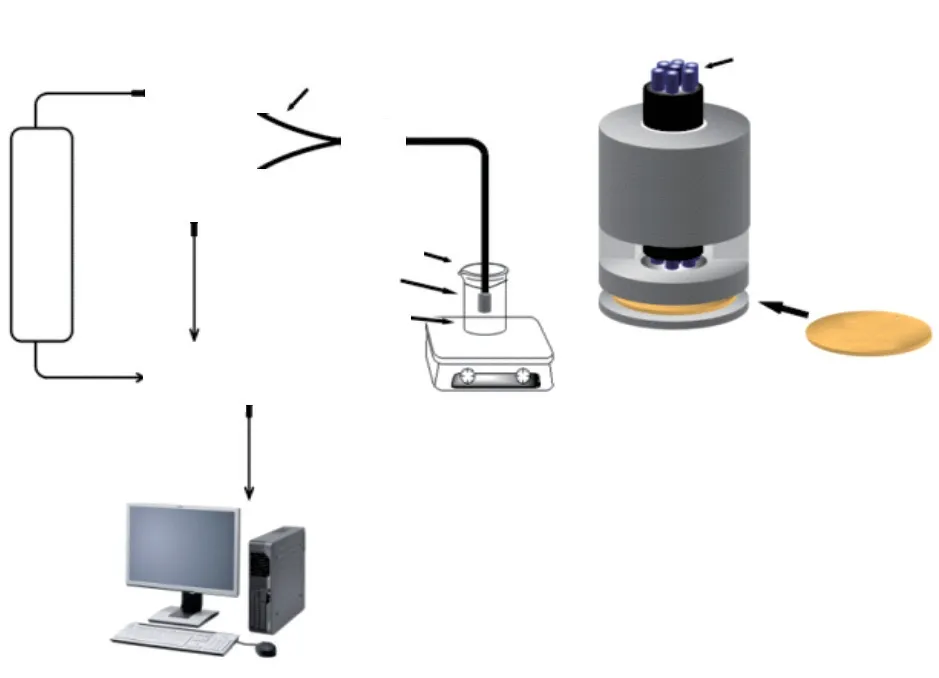
Fig.1 (a) Schematic representation of the dual-parameter fiber optic sensor; (b) The model of sensor head in its testing region
3 Results and discussion
3.1 Structure of hydrogel-SiO2 nanoparticles immobilized laccase
The typical transmission IR spectra of hydrogels are shown in Fig.2, Fig.2(a) is spectral line represented PNIPAAm hydrogel.A broad peak at 3 200-3 600 cm-1was attributed to N-H and O-H contractive vibration, the characteristic peak at 2 900 cm-1was C-H stretching vibration peak, the characteristic peak at 1 649 cm-1was attributed to carbonyl stretching vibration, the absorption peak at 1 548 cm-1showed N-H flexural vibration, and absorption peaks at 1 460 and 1 388 cm-1showed -CH(CH3)2absorption.In Fig.2(b), spectral line represented PNIPAAm hydrogel-SiO2nanoparticles immobilized laccase, compared with the absorption peaks at the same position in Fig.2(a),its peak values were enhanced, and the peaks were deformed sharply.In addition to the above peaks, the absorption peak at 830-910 cm-1showed the existence of Si-O stretching vibration, the absorption peak at 800 cm-1was stretching vibration peak of Si-O as well.The characteristic peak at 740-870 cm-1was Si-C stretching vibration peak, and the absorption peak at 1 069 cm-1was Si-O-Si absorption peak.These peaks proved that SiO2nanoparticles immobilized laccase has been synthesized in hydrogel.
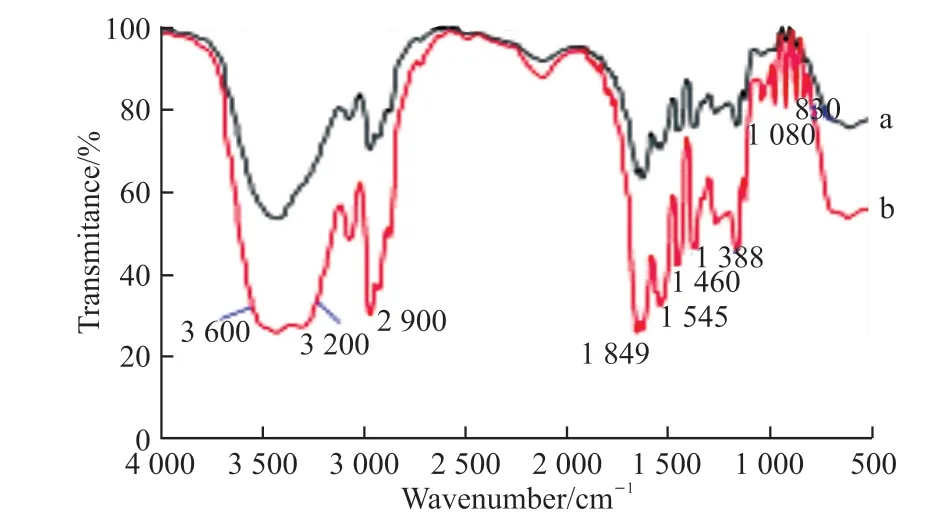
Fig.2 IR spectra of hydrogels
In order to observe the surface structure of the complex, scanning electron microscopy (SEM) was employed.Fig.3(a) is the SEM image of the crosssection of hydrogel without laccase.It can be seen that the inside of the hydrogel presents three-dimensional network hole structure, and most of the holes are connected to each other.Fig.3(b) shows the SEM image of cross-section of hydrogel-SiO2nanoparticles immobilized laccase.As shown in the figure, the SiO2nanoparticles immobilized laccase was existed on the inner wall of three-dimensional network pore,indicating that SiO2nanoparticles immobilized laccase were successfully fixed in the hydrogel.
3.2 Effect of different variables on fiber optic sulfide sensor
Our previous work documented that the different variables affect the sensor performance[31].For a fiber optic sulfide sensor,φis defined as the difference of phase delay shift when the sensor probe placement in a sulfide concentration is between 1.0×10-5and 0 mol/L.
pH influence on the phase delay change of fiber optic sensor was investigated in the range of pH 3.0-11.0 by using different buffer solution.As it could be noticed in Fig.4(a), the phase delay change of fiber optic sensor significantly increased with pH from 3.0 to 7.0, the maximal values ofφwere observed at pH 7.0-9.0.pH 7.0 was selected for our subsequent investigations.
The effect of catalyst MnSO4concentration on the change of phase delay is investigated (Fig.4(b)).The change of phase delayφincreased with the increasing concentrations of MnSO4added from 1.0×10-6to 5.0×10-6mol/L.The further increasing in MnSO4concentration could not obviously enhance change of phase delay.Therefore, 5.0×10-6mol/L of the catalyst was recommended for the sensor detection.
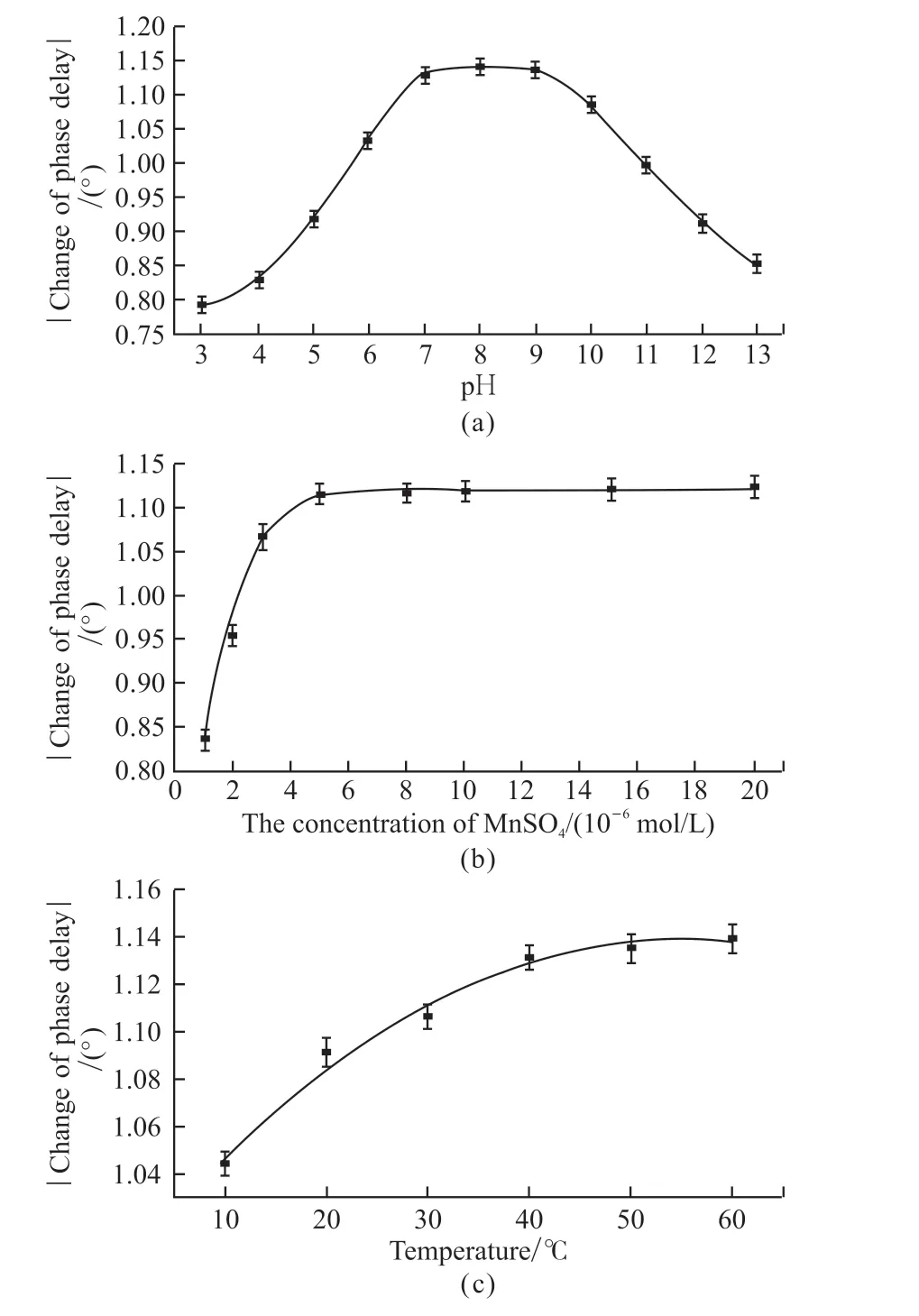
Fig.4 (a)Effect of pH on the fiber optic sulfide sensor (T = 40℃, MnSO4 concentration was 5.0×10-6 mol/L); (b)Effect of MnSO4 concentration on the fiber optic sulfide sensor(pH=7.0, T = 40 ℃); (c)Effect of temperature on the fiber optic sulfide sensor (pH=7.0, MnSO4 concentration was 5.0×10-6 mol/L)
MnSO4catalyzed reaction will be significantly influenced by temperature.Therefore, the effect of temperature on the phase delay change of fiber optic sensor was investigated as shown in Fig.4(c).It could be seen that as the temperature increased from 10 to 40 ℃, the phase delay changes of fiber optic sensor increased significantly.When the temperature continued to increase to 60 ℃, change of phase delay could not obviously enhance.The oxygen sensing film will be destroyed when the temperature was higher than 50 ℃[32].In order to prolong the lifetime of the sensor, 40 ℃ was recommended for our subsequent investigations.
3.3 Dynamic response of dual-parameter fiber optic sensor
At 40 ℃, hydrogel-SiO2nanoparticles immobilized laccase has no effect on the DCP in the solution.At this time, only sulfide in the solution was catalyzed by MnSO4, and the sensor was responded with the different concentrations of sulfide.Fig.5(a)depicts the relationship between the change of phase delayφand sulfide concentrations in the concentration range from 5.0×10-6to 1.2×10-4mol/L at 40 ℃.A good linear relationship and small standard deviation can be obtained in the detection range of sulfide concentration, the sensing calibration curve isY=0.011 3X+0.978 9, and the linear correlation coefficientR2=0.992 8.The response time of the sensor is taken as 250 s because most of sulfide was oxidized in this time.The detection limit is 2.0×10-6mol/L(S/N=3).Since the integrated waste water discharge standard of China for sulfide is 2.94×10-5mol/L, the sensor can meet the demand of practical application.
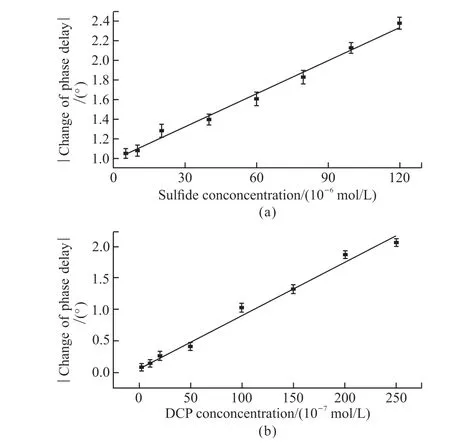
Fig.5 (a)Typical calibration curve of the fiber optic sensor at various concentration of sulfide (pH=7.0, T=40 ℃, MnSO4 concentration was 5.0×10-6 mol/L); (b)Typical calibration curve of the fiber optic sensor at various concentration of DCP (pH =5.0, T=25 ℃, laccase concentration was 2.0×10-5 mol/L in 10 mg carrier)
In our previous work, we studied the optimum test conditions for optical fiber sensor based on laccase catalyzed DCP (T=25 ℃, pH of the reaction solution was 5.0, laccase concentration was 2.0×10-5mol/L)[33].When the sulfide in the solution was oxidized completely, the temperature of the mixed solution to be tested was lowered to 25 ℃ (lower than the LCST of the hydrogel), laccase had obvious catalytic effect on DCP, so that the DCP in solution was oxidized and consumed oxygen, and the sensor was responded with the different concentrations of DCP at this time.Fig.5(b) shows the linear relationship between the change of phase delayφand different concentrations of DCP at 25 ℃.There is a good linear relationshipφand DCP concentration in the range of 3.0×10-7-2.5×10-5mol/L, and the equation of linear regression isY=0.008 4X+0.072 4,R2=0.988 6, the response time is 250 s.The lower detection limit of the sensor is 2.5×10-7mol/L (S/N=3).Since the integrated waste water discharge standard of China for DCP is 3.68×10-6mol/L, the sensor can meet the demand of practical application.
In consequence, the dual-parameter fiber optic sensor enabled the detection of sulfide and DCP by controlling different temperatures.We achieved the leap from a single parameter detecting fiber optic sensor to a fiber optic sensor that can continuously detect two kinds of parameters, which are significant improvements of sensor performance and application.
3.4 Properties of dual-parameter fiber optic sensor
3.4.1 Repeatability and reversibility
The reversibility of the sensor was assessed by exposing the sensor to five cycles of buffer, 1.0×10-5mol/L sulfide and 5.0×10-6mol/L DCP , respectively.The results showed that it has a highly reproducible and reversible response to sulfide (RSD=4.84%)and DCP solution (RSD=3.95%), indicating the good reversibility of the dual-parameter sensor.The repeatability of the response of the sensor was also investigated by subjecting the sensor to a 1.0×10-5mol/L sulfide and 5.0×10-6mol/L DCP solution.The relative standard deviations (RSDs) are 4.52% and 4.27% for 10 successive measurements, respectively,also indicating the good repeatability of the dualparameter sensor.
3.4.2 Long-term stability
To investigate the lifetime of the sensor, the response values of the oxygen sensing film in contact with 1.0×10-5mol/L sulfide and 5.0×10-6mol/L DCP solution were recorded after keeping the film in water for 7 days.The results showed that the change of phase delay of the sensor under optimal conditions only decreased by 3.21% (sulfide solution) and 2.78% (DCP solution), respectively.The indicator leaking from the film might be responsible for the loss of sensitivity.The response time of the sensor is 250 s, the single detection time is short, the indicator leakage can be ignored, which ensuring the dual-parameter sensor test multiple batches of samples effectively.
3.4.3 Selectivity against interference
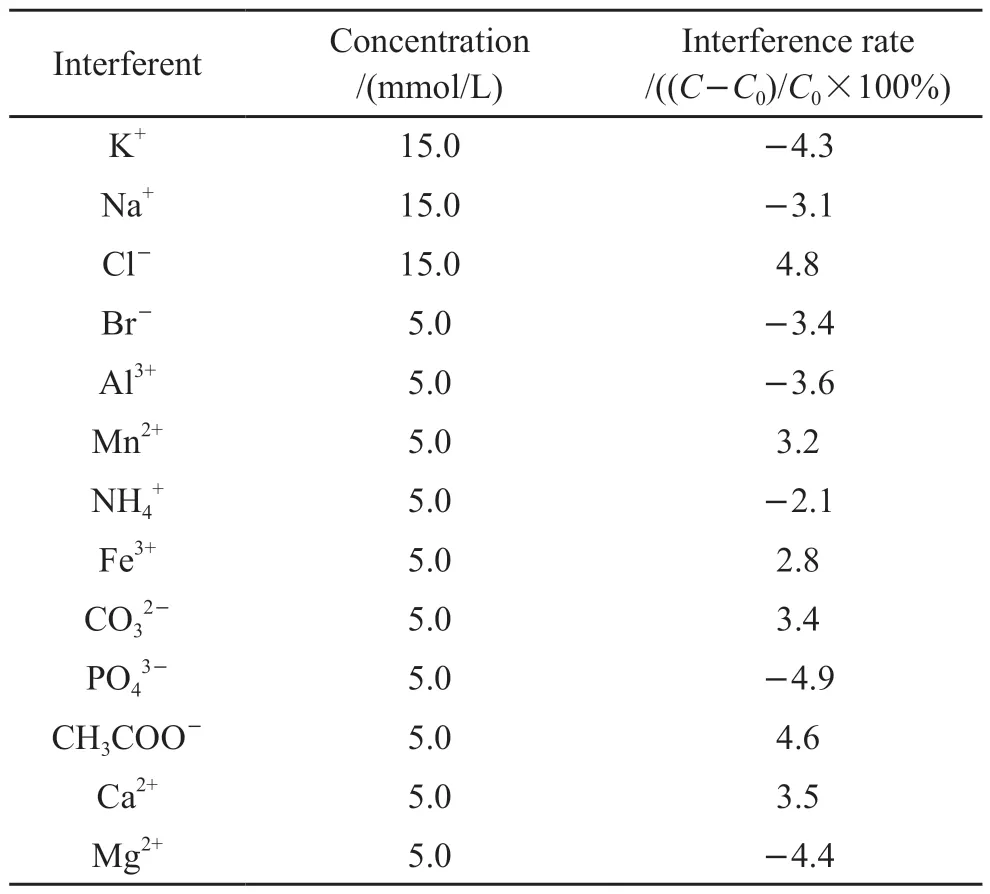
Table 1 Interference test of the sulfide fiber optic sensor on exposure to various interferents
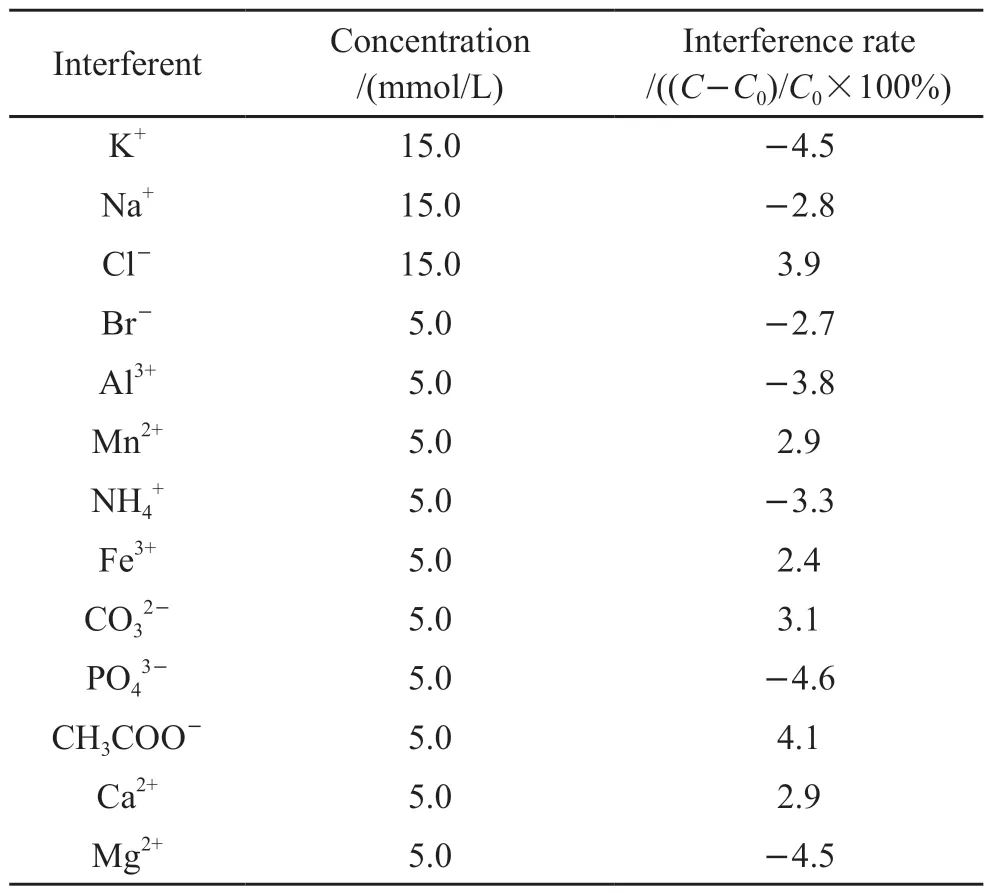
Table 2 Interference test of the DCP fiber optic sensor on exposure to various interferents
The selectivity against interference of sensor is an important performance.1.0×10-5mol/L was selected as the actual concentration of sulfide, 5.0×10-6mol/L was selected as the actual concentration of DCP,and ions commonly found in sewage such as K+, Na+,Cl-and so on were selected as potential interferents.The interference rate in the presence of potential interferents with different concentrations were used as indicators for selectivity of the sensor.The results are summarized in Table 1 and Table 2.We could see that all the interferents did not cause significant interference on the response of the sensor, the interference rates were within ±5%, demonstrating a good selectivity for the dual-parameter sensor.
3.4.4 Detecting in real water samples
The dual-parameter sensor was validated by applying it to the determination of sulfide and DCP in samples from sewage disposal plant in Wuhan.The standard addition method was adopted, different concentrations of sulfide and 5.0×10-6mol/L concentration of DCP were added to the samples of six groups, each group was tested three times using the proposed sensor.The results were shown in Table 3.As can be seen from Table 3, the recovery range of sulfide concentration detected by the sensor in each group is 94.78%-102.12%, which showed that the sensor was effective in the detection of sulfide in practical samples.
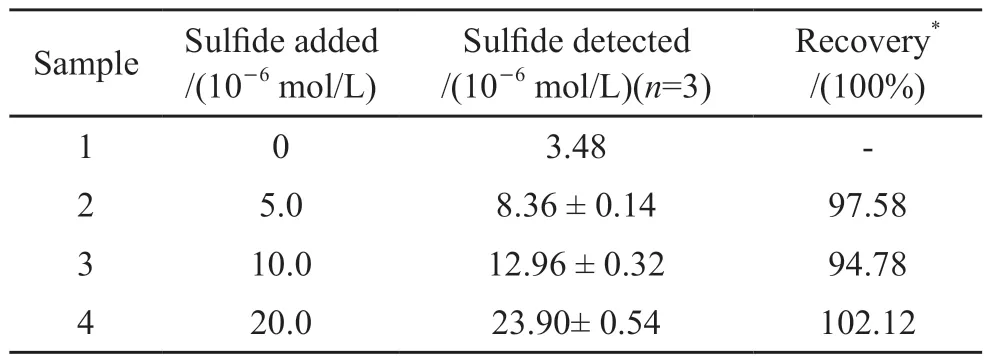
Table 3 Detection of sulfide in practical samples using the proposed sensor
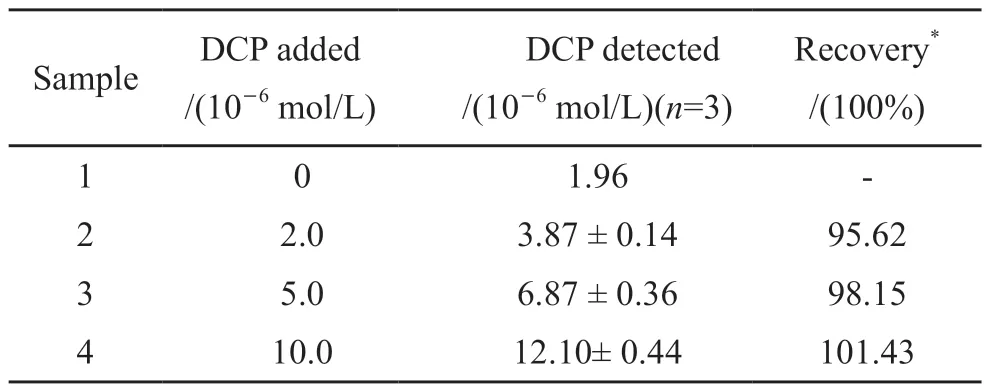
Table 4 Detection of DCP in practical samples using the proposed sensor
In order to study the detection effect of the sensor on DCP concentration in practical samples, the standard addition method was used.Different concentrations of DCP and 1.0×10-5mol/L concentration of sulfide were added to the samples of six groups, each group was tested three times using the proposed sensor.As can be seen from Table 4, the recovery range of DCP concentration detected by the sensor in each group is 95.62%-101.43%.It can be concluded that this sensor is suitable for the determination of DCP in practical samples.
3.4.5 Comparison of sensor with other methods
We compared the proposed sensor with other detection methods of sulfide and DCP, different detection methods (HPLC, electrochemical sensor and proposed sensor) were used to detect the concentrations of sulfide and DCP in samples, the results are shown in Table 5 and Table 6.The sample preparation for HPLC is much more complicated and the detection is a time-consuming process.The cost of HPLC is rather high, and it can not be used for real-time and on-line detection.Compared with the proposed sensor, the detection limits of electrochemical sensor are 1.8×10-4mol/L(for sulfide, S/N=3) and 2.5×10-5mol/L(for DCP,S/N=3), which are two orders higher than that of our sensor.And the response time of electrochemical sensor is 13.6 min, the response rate is much slow.Compared with other detection methods, the proposed sensor has good selectivity, repeatability and recovery.Due to the emission standard of sulfide and DCP are 2.94×10-5and 3.68×10-6mol/L respectively, the detection ranges of proposed sensor fully cover the above ranges, the proposed sensor is expected to have great application prospect in sewage detection.
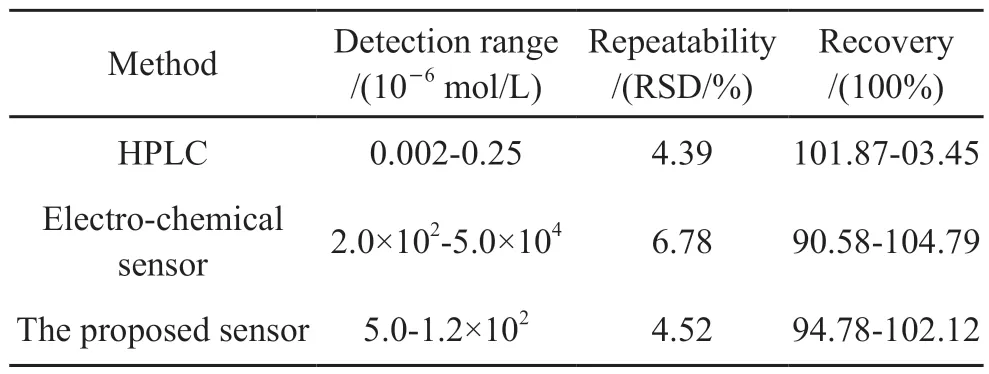
Table 5 Comparison of the proposed sensor with other sulfide detection methods
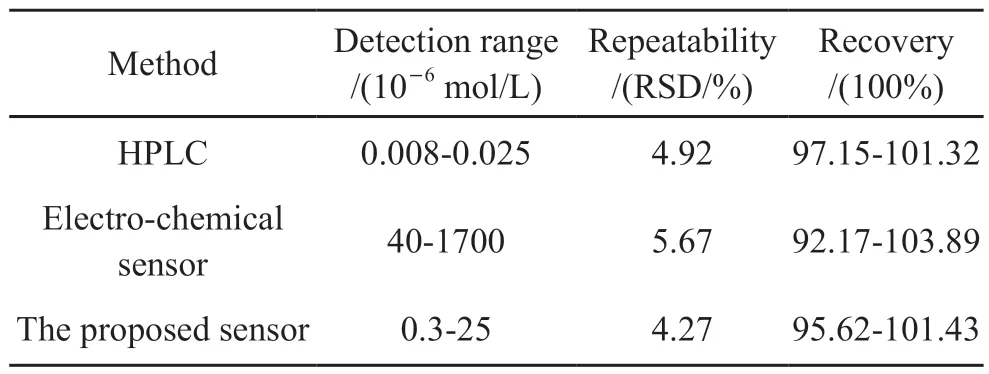
Table 6 Comparison of the proposed sensor with other DCP detection methods
4 Conclusions
In summary, we prepared hydrogel-SiO2nanoparticles immobilized laccase, and developed a dual- parameter fiber optic sensor based on hydrogelimmobilized enzyme complex.It is first time to report that this sensor can be used for the simultaneous measurement of dual-parameter.By controlling the temperature from high to low, the fiber optic sensor for the continuous detection of sulfide and DCP was realized.The sensor offered good linear dynamic in sulfide and DCP concentrations by means of the measured phase delayφon the fluorescence lifetimebased sensing, respectively.The sensing calibration curve betweenφand sulfide concentration wasY=0.011 3X+0.978 9 in the detection range of 5.0×10-6-1.2×10-4mol/L, the detection limit was 2.0×10-6mol/L (S/N=3) and the response time was 250 s.The sensing calibration curve betweenφand DCP concentration wasY=0.008 4X+0.072 4 in the detection range of 3.0×10-7-2.5×10-5mol/L, the detection limit was 2.5×10-7mol/L (S/N=3) and the response time was 250 s.Since the integrated waste water discharge standard of China for sulfide and DCP are 2.94×10-5mol/L and 3.68×10-6mol/L, the proposed sensor can meet the need of practical application.The dual-parameter sensor showed good repeatability, selectivity and stability.The dual-parameter sensor could be successfully applied to detect sulfide and DCP in real water samples,demonstrating a great application prospect for fast and accurate measurement of sewage pollutants.
Conflict of interest
All authors declare that there are no competing interests.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Flow Characteristics Analysis of TC18 Titanium Alloy during Hot Deformation Based on Phase Transformation
- Effect of Hydrated Calcium Aluminate Cement on the Chloride Immobilization of Portland Cement Paste
- Expansion Performance and Microstructure of High-performance Concrete using Differently Scaled MgO Agents and Mineral Powder
- Effect of Curing Age on Tensile Properties of Fly Ash Based Engineered Geopolymer Composites (FA-EGC) by Uniaxial Tensile Test and Ultrasonic Pulse Velocity Method
- Energy-dispersive X-ray Spectroscopy for the Quantitative Analysis of Pyrite Thin Specimens
- Bioprocess-inspired Actin Biomineralized Hematite Mesocrystals for Energy Storage
