Expansion Performance and Microstructure of High-performance Concrete using Differently Scaled MgO Agents and Mineral Powder
2024-01-03TIANChangjinWANGYouzhiQIUKaiYANGQilin
TIAN Changjin, WANG Youzhi, QIU Kai, YANG Qilin
(1. School of Civil Engineering, Shandong University, Jinan 250002, China; 2. China Construction Infrastructure Co., Ltd, Beijing 100089,China; 3. School of Transportation Science and Engineering, Harbin Institute of Technology, Harbin 150090, China)
Abstract: To investigate the assumptions proposed in this paper, the evolution law governing the strength and expansion performance of MgO and nano-MgO micro-expansive concrete in the environment of mineral powder was firstly observed in this study.Secondly, SEM, XRD, and TG-DSC microscopic tests were conducted to reveal the effects of the active mineral-powder admixture on the hydration degree and expansion performance of MgO and nano-MgO in HPC.Our experimental results successfully verified our hypothesis,which indicated that the expansion performance of macro-MgO and nano-MgO was indeed depressed by the addition of active mineral power admixtures, even though the mechanical property of concrete composites was effectively improved.Furthermore, the hydration test also demonstrated the negative interference on the mineral powders, which was induced by the expansion agents.It is found the amounts of hydrates tend to decrease because the mineral powder ratio reaches and exceeds 40%.Moreover, it is also concluded the effect of expansion agents is governed by the alkalinity cement paste, especially for the nano-MgO.In other words,the expansion performance of nano-MgO will vary more obviously with the hydration process, than MgO.The results of this study provide that effective experimental and theoretical data support the hydration-inhibition mechanism of magnesium expansive agents.
Key words: MgO; Nano-MgO; mineral powders; high-performance concrete; expansive agents;microstructures
1 Introduction
High-performance concrete (HPC) has been widely applied in bridge engineering and construction due to their good durability, high strength, and high corrosion resistance and, thus[1-3].However, the HPC structural system is prone to temperature shrinkage deformation owing to the relatively high cement amount and concentrated hydration heat release of cementitious materials, which may result in excessive shrinkage stress, and enhances the possibility of brittle cracking at the interface between the aggregate and cement slurry, subsequently affecting the durability and bearing capacity of the system and reducing the normal service life of the structure[4,5].The development of expansive concretes by using expansive agents and additives has proven to be effective in reducing shrinkage, decreasing crack formation and improving the service life of HPC[6-8].
MgO-based expansive agents have the characteristic of temperature excitation[9,10], which can compensate for the temperature and drying shrinkage of concrete; hence, their application in HPC is gradually becoming popular.Previous studies have revealed that the hydration of MgO expansion agents is slow during the initial stages, and the performance of expansive concrete cannot be effectively improved.However,after 28 d, the hydration of MgO is accelerated, and Mg(OH)2crystals filling the pores of the cement paste are continuously generated, optimizing the pore size distribution of the cement slurry and reducing detrimental porosity of the expansive concrete[11,12].Furthermore, Mg(OH)2crystals generated by the continuous hydration of MgO provide uniform expansion deformation for HPC, which effectively compensates for the shrinkage deformation in the process of setting and hardening of the paste structure,thus improving the strength, durability, and expansion performance of the concrete structure[13-16].
Compared with the MgO expansion agent,nano-MgO can improve the performance of concrete more effectively owing to its surface, small size, and quantum size effects[17,18].Studies have revealed that the microstructure of concrete is dense and uniform,and the mechanical properties and permeability of the structure can be significantly improved by the addition of nano-MgO to the cement composite[19-21].However, the amount of hydrotalcite in the system increases with the addition of nano-MgO, which has an adverse effect on the structural compactness[14].Therefore, the use of nano-MgO expansive agents seems to have some limitations.Moreover, compared with the hydration effect of lightly burned MgO in the cement environment, the volume stability, strength,and hydration expansion effect of nano-MgO are more distinct[15].Furthermore, the differences in the expansive performance, mechanism of compensating shrinkage, and microstructure of the MgO expansion agent and the nano-MgO expansion agent are not yet fully understood.
The thermal stress due to the large-volume pores and accumulated internal heat produced by the hydration of cement can cause cracking over time[22,23].To decrease the thermal stress in large-volume pores and improve the sustainability of the construction process, supplementary cementitious materials have been applied, such as mineral powder, fly ash (FA),limestone filler (LF), and ground granulated blast furnace slag (GGBS)[24,25].Mineral powder is a highquality active concrete admixture and cement mixture and is recognized as a high-quality material for HPC[26].The application of mineral powder in HPC can not only decrease the amount of generated hydration heat but also reduce the use of cement, which is in line with the basic national policy of conserving resources and protecting the environment[27].Moreover, the addition of mineral powder can improve the initial working performance of concrete along with its durability[28-30].In cement paste, the hydration of mineral powder,as an “inert material” is slow, and the water-binder ratio is considerably high in the mixing process of cementitious materials and cement-based materials;therefore, the fluidity and bleeding rate of the concrete mixture can be effectively improved.Mineral powder can also cause secondary hydration[31,32], further improving the mechanical properties, compactness, and frost resistance of concrete[33-35].
Recently, the combined use of supplementary cementitious materials and expansive agents has been studied.Based on the results, it can be concluded that for different contents of mineral admixtures, the expansion rates decrease with an increasing content of GGBS or FA[36].Moreover, the smaller influence of FA on expansion may be due to its lower initial reactivity compared to that of GGBS[37].LF has a substantial influence in the early stage of the hydration process,and this influence can be further enhanced by the expansive agents[38].
Based on the literatures, in this study, we selected MgO and nano-MgO as expansion agents and mineral powder as a supplementary cementitious material to prepare high-performance micro-expansive concrete.We evaluated the effects of mineral powder on the expansive effect and mechanical properties and employ thermogravimetry-differential scanning calorimetry(TG-DSC) and scanning electron microscopy (SEM)to analyze the hydration process and microstructures,respectively, of the hydration products.
2 Experimental
2.1 Raw materials
P·Ⅱ 52.5 grade portland cement with a specific surface area of 346 m2/kg, produced by the Taian Zhonglian cement plant, was used in this study.The coarse aggregate was gravel (Jinan Luping Building Materials Co., Ltd.) having a particle size of 5-16 mm,a crushing value of 12.8%, an apparent density of 2.727 g/cm3, and a loose bulk density of 1.50 g/cm3.The fine aggregate was river sand obtained from the Wenhe sand field of Taian City, and it had a mud content of 1.0%,a bulk density of 1.500 g/cm3, and an apparent density of 2.620 g/cm3.The active mineral admixture was S95 granulated blast-furnace slag powder (Shandong Shiheng Special Steel Co., Ltd.) with a density of 2.93 g/cm3and specific surface area of 438 m2/kg.Fig.1 depicts its main composition and crystal morphology,characterized by using X-ray diffraction (XRD) and SEM.Fig.1(a) shows that the blast-furnace slag powder is amorphous and has a very low degree of crystallinity and an irregular shape with rough surfaces[26,30].Fig.1(b)shows that mineral powder has a fairly high amount of C3S and SiO2.A polycarboxylate superplasticizer produced by Nanjing Bose Co., Ltd.was used as a water-reducing agent, which had a water reduction rate of 29% and density of 1.084 g/mL.MgO and nano-MgO (Shanghai McLean Biochemical Technology Co., Ltd.) was used as the expansion agent.The basic physicochemical properties are listed in Table 1.Figs.2 and 3 depict their main composition and crystal morphology, characterized by using X-ray diffraction(XRD) and SEM.Fig.2 shows that the purities of the MgO and nano-MgO products were high, and no diffraction peaks of other impurities could be observed.The purities of them exceeded 99%.Compared with the XRD detection patterns of MgO and nano-MgO,the XRD peak position of nano-MgO was consistent with that of MgO, but the diffraction peak width of nano-MgO was obvious and the peak type was sharp.Fig.3 show that MgO had a high crystallinity, complete crystal structure, and tight lattice[13].By contrast, the crystal size of nano-MgO was smaller and a greater number of lattice distortion defects could be observed.However, the structure dispersion was better, and no hard agglomeration phenomenon occurred.Combined with the analysis of the test results shown in Figs.2 and 3, the SEM results of MgO and nano-MgO were consistent with the calculated results of the XRD.In the preparation of MgO and nano-MgO, the crystal size increased with increasing calcination temperature and isothermal sintering time.The average particle size of the nano-MgO crystal was 50 nm under lowtemperature calcination and was greater than 400 nm under high-temperature calcination.The mix of test materials per cubic meter of concrete during the test is listed in Table 2.
2.2 Sample preparation
Based on the mix proportion of concrete presented in Table 2, gravel and sand were weighed and added to the wet concrete mixer and dry-mixed for 0.5 min.The cementitious materials (cement, expansive agent,slag powder, and active admixture) were mixed untilevenly distributed, and then for an additional 4 min.The mixture of high-performance polycarboxylate water reducer and water was mixed evenly for 2-3 min;then, the concrete mixture was poured onto the wet iron plate.Next, it was mixed manually 2-3 times, poured into the test mold with copper heads embedded at both ends, and vibrated until the surface was grouted.The mixture above the test mold along the top surface was scraped offand covered with a fresh-keeping film for 24 h, until the concrete was demolded.Finally, the concrete was numbered and placed in a curing room at 20 ± 2 ℃ and relative humidity (RH) ≥ 95% for curing.
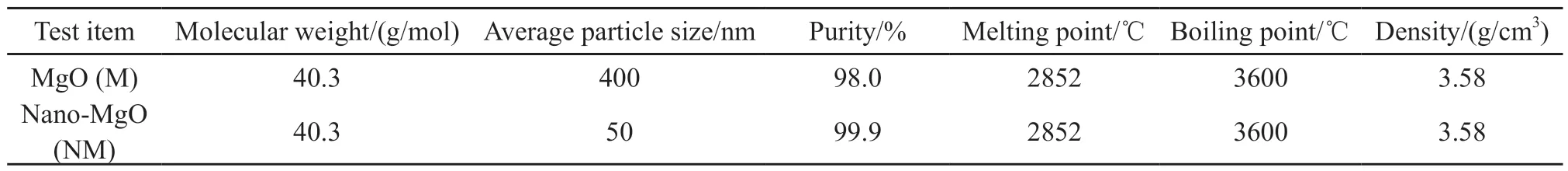
Table 1 Basic physicochemical properties of MgO and nano-MgO
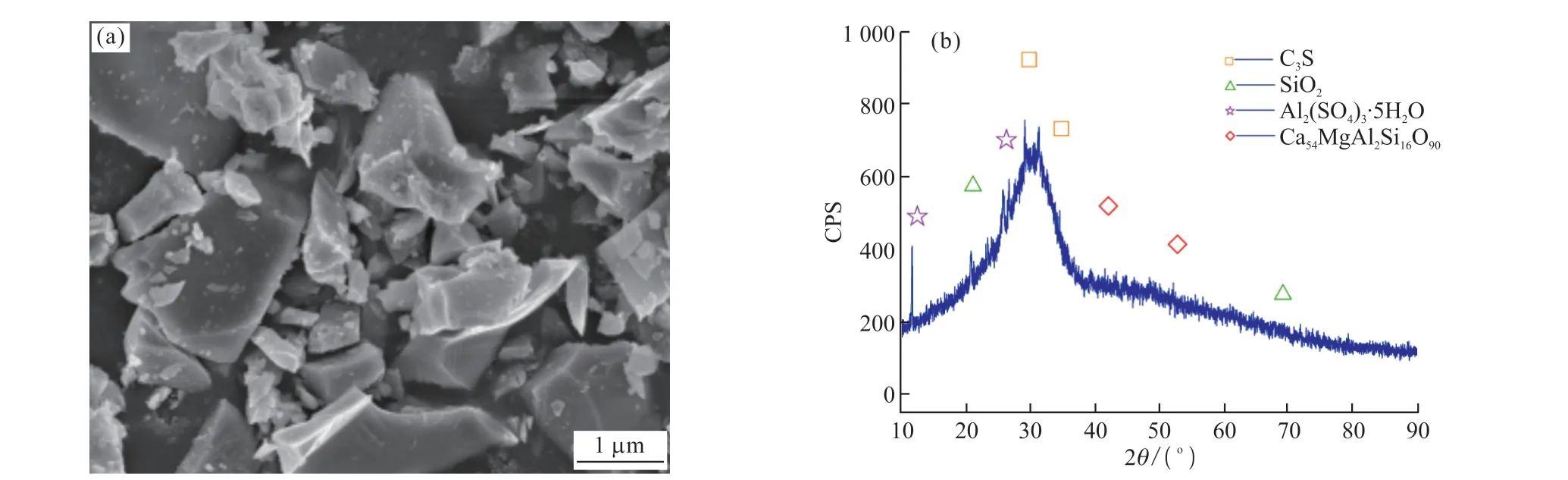
Fig.1 SEM and XRD analysis of mineral powder: (a) SEM image and (b) XRD pattern
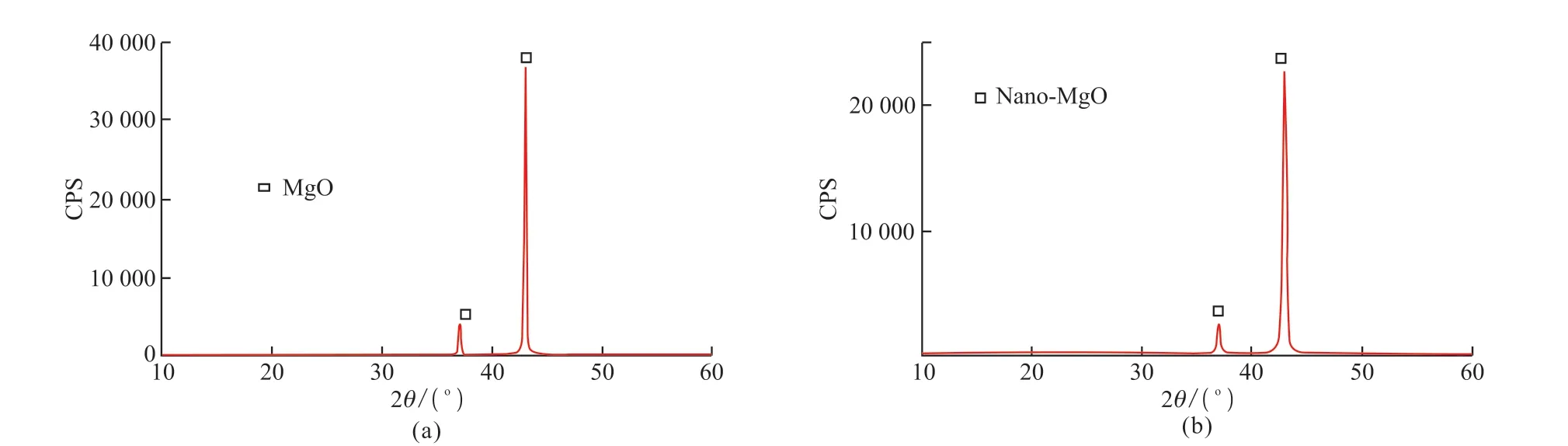
Fig.2 XRD patterns of (a) MgO and (b) nano-MgO
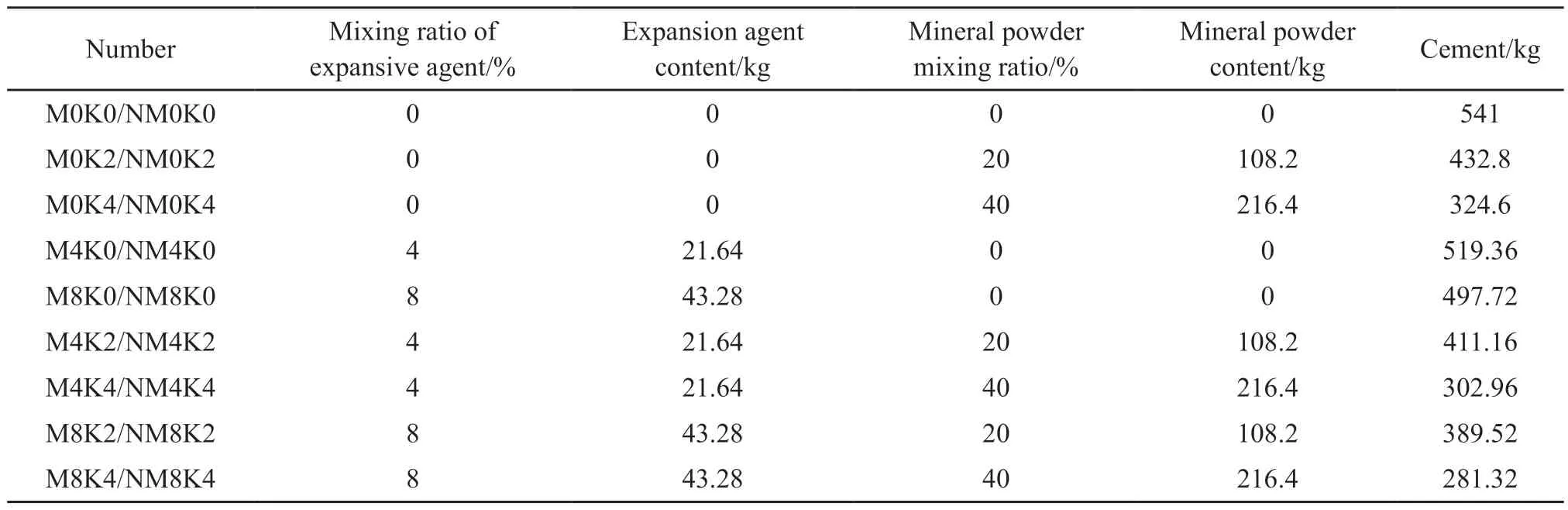
Table 2 Mix proportions
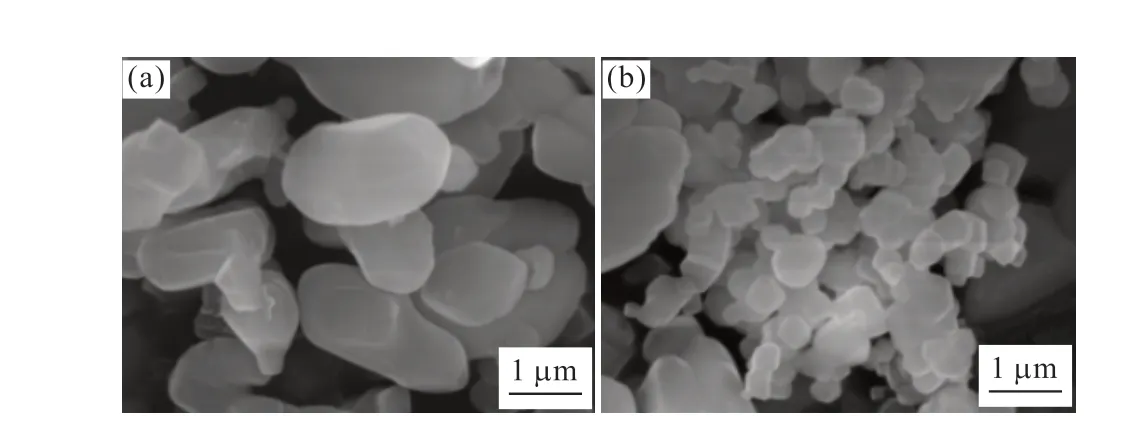
Fig.3 SEM images of MgO and nano-MgO: (a) MgO; (b) nano-MgO
2.3 Testing methodology
The compressive strength test of concrete was performed according to the GB/T50081-2002 “standard for test methods of mechanical properties of concrete”.A WEW-600D hydraulic universal testing machine was applied in the compressive test, and the test size of the specimen was 100 mm × 100 mm × 100 mm.The flexural strength of the concrete structure was measured by using the WEW-600D instrument according to the four-point bending test method, and the specimen size was 55 mm × 55 mm × 280 mm.The free-expansion deformation of the concrete structure was measured by using a BC156-300 concrete length meter and employing the contact method, and 55 mm× 55 mm × 280 mm concrete specimens were selected in accordance with the SL352-2006 hydraulic concrete test specifications.
To stop the hydration of MgO and nano-MgO, the specimens were fabricated into 1-mm-thick samples and immersed in absolute ethanol for 48 h.The samples were dried for 24 h in a constant-temperature blast oven at 65 ℃ and then placed in the ion-sputtering instrument for gold spray-treatment.The SEM analysis was performed using a JSM-7610F field-emission scanning electron microscope.For the TG-DSC test, a TGA/DSC1 1600 high-temperature thermogravimetric analyzer was used to test a cylindrical block ofΦ30 mm × 10 mm.The ratio of mixing water to cementitious material was 0.27, according to the watercement ratio of C60 micro-expansion concrete.The mix proportions of the cementitious materials are listed in Table 3.The cement paste was demolded immediately after molding, and the block was placed in the constanttemperature curing chamber.After curing to the specified age (1, 3, 7, and 28 d), some samples were removed, and the hydration process was terminated using absolute ethanol.The samples were dried in a digital-display electric blast-drying oven at 65 ℃ for 24 h.Powders with a surface area of 350-400 m2/kg were ground in a ball joint grinder for TG-DSC analysis.
3 Results and discussion
3.1 Effect of the mineral powder on mechanical property of MgO and nano-MgO HPC
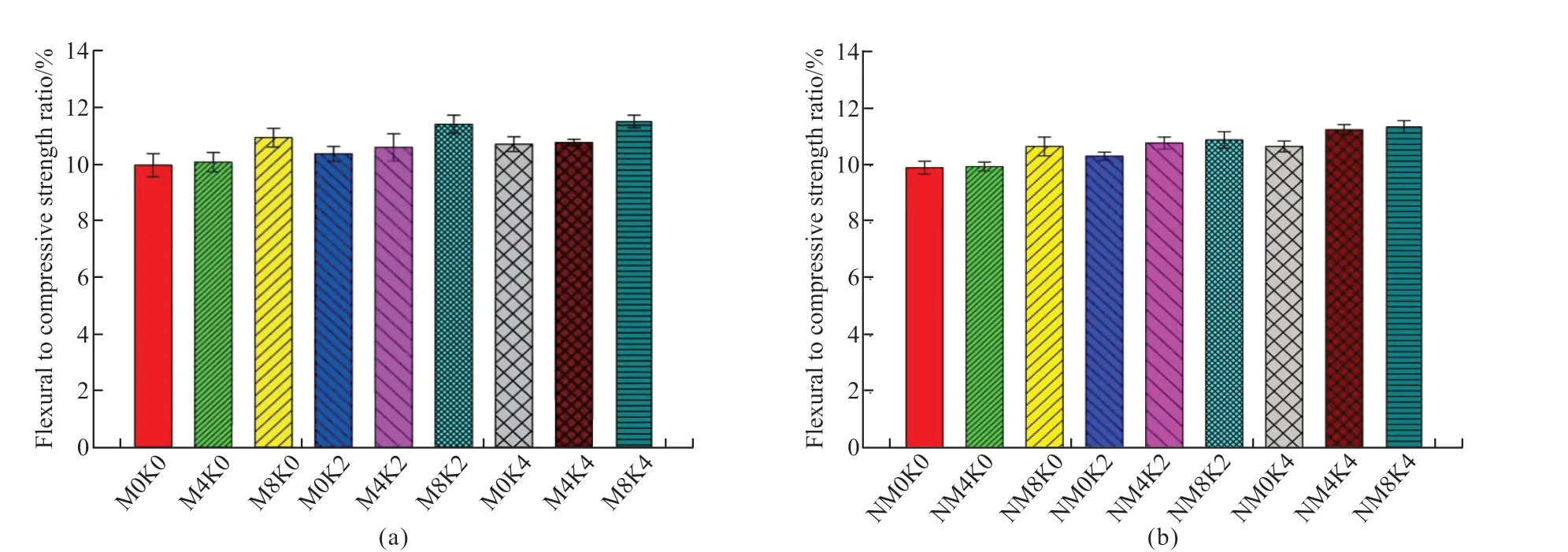
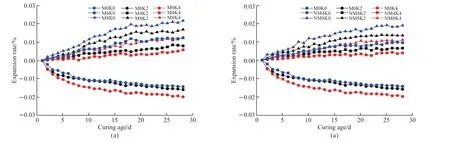
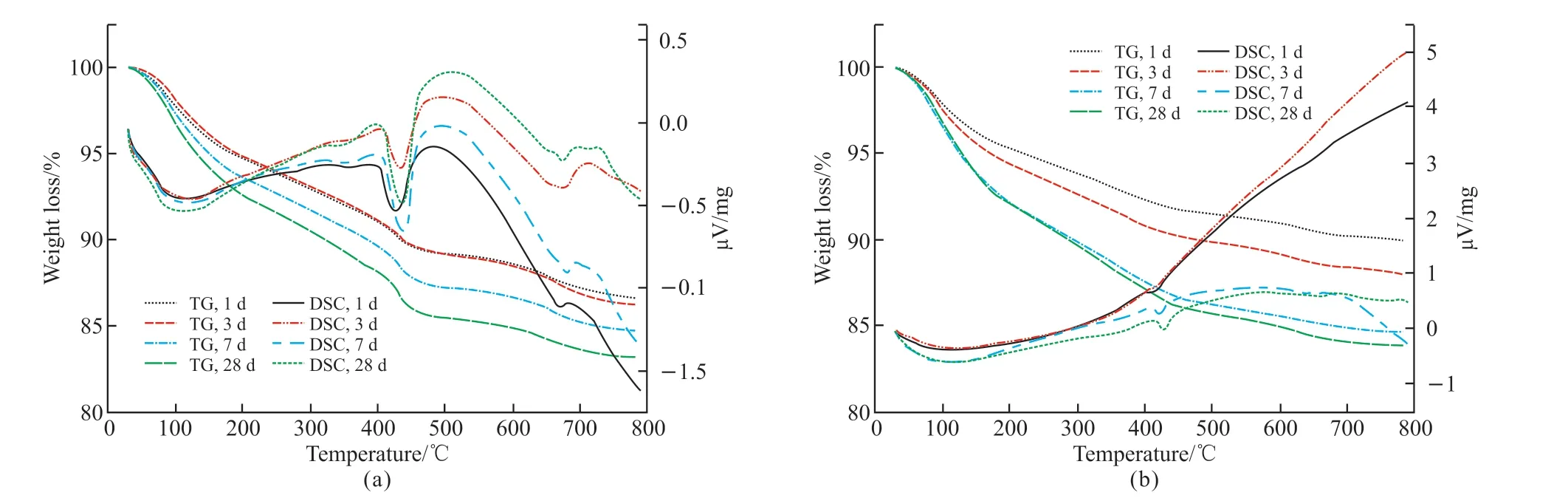
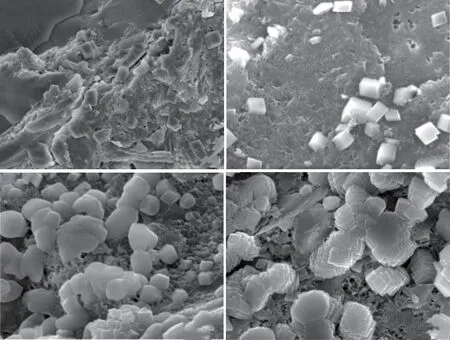
Fig.4(a) presents the compressive strength test results of MgO concrete with 20% and 40%mineral powder.It can be observed that the strength development trend of HPC under the single action of mineral powder is similar to that of the M0 reference group.When the cementitious material is hydrated for 3 d, the structural strength increases in the order M0K4 < M0K2 < M0K0 under the action of mineral powder; the hydration rate of the mineral powder becomes lower than that of Portland cement, and the proportion of mineral powder becomes indirectly proportional to the contribution to concrete strength.When the cementitious materials are hydrated for 7 and 28 d, stable hydration products are formed in the cement mineral powder system.The strength of the concrete structure increases in the order M0K4 Fig.4 (a) Compressive and (b) flexural strength of MgO concrete Fig.4(a) also shows that under the combined action of mineral powder and MgO, the compressive strength of the concrete at each curing age first increases and then decreases with an increase in the proportion of mineral powder and MgO.The compressive performance of the concrete structure is at its peak when 20% mineral powder and 4% MgO are added to the cementitious material.The compressive strength of M4K2 concrete at 28 d of aging is 80.05 MPa, which is 10.61% and 6.44% higher than those of M0K2 and M4K0, respectively.When 40% mineral powder and 8% MgO react together, the compressive strength of the concrete structure decreases significantly; this may be due to the distinct retardation effect when the mineral powder content is considerably high[39,40].Moreover,the expansion delay caused by the lower hydration of MgO cannot effectively compensate for the shrinkage deformation of concrete, deteriorating the strength[41].The compressive strength of M8K4 concrete at 28 d of aging is 70.43 MPa, which is 12.02% lower than that of M4K2.However, the compressive strength of the M8K4 group is superior to that of the single action of mineral powder and MgO, and the growth rate of the M8K4 group is 2.47% and 12.03% higher than those of M0K4 and M8, respectively. Fig.4(b) presents the flexural-strength test results of MgO concrete with 20% and 40% mineral powder.It can be observed that at the initial stage of structural hydration, the flexural strength of concrete under the single action of mineral powder is M0K4 < M0K2< M0K0.The proportion of mineral powder mixed with replacement cementitious materials is indirectly proportional to the flexural strength of the concrete.In the later stages of curing, the strengthening effect of mineral powder on concrete first increases and then decreases.The flexural strength follows the order M0K0 < M0K4 < M0K2; the flexural strengths of M0K2 and M0K4 are 7.29% and 5.21%, respectively,compared with that of the M0 base group. Fig.4(b) also shows that under the combined action of mineral powder and MgO, the change law for the flexural strength of concrete is similar to that of the compressive strength (Fig.2).The structural strength first increases and then decreases with an increase in the MgO and nano-MgO ratio.When the ratio of MgO is 4%, the flexural strength of concrete exhibits a clear turning point.When 20% mineral powder and 4%MgO react together, the flexural strength reaches its maximum.The flexural strength of M4K2 concrete is 8.47 MPa at 28 d, which is 13.06% and 11.93% higher than those of M0K2 and M4K0, respectively.When 40% mineral powder and 8% MgO act together, the flexural strength of the concrete decreases.The 28 d flexural strength of the M8K4 concrete group is 8.09 MPa, which is 4.45% lower than that of the M4K2 group; however, the flexural strength of the M8K4 group is higher than that of the single action of mineral powder and MgO, and the growth rates are 10.17%and 17.92% higher than those of M0K4 and M8K0,respectively. A comparison of Figs.4(a) and 4(b) reveals that in the initial stage of hydration, the hydration reaction of mineral powder and MgO is slow, the hydration degree of the mineral powder/MgO cementitious system is small, and the effective strengthening effect on the concrete is low.In the hydration stage,the mineral powder and MgO form stable hydration products in the cement paste.The internal structure of the concrete is dense, and its structural strength is better than that produced by the single effect of mineral powder and MgO.The hydration degree of the cement mineral powder/MgO system decreases when the proportion of mineral powder and MgO is excessively high.The hydration inertia of mineral powder and delayed expansion of MgO have adverse effects on the initial strength development of the structure.In the later stages of curing, the hydration expansion of MgO is excessively large, and the expansion stress concentration of the hydration product Mg(OH)2weakens the structure, resulting in a weakening of its mechanical properties[41].Therefore, the use of the maximum amount of MgO and mineral powder must be limited in the practical application process. Fig.5 (a) Compressive and (b) flexural strength of nano-MgO HPC Fig.5 presents the test results of the compressive and flexural strengths of concrete under the combined action of mineral powder and nano-MgO.Fig.5(a)shows that the compressive strength of the concrete structure is directly proportional to the curing age of the concrete.The compressive strength decreases with the increase in MgO and nano-MgO.The 3 and 7 d compressive strengths of NM8K4 were 43.37 and 54.64 MPa, respectively, which were 25.71% and 15.42%lower than those of the M0K0 group, respectively.When the cementitious material is hydrated to 28 days,the compressive strength of concrete increases with the increase of the content for nano-MgO.The 28 d compressive strength of the NM8K2 concrete group is 75.36 MPa, which is 3.97% and 4.13% higher than those of the NM8K0 and M0K2 groups, respectively.Thus, the development rule for the compressive strength of the structure is as follows: NM4K4 Under the joint action of mineral powder and nano-MgO, the change rule for structural strength is NM4K0 < NM4K4 < NM4K2 and NM8K0 < NM8K4< NM8K2.An excessively high proportion of mineral powder adversely affects the structural flexural strength.Therefore, the flexural strength of concrete under the actions of 20% mineral powder and 8%nano-MgO is the highest.The 28 d flexural strength of the NM8K2 concrete group is 8.13 MPa, which is 4.99% and 8.56% higher than that of the NM8K0 and M0K2 groups, respectively.Based on the analysis of the strength development trend of concrete containing different proportions of mineral powder and nano-MgO, it was discovered that the addition of mineral powder and nano-MgO in cement and cementitious materials enhances the structural strength of the concrete.In the cement paste, the continuous hydration of mineral powder and nano-MgO improves the density of the interface structure and the bonding force between the cemented paste and aggregate, effectively increases the structural strength[20,42], and meets the normal use requirements of the structure.When the mineral powder ratio is excessively high, the hydration rate of the cementitious materials is relatively reduced,leading to a decrease in the overall hydration degree of the cement and cementitious materials, which has a negative impact on the development of structural strength[39,40]. Fig.6 Ductility of (a) MgO and (b) nano-MgO HPC Relevant data show that the ductility of HPC can be indicated by the ratio of the flexural strength to the compressive strength of the structure within the specified curing period[43].The higher the ratio of the flexural strength to compressive strength, the better is the deformation capacity of the concrete structure.As shown in Figs.4 and 5, based on the flexural strength and compressive strength data of the micro-expanded concrete, the flexural-to-compressive-strength ratio of the structure cured for 28 d is calculated.The ductility analysis results of MgO concrete and nano-MgO concrete in the mineral powder environment are shown in Fig.6. Fig.6(a) reveals that under the action of 20% and 40% mineral powder, the flexural-to-compressivestrength ratios of MgO concrete are significantly improved.The greater the proportion of mineral powder and MgO in cementitious materials, the greater is the ductility of the structure.The flexural-to-compressivestrength ratio of M8K4 is 11.49%, which is 5.22%and 7.48% higher than those of M0K4 and M8K0,respectively.As shown in Fig.4, under the action of 8% MgO, the expansion effect of hydration products inside the structure is large, weakening its mechanical properties and considerably increasing the flexural-tocompressive-strength ratios; under the effect of 40%mineral powder, the proportion of cement replacement is large, leading to insufficient effective strength and a relative increase in the flexural-to-compression-strength ratio of the structure. Fig.6(b) shows that the ductility of the nano-MgO concrete structure increases with the increase in mineral powder and nano-MgO ratio.When nano-MgO and mineral powder act together, the hydration degree and structural compactness of internal cementitious materials are higher, effectively improving the mechanical properties and ductility of concrete.However, when the content of mineral powder is 40%,the hydration process of the cementitious system inside the structure is slow and the mechanical properties degrade, resulting in a relative increase in the flexural compression ratio of the structure. A combined analysis of Figs.6(a) and 6(b)reveals that the NM8K2 concrete group has the best comprehensive performance in terms of structural ductility and strength.When 20% mineral powder and 8% nano-MgO are mixed into cement-based materials,the 28 d compressive strength of the concrete structure is 75.36 MPa, the flexural strength is 8.23 MPa, and the flexural-to-compressive-strength ratio is 10.92%, thus significantly improving ductility compared with that of the M0 reference group and NM8 group, which have flexural-to-compressive-strength ratios of 9.76% and 2.21%, respectively. Fig.7 illustrates the curve representing the influence of mineral powder on the expansion rate of MgO and nano-MgO concrete.Fig.7(a) shows that the proportion of mineral powder in the cementitious material is directly proportional to the significance of the shrinkage deformation of the concrete specimen,that is, the shrinkage rate of the concrete structure is M0 < M0K2 < M0K4.MgO concrete can expand stably when a certain proportion of mineral powder is added to cement and cementitious materials.The volume deformation of the concrete blocks increases in the following order: M4K4 < M4K2 < M8K4 Fig.7(b) reveals that the concrete structure has stable expansion volume deformation under the combined action of mineral powder and nano-MgO,and this is consistent with the change trend of the expansion rate under the single action of nano-MgO.At the same proportion of mineral powder, the expansion rate of nano-MgO concrete increases with the increase in the nano-MgO amount.The volume deformation of nano-MgO concrete is less than the hydration expansion effect of MgO; the mineral powder has a negative effect on the hydration expansion of nano-MgO.The greater the amount of mineral powder, the greater is the resistance of the nano-MgO concrete expansion efficiency. The basic performance parameters of materials were used to analyze the expansion development trend of the concrete system.The observed change trend of the MgO and nano-MgO high-performance concrete structure can be mainly attributed to the inhibition of the hydration expansion reaction of MgO and nano-MgO by the mineral powder; the hydration degree of MgO and nano-MgO, along with the stability of Mg(OH)2hydrate, are adversely affected by the mineral powder[44].Therefore, the macro expansion volume deformation of the system is relatively reduced after concrete setting and hardening. The experimental results indicate that 8% MgO or nano-MgO yield a greater improvement in ductility and expansion performance.In the subsequent analysis,M8K0, M8K4, NM8K0, and NM8K4 were used and compared to study the mechanism of the effect of mineral powder on the hydration expansion of MgO and nano-MgO. Fig.7 Analysis of the influence of mineral powder on the expansion rates of (a) MgO and (b) nano-MgO concrete Fig.8 presents the TG-DSC curves of the M8K0 and M8K4 ages.Two clear mass-loss peaks can be observed in the TG curve.Therefore, it can be inferred that the thermal decomposition of the hydration product of MgO and nano-MgO, mainly undergoes two mass loss stages: the first stage is mainly concentrated at 340℃, and the hydration product Mg (OH)2of MgO and nano-MgO begins thermal decomposition, whereas the second is mainly concentrated at 450 ℃, which is the weight-loss curve for the thermal decomposition of Ca(OH)2. Fig.8(a) shows that with an extension of the curing time, the thermal-decomposition weight loss of Mg(OH)2becomes significant at 340 ℃, and the hydration degree of MgO increases.The hydration degree of MgO is low after curing for 1 or 3 d; this is consistent with the delayed expansion characteristics in the expansion curve.The weight-loss trough of the hydration product, Mg(OH)2, at 28 d is the largest in the differential thermal gravimetric analysis curve.The amount of Mg(OH)2generated by the continuous hydration of MgO in the cement environment gradually increases, and this is conducive for improving the strength, toughness, and expansion performance of the structure.However, the specific gravity of Ca(OH)2in the temperature range of 410-470 ℃ initially increases and then decreases.After curing for 7 d, the hydration degree of MgO in the system increases, and the amount of consumed OH–increases continuously, leading to a decrease in the alkalinity of the system, consistent with the development trend of the macro-performance of MgO concrete. Fig.8(b) depicts the TG-DSC curves for the M8K4 group.It can be observed that with an extension of the hydration time, the amount of Mg(OH)2in the cementitious system increases continuously; however,the change in the trend of Ca(OH)2is different from that of Mg(OH)2.In the initial stage of hydration,owing to the inertia of mineral powder particles and the delayed expansion of MgO, the water to binder ratio is relatively increased, the cement hydration in the cementitious system is sufficient, and the amount of Ca(OH)2is relatively high.Owing to the development of the pozzolanic and hydration reactions of MgO,the specific gravity of the hydration product Ca(OH)2decreased, thus inhibiting the alkali aggregate reaction. Compared with the differential thermal gravimetric analysis curves of M8K0, the DSC trough values of Mg(OH)2and Ca(OH)2in M8K4 were significantly reduced, and the hydration degree of MgO was lower, resulting in a lower macroscopic expansion effect compared to that of MgO only.When 40% mineral powder and 8% MgO were added to the cement, the amount of Ca(OH)2generated by hydration of the composite cementitious system was relatively reduced.The secondary reaction of mineral powder and Ca(OH)2in the cement slurry reduces the OH–concentration and hinders the hydration expansion process of MgO[45]. Fig.9 illustrates the differential thermal gravimetric analysis curves of NM8K0 and NM8K4 in cement paste.Fig.9 (a) shows that the formation of hydration products of Mg(OH)2and Ca(OH)2gradually increases with the curing time.During the specified curing period, the DSC weight-loss troughs of Mg(OH)2and Ca(OH)2in the NM8K0 group are larger than those in the M8K0 group, and the amounts of Mg(OH)2and Ca(OH)2hydration products increase significantly.Compared with MgO, the activity of nano-MgO in the NM8K0 group is higher, effectively promoting the hydration reaction of the cementitious system and increasing the proportion of hydration products such as Ca(OH)2and Mg(OH)2in the hydration stability period;therefore, the structural compactness and mechanical properties of nano-MgO concrete are improved[20],which is consistent with the macroscopic performance test results of nano-MgO concrete. Fig.8 Differential thermal gravimetric analyses: (a) M8K0 and (b) M8K4 Fig.9(b) presents the differential thermal gravimetric analysis curve of the NM8K4 group in cement paste.It can be observed that the mass loss of the TG and DSC curves of NM8K4 is low at the initial stage of hydration, and the amount of Ca(OH)2and Mg(OH)2in the cemented slurry is extremely low; with an extension in the hydration time, the TG weight-loss ratio of Ca(OH)2and Mg(OH)2in the thermal decomposition process increases continuously.Compared with the thermal analysis curve of NM8K0,the thermal-decomposition weight-loss troughs of Ca(OH)2and Mg(OH)2decrease in varying degrees when 40% mineral powder and 8% nano-MgO are added to the system; the hydration reaction of cement and nano-MgO is hindered by the addition of excess mineral powder.Furthermore, the expansion and mechanical properties of nano-MgO concrete are adversely affected. Fig.9 Differential thermal gravimetric analyses: (a) NM8K0 and (b) NM8K4 A comparison and an analysis of the TGDSC test results reveal that the DSC wave trough of Ca(OH)2in the M8K0 and NM8K0 groups is larger;however, after 40% mineral powder is added to the cement, the amount of crystalline Ca(OH)2in the M8K4 and NM8K4 group samples becomes lower,causing a decrease in the alkalinity of the cement paste.The results indicate that the decrease of Ca(OH)2concentration in cement paste has an adverse effect on the hydration degree of MgO and nano-MgO.Nano-MgO is more sensitive to changes in the alkalinity of the cement paste.The decrease in the OH–ion concentration has the highest adverse effect on the hydration of nano-MgO, significantly hindering the hydration process[45]. Fig.10 shows the mass loss of Mg (OH)2and Ca(OH)2during the thermogravimetric analysis.Fig.10 (a)depicts that the lower the content of mineral powder,the greater is the mass losses of Mg (OH)2, indicating that the mineral powder has a distinct inhibitory effect on the hydration of MgO at early age (1 and 3 d).Compared with MgO, the hydration degree of nano-MgO is higher due to the high molecular activity,which shows that nano-MgO can provide more stable expansion deformation at early age.This is consistent with the test results in Fig.7.Fig.10(b) depicts that mineral powder consumes a large amount of Ca (OH)2through secondary reaction in cement environment, as shown in Eq.(1): Fig.10 The mass losses of Mg (OH)2 and Ca (OH)2: (a) Mg (OH)2 and (b) Ca (OH)2 And, the main phase component SiO2can adsorb Ca (OH)2in cement slurry and consume the alkali content in cement hydration products.Moreover, the contents of Ca(OH)2contained in the cement pastes could be estimated according to the Eq.(2) Therefore, a large amount of OH–ions in the cement environment are consumed, and the decrease in the alkalinity of the cementation system inhibits the hydration expansion reaction of MgO and nano-MgO,resulting in the situation depicted in Fig.7. Fig.11 illustrates the SEM analysis of the hydration process of 20% mineral powder in the cement environment.It can be observed that when the cementitious material is hydrated for 3 d, the cement paste structure becomes loose, and the hydration reaction of C3S and C2S components in the cement are incomplete.The concentration of free Ca(OH)2in the cement paste is low, which cannot react with the mineral powder, resulting in a high number of nonhydrated mineral powder particles in the concrete structure.Moreover, as seen in Figs.11(a) and 11(b),many micro cracks and pores were observed in the cementitious material structure, which adversely affects the mechanical properties of early age concrete.This is probably caused by the shrinkage of mineral powder and incomplete hydration of early age cementitious materials.After 7 d of hydration, the hydration reaction of cement and cementitious materials continues, the alkalinity of the system increases, the mineral powder continues to hydrate in the Ca(OH)2environment,and the crystal edges and corners gradually hydrate and blur.After 28 d of hydration, the mineral powder particles are completely hydrated and form stable layered hydration products interlaced with the crystals,improving the structure of the interface between cement paste and aggregate and the strength and toughness of the concrete structure[26].Moreover, as seen in Fig.11(d), Many thick rod-shaped Aft crystals were observed in the structure of cement-based materials.This is due to the addition of mineral powder increases the concentration of sulfate in the hydration liquid phase, reduces the alkalinity, increases the supersaturation of Aft crystallization, reduces the aspect ratio of crystal growth and presents a rod needle shape.Moreover, Aft is disorderly distributed in the cement stone structure, which is equivalent to adding fiber into the cement, so as to improve the flexural strength of the cement stone. Fig.11 Hydration process of mineral powder in cement environment:(a) Hydration state of cement; (b) Hydration state of mineral powder after 3 d; (c) Hydration state of mineral powder after 7 d; (d) Hydration state of mineral powder after 28 d Fig.12 Hydration products of MgO and nano-MgO in mineral powder environment: (a) MgO hydration products after 7 d; (b) MgO hydration products after 28 d; (c) Nano-MgO hydration products after 7 d; (d) Nano-MgO hydration products after 28 d Fig.12 illustrates the SEM analysis of 8% MgO and nano-MgO hydration products in a 40% mineral powder environment.When the specimens are cured for 3 d, they are difficult to detect using SEM.There are several non-hydrated slag particles in the internal structure of the concrete, whereas there are fewer hydration products of MgO and nano-MgO, which make a weak contribution to the initial strength of the concrete.Therefore, an SEM analysis of the hydration state of MgO and nano-MgO at 7 and 28 d under a mineral powder environment was performed.Figs.12(a)and 12(b) indicate that during the hydration reaction,MgO forms hexagonal, plate-like Mg(OH)2in the cement and crystalline mineral powder environment[11],significantly improving the strength and toughness of the concrete structure.However, when 40% mineral powder and 8% MgO were added to the cementitious materials, a significant number of hydration products were generated within the structure with a concentrated distribution and high local expansion stress, resulting in internal porosity; this negatively impacted the development of the structural performance. Figs.12(c) and 12(d) show that in the cement environment that contains mineral powder, the hydration process of nano-MgO is consistent with that in the cement environment.Compared with the M0K0 reference group and MgO concrete, the structure of nano-MgO concrete under the action of mineral powder is dense, which effectively improves the toughness of the hardened cement paste and the strength of the concrete structure.Under the joint action of mineral powder and nano-MgO, the structure can produce an effective expansion effect[20]. In this study, the effects of mineral powder on the expansion performance, mechanical properties and microstructure of MgO and nano-MgO expansive agents were investigated.Futher, the expansive effect and mechanism of the MgO and nano-MgO in the environment of mineral powder were evaluated and compared.Based on the results, the following conclusions can be drawn: HPC that contains 20% mineral powder and 4%MgO expansive agent, as well as 20% mineral powder and 8% nano-MgO expansive agent exhibits the best mechanical properties. b) The addition of mineral powder, MgO or nano-MgO can significantly improve the strength and toughness of concrete.However, excessive mineral powder will inhibit the hydration expansion reaction of MgO and nano-MgO, and adversely affect the hydration product, Mg(OH)2.Therefore, the content of mineral powder should be controlled in practical engineering applications. c) The nano-MgO expansive agent yields better expansive effects than MgO expansive agent for samples blended with mineral powder. d) Nano-MgO is sensitive to changes in the alkalinity of the cement paste, and the decrease in OH–ion concentration significantly hinders the hydration process of nano-MgO, which is not conducive to improving strength, toughness, and expansion properties of the structure. Conflict of interest All authors declare that there are no competing interests.

3.2 Effect of mineral powder on expansion performance of MgO and nano-MgO
3.3 Effect of mineral powder on the hydration rate of MgO and nano-MgO

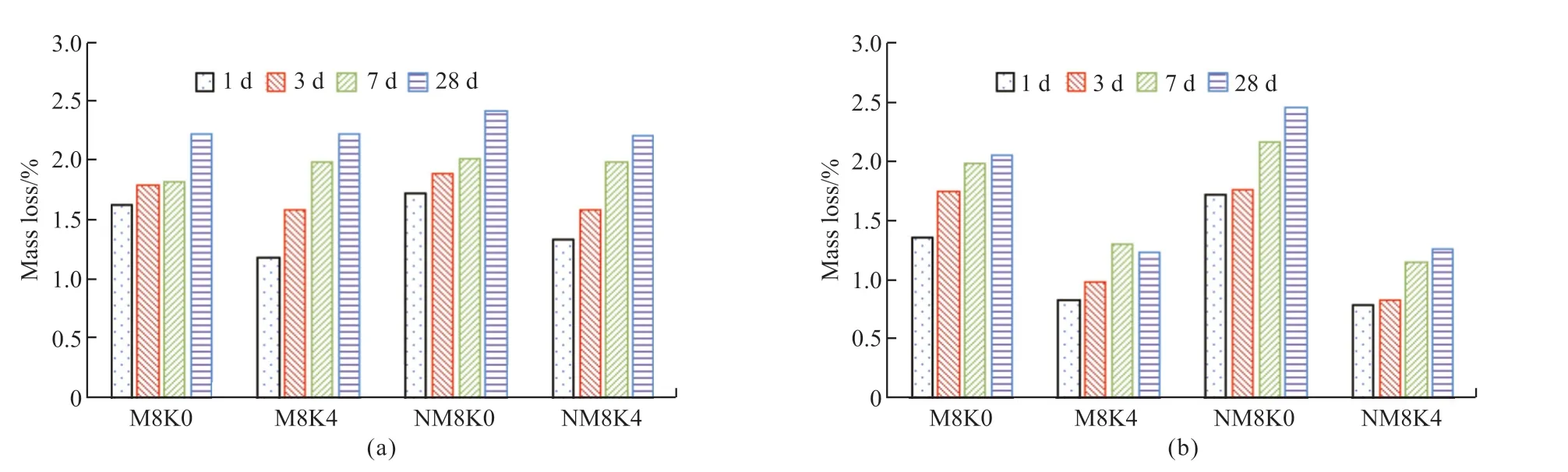
3.4 Microanalysis of MgO and nano-MgO HPC under the action of mineral powder
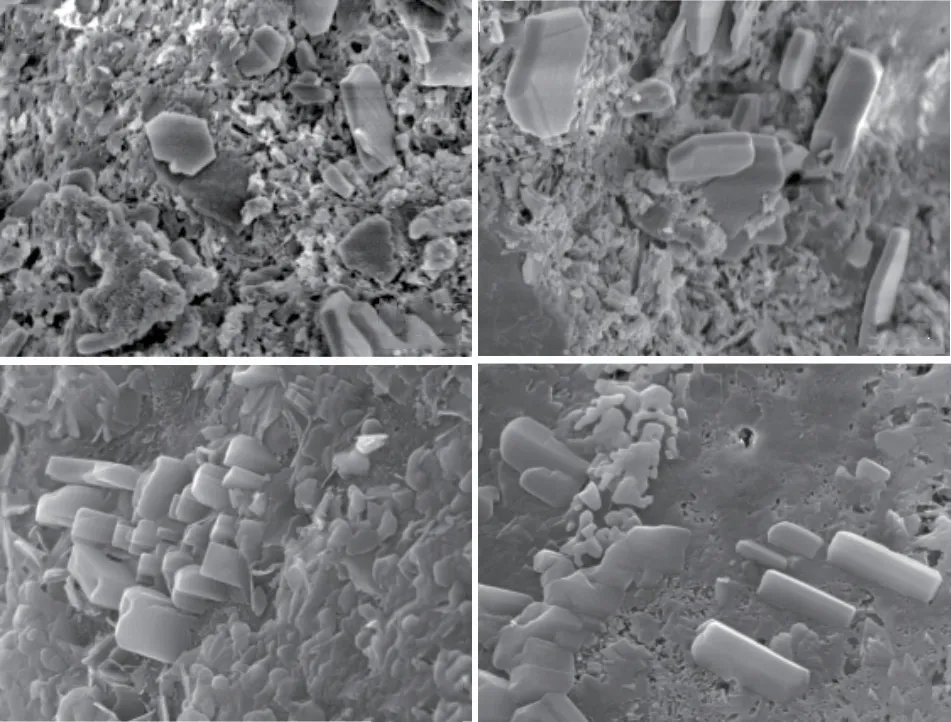
4 Conclusions
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Flow Characteristics Analysis of TC18 Titanium Alloy during Hot Deformation Based on Phase Transformation
- Effect of Hydrated Calcium Aluminate Cement on the Chloride Immobilization of Portland Cement Paste
- Effect of Curing Age on Tensile Properties of Fly Ash Based Engineered Geopolymer Composites (FA-EGC) by Uniaxial Tensile Test and Ultrasonic Pulse Velocity Method
- A Fiber Optic Sensor for the Simultaneous Measurement of Dual-parameter Based on Hydrogelimmobilized Enzyme Complex
- Energy-dispersive X-ray Spectroscopy for the Quantitative Analysis of Pyrite Thin Specimens
- Bioprocess-inspired Actin Biomineralized Hematite Mesocrystals for Energy Storage
