Preparation and Performance of Chitosan/Citric Acid Modified Magnesium Oxychloride Cement
2024-01-03SONGZijianLIULangYUPeipeiZHANGYunshengLIXinchengCHUHongqiangJIANGLinhua
SONG Zijian, LIU Lang, YU Peipei, ZHANG Yunsheng, LI Xincheng,CHU Hongqiang, JIANG Linhua
(1. College of Mechanics and Materials, Hohai University, Nanjing 211100, China; 2. School of Civil Engineering, Lanzhou University of Technology, Lanzhou 730050, China; 3. Yunnan Institute of Building Research Co., Ltd, Kunming 650223, China)
Abstract: Citric acid (CA) and chitosan (CS) were employed to modify magnesium oxychloride cement(MOC).Multiscale measurements were implemented to study the properties of the modified MOC pastes.Results show that the addition of CA/CS significantly changes the content of each phase and the microstructure of phase 5.The single addition of CA can effectively increase the compressive strength of MOC after 7 d curing,while CS exerts no obvious effect on the compressive strength.As to the simultaneous addition of CA and CS,the compressive strength of MOC gradually decreases with the increasing content of CS.Interestingly, mixing CA and CS significantly enhances the water resistance of MOC and decreases the degradation rate of MOC in phosphate buffered solution, which can be ascribed to the low specific surface area of the plate-like crystals in the modified MOC and the reduction of pores in the structure.
Key words: magnesium oxychloride cement; modifier; citric acid; chitosan
1 Introduction
Magnesium oxychloride cement (MOC) is an air-hardening cement made by mixing light-calcined magnesia powder, magnesium chloride, and water in a certain proportion[1].China has abundant reserves of magnesium, accounting for approximately 30% of global reserves and ranking first in the world[2].Moreover, the net CO2emissions of MOC are about 73%lower than those of Portland cements, which makes MOC a green and environmental-friendly cement[3].Hence, the rational utilization of MOC has great potential to alleviate resource wastes and environmental pollution caused by the production and use of Portland cements[4].
Due to their excellent mechanical properties, fire resistance, and abrasion resistance[5-7], MOC products have been widely used in many fields such as transportation, building materials, and decorative materials[8,9].However, it is also known that the poor water resistance of MOC products seriously hinders their further applications in civil engineering[10,11].Soaking MOC in water will cause significant degradation of the microstructure, directly affecting its performance in humid environments[10].Fortunately, this degradation phenomenon highlights its potential as an absorbable bone cement biomaterial.
The current bone cement biomaterials (eg, polymethyl methacrylate bone cement, calcium phosphate bone cement) suffer from multiple limitations, such as high heat release, poor biocompatibility with human tissues, non-spontaneous degradation, mismatched mechanical stability, and poor adhesion with the vertebral body[12].MOC exhibits good biocompatibility and no biological toxicity, which has gradually attracted the attention of researchers[13].However, as mentioned above, the degradation rates of ordinary MOC products are too fast to achieve stable degradation in humid environments.Therefore, it is of practical significance to study the addition of modifiers in MOC to control its degradation rate in liquid environments in the hope of obtaining a type of magnesium oxychloride bio-cement.
MOC modifiers mainly include inorganic and organic modifiers, as well as fiber-reinforced modifiers[14,15].The modifiers for bio-cement must meet the requirements of good biocompatibility, non-toxicity, and biodegradability.Citric acid (CA), as an already-existed organic acid in the human body, plays an important regulatory role in bone formation and induction[16].Related studies have found that CA has a slight effect on the mechanical properties of MOC, but significantly improves its water resistance[17].Chitosan (CS), a natural alkaline amino polysaccharide widely present in nature, also has gained attention from researchers in the field of biomaterials due to its biocompatibility,biodegradability, and excellent antibacterial properties[18].The degradation products of CS are neutral or slightly alkaline, which will not induce anemia or local metabolic disturbances in the implantation site of the body[19-21].Additionally, the hydrophilic surface of CS is conducive to cell adhesion, proliferation, and differentiation[22].Based on these characteristics, CS has recently been employed to improve the performance of calcium phosphate cements (CPCs) that is a bone cement used in clinical practice[23].However, the role of CS in improving the degradability and pore size distribution of this bone cement is not very ideal[23].
So far, few studies concerning the modification effect of CS on MOC are available.In particular, the variation in MOC performance and structure under the simultaneous modification of CS and CA is not well understood.For this purpose, Citric acid (CA)/chitosan (CS) MOC pastes were prepared and their phase composition, microstructure, mechanical properties,water resistance, and degradation rate were elucidated by various experimental approaches.The modification mechanisms of CA and CS on MOC were discussed.This study aims to contribute to augmenting the performance of MOC and providing new insight into achieving its further application as bio-cement.
2 Experimental
2.1 Raw materials
The active MgO (99% purity) was purchased from Shanghai Yuanjiang Chemical Co., Ltd, with a specific surface area of around 20 m2/g.Magnesium chloride hexahydrate (MgCl2⋅6H2O, 98% purity) was purchased from Chengdu Kelong Chemicals Co., Ltd.CA is citric acid monohydrate (C6H8O7•H2O), which was purchased from Chengdu Kelong Chemicals Co., Ltd.CS is carboxymethyl chitosan (C8H15NO6) with high solubility in neutral and alkaline solutions, which was purchased from Shanghai Yien Chemical Technology Co., Ltd.Phosphate buffered solution (PBS) selected was produced by Tianjin Zihan Biotechnology Co., Ltd.The PBS was used to provide a relatively stable soaking environment for the specimens.
2.2 Specimen preparation
To maximize the content of phase 5 in the hydration product system, a molar ratio ofn(MgO):n(MgCl2):n(H2O) was selected as 9:1:15.The dosage of CA was set at 0.5wt% of the active MgO powder.To maintain the good workability of the slurry, the amount of CS added should not exceed 0.5wt%.In the experiments of single addition of CS, its dosage was set at 0, 0.1wt%, 0.25wt%, and 0.5wt%, respectively.In the experiments of mixed addition of CA and CS,the dosage of CS was set at 0, 0.1wt%, 0.3wt%, and 0.5wt%, respectively while CA was fixed at 0.5wt%.For the above dosages, the control specimen without the addition of modifiers was labeled as N-MOC.The specimen with only CA was labeled as CA-MOC.The specimens with only CS were labeled as CS0.1%-MOC,CS0.25%-MOC, and CS0.5%-MOC according to the concentration of CS.The specimens with both CA and CS were labeled as CA/CS0.1%-MOC, CA/CS0.3%-MOC, and CA/CS0.5%-MOC.
A forced cement mixer (NJ-160A) was used to prepare CS/CA modified MOC.In order to reduce the influence of pH on the solubility of the modifiers, CS was completely dissolved in distilled water first, then CA and MgCl2were added and stirred evenly.Subsequently, the mixture was combined with reactive MgO powder and placed into the cement slurry mixer.After 60 s of slow stirring and 60 s of rapid stirring, the slurry was poured into a mold measuring 40 mm × 40 mm× 160 mm and vibrated using a ZS-15 cement mortar vibrating table for 60 s (15 mm amplitude, 60 cycles/60 s frequency) to make the MOC slurry dense.After standing in the air for 1 d, the specimens were demolded, and a batch of specimens was tested for compressive strength.After 1 d of air curing, the remaining specimens were transferred to a curing chamber with a temperature of 37 ℃ and relative humidity of 100%and continued to be cured for the required curing age.
2.3 Test methods
2.3.1 Macroscopic characterization
To test the compressive strength of the specimens,the MOC specimens measuring 40 mm × 40 mm × 160 mm were demolded after 24 h and cured under the corresponding conditions (air curing, standard curing) for the required age (1 d, 7 d, and 14 d).The corresponding compressive strength was tested using the following equation:
where,Krepresents the compressive strength of the specimen (MPa),Pthe failure load (N);Athe bearing area of the specimen (mm2).
To quantify the water resistance of the MOC specimens, the specimens were completely immersed in distilled water for 14 d after being cured for 14 d in the curing chamber to measure the residual compressive strength after soaking.The softening coefficient(Kf) was calculated using the following equation:
where,Kis the compressive strength of the MOC specimen cured for 14 d (MPa), andKrthe residual compressive strength of the 14 d-cured MOC specimen after immersion in distilled water for another 14 d (MPa).Moreover, a higher value ofKfindicates a better water resistance of the MOC specimens.
To measure the degradation ratio of the different MOC specimens, several crushed MOC fragments from each group in the compressive strength test were taken and divided into five groups based on the type of modifier.They were labeled as N-MOC, CA-MOC, CA/CS0.1%-MOC, CA/CS0.3%-MOC, and CA/CS0.5%-MOC,with several fragments per group.The obtained fragments were immersed in a PBS solution, and the PBS solution was replaced and refreshed every two days.The mass loss of each MOC fragment specimen was measured, and the entire process lasted for 28 days.Subsequently, the degradation rate of the specimens was fitted and the final degradation days were further estimated.
2.3.2 Microscopic characterization

Fig.1 XRD patterns of N-MOC, CA-MOC, and CA/CS0.5%-MOC after 1 d (a) and 7 d (b) curing
MOC fragments collected after the compressive strength tests were immersed in anhydrous ethanol for 24 h, and the resulting specimens were dried in an electric blast drying oven at 40 ℃ for an additional 24 h to cease the hydration of MOC[13].The samples for XRD(Rigaku Ultima IV) were obtained by grinding these specimens, which were stored in isolation from the air.XRD analysis with Cu Kα radiation was conducted over a 2θrange of 5°-120° with a working voltage of 40 kV and a working current of 30 mA.In addition, the samples for Fourier transform infrared (FTIR) spectroscopy (Vector-22) were obtained by crushing the dry fragments into powder.FTIR spectra were collected in the range of 500-4 000 cm-1with a spectral resolution of 1 cm-1.For the microstructure analysis, SEM was performed using dry flat fragments that were sprayed with gold on the surface (ZEISS Sigma 300).All specimens for microscopic characterization were sampled from the center of the crushed MOC fragments to avoid inconsistencies.
3 Results and discussion
3.1 Characterization of CS/CA modified MOC
3.1.1 Phase analyses
Fig.1 shows the XRD patterns of N-MOC, CAMOC, and CA/CS0.5%-MOC after 1 and 7 d curing.Obviously, characteristic peaks of Mg(OH)2can be observed at 18.716°, 38.097°, and 50.901°, while characteristic peaks of phase 5 can be observed at 11.955°,21.470°, and 37.140°.This indicates that the mineral phases in these three MOC specimens mainly consist of phase 5 and Mg(OH)2crystals, and the modifiers exhibit no obvious corresponding peaks due to their low content.Compared with N-MOC, the Mg(OH)2peaks in CA-MOC spectra are smaller, while the phase 5 peaks are relatively pronounced, indicating that the content of phase 5 in CA-MOC is higher than that in N-MOC.The reason may be that the polar groups in the citrate ionized from citric acid undergo chelation reaction with Mg2+, forming an organic magnesium complex layer on the surface of active MgO, which decreases the rate of generating Mg(OH)2phase[24], thus promoting the hydration reaction to the direction of generating the main products.Notably, for the phase composition after 1 d curing, the phase 5 peaks of CA/CS0.5%-MOC are stronger in comparison to N-MOC and CA-MOC, indicating a higher content of phase 5 in CA/CS0.5%-MOC specimen.However, after 7 d curing, the peak intensity of phase 5 of CA/CS0.5%-MOC is no longer higher than that of CA-MOC, and the peak intensity ratio of phase 5/Mg(OH)2is also lower than that of CA-MOC, which may be attributed to the decomposition of phase 5 into Mg(OH)2after water penetration in the high humidity curing environment.
3.1.2 Microstructure
Fig.2 displays the microstructure of N-MOC after 1 d air curing.It can be seen from Fig.2 that the MOC matrix is porous, which provides a place for nutrient transfer and metabolism.Moreover, there is a sign of crystal aggregations on the inner wall of the holes,which are the well-developed short rod-shaped and fibrous bundle-like phase 5 crystals with no obvious orientation[25].The distribution of short rod-shaped phase 5 crystals is irregular, with the phenomenon of point contacts and mutual overlaps between crystals.The fibrous bundle-like phase 5 crystals are tightly connected to each other, forming a fibrous network skeleton,and some pores in the network structure are filled with Mg(OH)2.Additionally, granular phase 5 can be observed on the surface of lamellar and blocky Mg(OH)2crystals, which may be due to the phase transformation between phase 5 and Mg(OH)2at the initial stage of hydration.

Fig.2 Microstructures of N-MOC after 1 d air curing

Fig.3 Microstructures of CA-MOC after 1 d air curing

Fig.4 Microstructures of CA/CS0.5%-MOC after 1 d air curing
Fig.3 depicts the microstructure of CA-MOC after 1 d air curing.Compared with the N-MOC specimen,the crystal morphology of phase 5 in the CA-MOC specimen is converted from a short rod-shaped structure into a needle-like fine fiber structure with a higher aspect ratio, which may be attributed to the hydrogen bonding between the ester groups in CA and the hydroxyl groups in phase 5, or the complexation between the ester groups in CA and Mg2+.As the cross-stacked accumulation of the needle-like fine fiber crystals, they overlap into hydration products.Ultimately, these hydration products further overlap and combine, resulting in the formation of a sophisticated multiphase porous network structure.
Fig.4 illustrates the microstructure of CA/CS0.5%-MOC after 1 d air curing.Evidently, under the combination of CA and CS, another transformation in the crystal morphology of phase 5 in the CA/CS0.5%-MOC specimen can be observed in comparison to the CAMOC specimen.The needle-like fine fiber structure transitions into a flat plate-like structure with no apparent crystal edges, exhibiting a lamellar morphology.The hydration products interconnect and appear in a gelatinous state.The alteration in crystal morphology of phase 5 may be due to the hydrogen bonding between the amino groups in CS and the hydroxyl groups in phase 5, or the complexation between the amino groups in CS and Mg2+.Furthermore, the connection area of the hydration products is larger than that of N-MOC and CA-MOC, while the pore size is smaller than that of the branched structure observed in CA-MOC.Both the pore structure and distribution of hydration products become more uniform compared with the CAMOC specimen.
Based on the above observations, it can be found that the addition of modifiers CA and CS causes the variation of crystal morphology during the hydration process of MOC.The alteration of microstructure leads to modifications in the specific surface area of the hydration products, consequently affecting the hydrolysis reaction area of hydration products.The variation in pore structure influences the pathways for moisture infiltration and ion transport.These factors are bound to exert an impact on the compressive strength, water resistance, and degradation rate of MOC in liquid environments, which will be further discussed in subsequent analyses.
3.1.3 FTIR analysis
Fig.5 shows the FTIR spectra of N-MOC and CS0.5%-MOC specimens after curing for 7 d.Obviously, these two MOC samples exhibit similar absorption bands from 500 to 4 000 cm-1.The absorption peaks at 3 735.97 and 3 606.97 cm-1are features of the hydroxyl group stretching vibration in the phase 5 crystals, and the strong absorption band from 3 000 to 3 600 cm-1is caused by the stretching vibration of the numerous hydroxyl groups[26].The absorption peaks from 1 610.07 to 1 648.05 cm-1can be attributed to the H-O-H bending vibration[27].The Si-O-Si stretching vibration at 1 026.36 cm-1reveals the presence of silicate or quartz in the MOC substrate[28].Compared with the N-MOC specimen, it can be observed from the characteristic peaks that the incorporation of CS induces no substantial alteration in the chemical composition of MOC.However, in the spectrum of CS0.5%-MOC, there is a significant increase of the transmittance at 3 735.97 and 3 606.97 cm-1, indicating an elevated content of phase 5 in CS0.5%-MOC specimen, which is probably due to the complex reaction between free amino groups in CS and Mg2+, promoting the reaction process of active magnesium oxide generating phase 5.The above results are consistent with the findings obtained from XRD.

Fig.5 FTIR spectra of N-MOC and CS0.5%-MOC after 7 d curing
3.2 Performance of CS/CA modified MOC
3.2.1 Compressive strength

Fig.6 Compressive strength of N-MOC and CA-MOC specimens after 1 and 7 d curing
Fig.6 illustrates the compressive strength of N-MOC and CA-MOC specimens after 1 and 7 d curing.It can be seen from Fig.6 that the compressive strength of the CA-MOC specimen cured in the air for 1 d is slightly lower than that of the N-MOC specimen.This can be attributed to that the addition of CA changes the pH value of the liquid phase, which to some extent affects the formation of phase 5[29].For the N-MOC specimen, under the high humidity curing condition,the compressive strength after 7 d curing decreases by 42.8% compared to that after 1 d curing owing to the rapid diffusion of moisture into the MOC matrix through surface pores[30].In contrast, the addition of 0.5wt% CA effectively prevents this trend.The CAMOC specimen exhibits a 46% increase in compressive strength after 7 d curing compared to that after 1 d curing.This is probably due to the fact that the carboxyl groups of citrate ions chelate with magnesium ions to form stable chelates, thereby forming an organic magnesium complex layer that slows down the rate of Mg2+reacting with OH-to form unstable magnesium hydroxide phases.This factor prolongs the hydration process and facilitates the reaction process of active magnesium oxide generating phase 5[29], thereby enhancing the compressive strength of MOC.

Fig.7 Compressive strength of N-MOC, CS0.1%-MOC, CS0.25%-MOC,and CS0.5%-MOC specimens after 1 and 7 d curing

Fig.8 Compressive strength of N-MOC, CA-MOC, CA/CS0.1%-MOC, CA/CS0.3%-MOC and CA/CS0.5%-MOC specimens after 1 and 7 d curing
Fig.7 shows the compressive strength of MOC mixed with different amounts of CS after 1 and 7 d curing.It is obvious that CS has no significant effect on the compressive strength of MOC.As can be seen from Fig.7, the addition of 0.1wt% CS slightly decreases the compressive strength of MOC, while the addition of 0.25% CS reduces the compressive strength by 12.0%and 16.6% after 1 and 7 d curing, respectively.The addition of 0.5wt% CS slightly increases the compressive strength of MOC.These results indicate that CS at a lower dosage has no significant effect on the compressive strength of MOC.The 0.25wt% dosage has the most obvious strength reduction effect on MOC,while the 0.5wt% or higher dosages may improve the compressive strength of MOC.At a lower dosage,CS affects the crystallization process and inhibits the formation of phase 5, resulting in a slight decrease in compressive strength.At the 0.5wt% or higher dosages, the free amino groups of CS undergo a complex reaction with Mg2+, promoting the reaction process of active magnesium oxide generating phase 5[31], which is consistent with FTIR analysis.
Fig.8 shows the compressive strength of CA/CS modified MOC after 1 and 7 d curing.It can be seen that adding CS will reduce the compressive strength of CA modified MOC.As the amount of CS increases,the negative impact on compressive strength becomes greater.Subsequently, the decreasing trend of compressive strength of MOC slows down and gradually tends towards a stable value.The possible mechanism is that the ester groups in CA react with the amino groups in CS, hindering the chelation of CA and CS with Mg2+,thereby inhibiting the formation of organic magnesium complex layer, which is not conducive to the formation of more phase 5.
3.2.2 Surface morphology
Fig.9 shows the morphological differences between N-MOC and CS-MOC after 7 and 14 d curing.It can be observed from Figs.9(a) and 9(b) that there is a sign of halogenation and frost on the surface of the N-MOC specimens.Furthermore, as the curing age increases, the halogenation area increases and the frost phenomenon becomes more severe[11].In contrast, the surface of the specimens mixed with CS is relatively flat and smooth, with only slight frost, as shown in Figs.9(c) and 9(d), revealing that the addition of CS enhances the volume stability of the MOC specimens.Additionally, a slight warping phenomenon can be observed on the side of the formed specimen (Fig.9(b)).

Fig.9 Surface morphology of MOC with and without CS after 7 and 14 d curing: (a) 7 d N-MOC; (b) 14 d N-MOC; (c) 7 d CSMOC; (d) 14 d CS-MOC
3.2.3 Softening coefficient
Fig.10 illustrates the residual compressive strength and softening coefficient of N-MOC, CAMOC and CA/CS-MOC specimens after soaking.The residual compressive strength of CA-MOC, CA/CS0.1%-MOC, CA/CS0.3%-MOC, and CA/CS0.5%-MOC is 8.7,8.1, 9.3, and 8.5 times that of N-MOC, respectively,and the softening coefficients are 3.4, 4.7, 6.2, and 5.8 times that of N-MOC, respectively.This demonstrates that the addition of CA and CS greatly improves the water resistance of MOC, which can be attributed to the low specific surface area of the plate-like crystal shape of the CA/CS-MOC specimen and the block effect of gel state substances formed by the combination of CS and hydration products.It is noteworthy that the 0.3% dosage of CS has the highest improvement in the water resistance of MOC.Accordingly, these results are in good agreement with the findings of SEM images(Figs.2-4).

Fig.10 Residual compressive strength and softening coefficient of N-MOC, CA-MOC, CA/CS0.1%-MOC, CA/CS0.3%-MOC, and CA/CS0.5%-MOC specimens after soaking
3.2.4 Degradation rate
Fig.11 shows the mass loss of N-MOC, CA-MOC,and CA/CS-MOC specimens during 28 d of immersion in PBS solution.Evidently, the mass loss curves of all specimens display a monotonic decreasing trend during the immersion.As can be seen from Fig.11, the N-MOC specimen exhibits the fastest degradation rate, and the addition of CA significantly slows down the degradation rate of MOC.Moreover, the distribution of the mass loss curves of the specimens mixed with CA and CS is slightly complicated.The decrease in degradation rate is not proportional to the increase in CS dosage,but the degradation rate of the CA/CS-MOC specimen is still slower than that of the N-MOC specimen.
By fitting the obtained data, all the experimental groups display a linear relationship between mass loss and time (R2> 0.94).The linear equation can be used to predict the time required for the complete degradation of each specimen, and the predicted results are shown in Table 1.Compared with the N-MOC specimen,the predicted degradation completion time of the CA/CS0.1%-MOC specimen increases by 130 d, indicating that the degradation rate of MOC can be regulated by adding CA and CS.This can be attributed to the alteration in crystal morphology of phase 5 after the addition of CA and CS.At higher CS dosages (0.3wt%and 0.5wt%), the degradation rates of CA/CS-MOC specimens are higher than that of the CA-MOC spec-imen.Meanwhile, at the lower CS dosage of 0.1wt%,the degradation rate is slowed down.This rule indicates that the 0.1wt% CS dosage exhibits the optimal effect on improving the degradation rate of MOC.
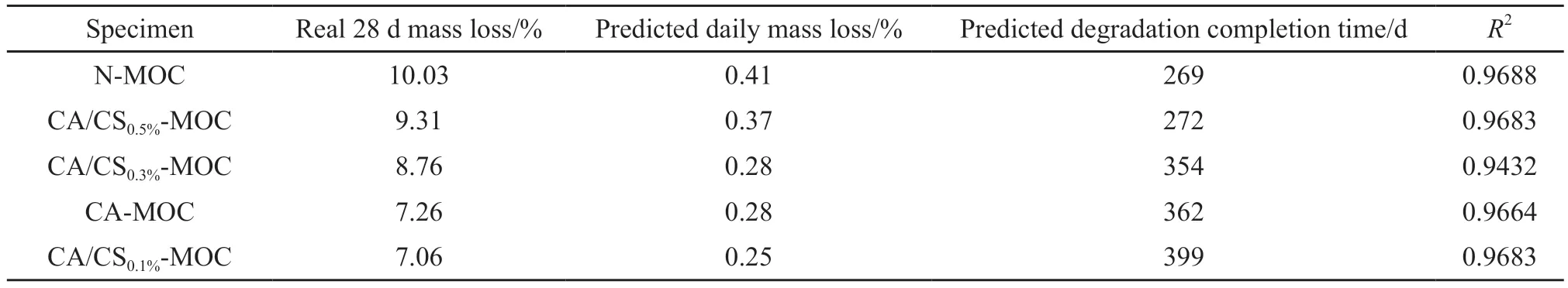
Table 1 Measured and predicted degradation data of specimens.

Fig.11 Residual mass ratio of N-MOC, CA-MOC, CA/CS0.1%-MOC, CA/CS0.3%-MOC, and CA/CS0.5%-MOC specimens during 28 d of immersion in PBS solution
3.3 Discussion
According to the Refs.[26,32], the hydration process of MOC is an ion reaction process that can be divided into three stages: neutralization, hydrolysis, and crystallization.It is generally considered that phase 5 is the main component responsible for the mechanical strength of MOC, and the crystal morphology of hydration products and the pore size distribution also play a crucial role in the mechanical properties of MOC[33].The main hydration product of N-MOC (phase 5)exhibits a short rod-shaped crystalline morphology(Fig.2), with a larger specific surface area and a larger reaction area in contact with water, resulting in the high sensitivity of N-MOC to water.Therefore, in high humidity or aqueous environments, water rapidly invades the structure of N-MOC from pores, causing a continuous hydration reaction of active magnesium oxide,which directly affects volume stability and leads to the development of cracks.
After the addition of CA, the microstructure of CA-MOC becomes more uniform, and the short rodshaped crystals transform into needle-like crystals with a high aspect ratio.The contact points of the hydration products become denser, forming a multiphase fiber network structure.These can be verified by the morphologies shown in the SEM image (Fig.3).In addition,CA ionizes into citrate ions, which combine with Mg2+to form stable chelates, thus reducing the concentration of Mg2+in the system[24].This delays the hydrolysis reaction of phase 5, consequently decreasing the degradation rate of MOC, which can be well supported by the results of Table 1.
After mixing CA and CS into the MOC, the crystal morphology of phase 5 is converted from a short rod-shaped structure in N-MOC into a flat plate-like structure with no apparent crystal edges that exhibits a smaller specific surface area compared with the N-MOC and CA-MOC, which is probably due to the hydrogen bonding of the ester groups in CA and the amino groups in CS with the hydroxyl groups in phase 5, or the complexation of the ester groups in CA and the amino groups in CS with Mg2+.This can be well supported by the SEM image (Fig.4).The phases in CA/CS-MOC bond to each other in gelatinous state,resulting in a more uniform distribution of hydration products and pores.Moreover, the gelatinous state substances generated by CS and hydration products block some of the internal capillary pores, reducing the pathways for water infiltration, thereby improving the water resistance and decreasing the degradation rate of MOC(Figs.10 and 11).Notably, the ester group in CA may undergo a condensation reaction with the amino group in CS to generate the amide group and form a network cross-linked structure, which inhibits the combination of Mg2+with CA and CS.This is not conducive to alleviating the formation of Mg(OH)2with poor stability and hinders the reaction process of more active magnesium oxide forming phase 5.Therefore, compared with the addition of CA, the addition of CA and CS has a negative impact on the compressive strength of MOC(Fig.8).
4 Conclusions
a) The MOC with a molar ratio ofn(MgO):n(MgCl2):n(H2O) of 9:1:15 is mainly composed of phase 5 and Mg(OH)2phase.The addition of CA/CS has no obvious impact on the composition of the MOC phase, but significantly changes the microstructure of phase 5 and the content of each phase.
b) For the N-MOC specimen, the compressive strength decreases by 42.8% after 7 d curing compared to that after 1 d curing.When adding 0.5wt% CA, a 46% increase in compressive strength can be recorded for the CA-MOC specimen.The simultaneous addition of CA and CS reduces the compressive strength of MOC in comparison to the single addition of CA.
c) The addition of CA and CS significantly enhances the water resistance of MOC, with the residual strength after immersion being approximately 8 times that of the N-MOC, and the softening coefficient being about 3-6 times higher than that of the N-MOC.The inclusion of 0.3wt% CS shows an optimal improvement in the water resistance of MOC.
d) The addition of CA and CS can effectively slow down the degradation rate of MOC.The degradation rate of MOC is as follows: CA/CS-MOC group(lower CS dosage) < CA-MOC group < CA/CS-MOC group (higher CMCS dosage) < N-MOC group.The addition of 0.1wt% CS dosage has the optimal effect on decreasing the degradation rate of MOC.
Conflict of interest
All authors declare that there are no competing interests.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Enhanced Electrochemical Performances of Ni Doped Cr8O21 Cathode Materials for Lithium-ion Batteries
- Design on the Prestressed Concrete Frame Beam-column
- Synthesis and Flocculation of Polyacrylamide with Low Water Absorption for Non-dispersible Underwater Concrete
- Experimental Behavior of Recycled Aggregate Concrete Filled Steel Tubular Columns
- Impact-abrasive Wear Behavior of ZTA and NbC Reinforced Fe60 Matrix Composites
- Synthesis and Characterization of Hollow Strontium Carbonate Pompons by Composite Soft Template Method
