Preparation of Chiral Silica Nanostructures with Radial Pores through Single-templating Approach
2024-01-03ZHAXinlinFANHuiCHENYuanli
ZHA Xinlin, FAN Hui, CHEN Yuanli
(Key Laboratory of Textile Fiber and Products(Wuhan Textile University), Ministry of Education, Wuhan 430073, China)
Abstract: A chiral low-molecular-weight gelator (LMWG) L-16Ala5PyPF6 was synthesized from L-alanine, which can cause physical gel in n-propanol, ethyl acetate, butylene oxide, water, benzene, 1,4-dioxane and chloroform.The sol-gel reactions were carried out in a mixture of stronger ammonia water and n-propanol at the volume ratio of 2:8.Single-handed twisted silica nanostructures with pore channels vertical to the wall surfaces were first prepared through a single-templating approach comparing with the reported double template method.The formation mechanism of radial pore structure was studied by transmission electron microscopy at different reaction time intervals, which indicated that the radial pore structure was formed via a structural transition in the sol-gel transcription process.
Key words: sol-gel preparation; radial pores; vertical pore channels; single-handed; structural transition
1 Introduction
In the past few decades, mesoporous nanomaterials have experienced rapid development[1-3].Especially, the single-handed helical mesoporous silica nanostructures have been extensively explored due to their potential application in the fields such as asymmetric catalysis[4], chiral recognition[5], and enantioseparation[6].To date, single-handed twisted silica nanoribbons, helical and nanofibers have been reported using soft-templates[7,8].Generally,the Brunauer-Emmett-Teller (BET) surface areas of conventional chiral silica nanomaterials were low.As a result, the preparation of chiral silica nanomaterials with high BET surface areas is particularly attractive to researchers.Though helical silica nanostructures with BET about 705 m2·g-1were also prepared, the pores were parallel to the axis not vertical to the walls,which seriously affected the mass transfer and the interactions between the host and guest[8].Therefore,designing a simple method to fabricate chiral silica nanostructures with pore channels perpendicular to the walls and high BET is highly desirable for increasing mass transfer.
So far, some literatures have reported silica nanostructures with radiation holes on the surface[9-16].Up to now, the dual template method is the most commonly used method for the preparation of radiation pore materials.Guo’s group reported silica with mesopores vertical to the walls using P123 as template through interfacial growth method by changing the channel diameter of PAA or PC membranes[15].Che group reported mesoporous silica film with vertical channels on substrate using gemini surfactants[17].In recent years, various of vertical channels have been reported by Kao[18]and Xionget al[19]But there were few reports about chiral nanomaterials with radiation holes on the surface.Although there was a study generating chiral silica with vertical pores through a single-templating approach, the samples were a mixture of nanotube and nanosphere rather than a uniform tubular structure[8].That is to say, there is currently no research on the preparation of well-shaped,morphologically homogeneous single-handed helical or twisted nanostructures with vertical pore channels to the wall surfaces through a single-templating method.
Herein, for the first time, single-handed twisted silica tubular nanoribbons with radical pore channels were first formed through a single-templating approach.Supramolecular self-assemblies of chiral alanine amphiphilic small molecules acted as templates to control the formation of pore channels vertical to the walls.Importantly, the perpendicular pore channels were formed by removing the templates via a structural transition in the sol-gel transcription process.
2 Experimental
2.1 Materials
Tetraethyl orthosilicate,n-propanol, isopropanol,absolute methanol, 1,4-dioxane, tetrahydrofuran(THF), N, N-Dimethylformamide (DMF), absolute ethanol, ammonia (25.0wt%) and hydrochloric acid (35.0wt%-37.0wt%) were purchased from the Sinopharm Chemical Regent Co.Ltd.The chiral alanine amphiphilic low-molecular-weight gelator L-16Ala5PyPF6[8]L-16Ala5PyClO4[20]were synthesized according to the previous literatures.
2.2 Characterization methods
FT-IR spectrum was taken on a Bruker Vertex70 spectrometer.1HNMR spectrum was recorded on a Bruker AVANCE 400 spectrometer in DMSO-d6 solutions using TMS as an internal standard.Elemental analyses were performed on a Perkin Elmerseries II CHNS/O analyzer 2400.TEM images were obtained using a TecnaiG220.Field emission scanning electron microscopy (FESEM) images were taken on a JEOL-7800F.Specific surface areas and pore-size distribution were determined by the Brunauer-Emmett-Teller(BET) and Barrett-Joyner-Halon (BJH) methods using N2adsorption-isotherm measured by a Micromeritics ASAP 2460 instrument.Small-angle X-ray diffraction(SAXRD) patterns were taken on a Bruke D8 Advance X-ray diffractometer.Circular dichroism (CD) and ultraviolet-visible (UV-Vis) spectra were taken on a JASCO J-1500 CD spectrophotometer.
2.3 Characterization of LMWA L-16Ala5-PyPF6
FT-IR (KBr): 3 286 cm-1(N-H, amide A),1 637 cm-1(CO, amide I), 1 548 cm-1(N-H, amide II).1H-NMR (400 MHz, DMSO-d6, TMS, 25 ℃):δ= 0.856(t, J = 4.8 Hz, 3H; CH2CH3), 1.16 (d, J = 4.8 Hz, 2H;NHCHCH3), 1.237 (br, 26H; alkyl), 1.347-1.368 (m,2H; CONHCH2CH2), 1.486 (m, 2H; CH2CH2CONH),1.904 (m, 2H; PyCH2CH2), 2.173 (t, 2H; CH2CONH),2.984-3.049 (m, 2H; CONHCH2), 4.228 (m, J = 4.8 Hz, 1H; NHCHCOCH3), 4.606 (t, J = 4.8 Hz, 2H;CH2Py), 7.826 (t, J = 3.6 Hz, 1H; CONHCH2) 7.969 (d,J = 5.2 Hz, 1H; CONHCH), 8.170 (t, J = 4.8 Hz, 2H;3-PyH), 8.613 (t, J = 5.2 Hz, 1H; 2-PyH), 9.087 (d, J =4.0 Hz, 2H; 2-PyH).Elemental analysis calcined (%):C29H52F6N3O2P, C, 56.21; H, 8.46; N, 6.78.Found: C,56.78; H, 8.54; N, 6.66.
2.4 Preparation of single-handed twisted silica tubular nanoribbons with radical pore channels
In a typical synthesis, L-16Ala5PyPF6(12.5 mg,0.020 mmol) was dissolved inn-propanol (0.2 mL).Then concentrated ammonia (0.8 mL, 25.0wt%) was added to form a homogeneous solution and put into a 0 ℃ ice-water bath.Subsequently, 20 mg (0.096 mmol) of tetraethyl orthosilicate (TEOS) was added into the above solution with vigorous stirring by vortex agitator for 15 s and kept still at 0 ℃ for 2 hours.Then, the reaction system was heated up to 80 ℃ and aged for 4 days.The reaction mixture was filtered and washed with a mixture of ethanol and concentrated hydrochloric.Finally, the resultant white power was calcined at 550 ℃ for 5 h to remove the templates and denoted as TSTV.
2.5 Ibuprofen adsorption and release
Typically, for adsorption study, 100 mg TSTV degassed at 100 ℃ for 24 h was added into 20 mL of 10 mg/mL ibuprofen hexane solution.Then, the mixture was sonicated for 40 min and stirred overnight at room temperature to ensure complete adsorption.Subsequently, the loaded TSTV was separated through centrifugation and the loading capacity was calculated by UV-Vis analysis of the supernatant.For release research, the dried solid products obtained in the above procedures were re-dispersed in 250 mL PBS buffer solution (pH 7.4) under constant stirring.At each specific time point, 10 mL of the mixed solution was taken out and centrifuged for 3 minutes.The real-time IBU concentration was analyzed using the absorption value of 2.6 mL supernatant at a wavelength of 264 nm measured by UV-Vis spectrophotometer.It should be noted that the 10 mL of the removed mixture should be returned to the buffer solution immediately after the test was completed.
3 Results and discussion
3.1 Circular dichroism (CD) spectroscopy and scanning electron microscopy(SEM) study
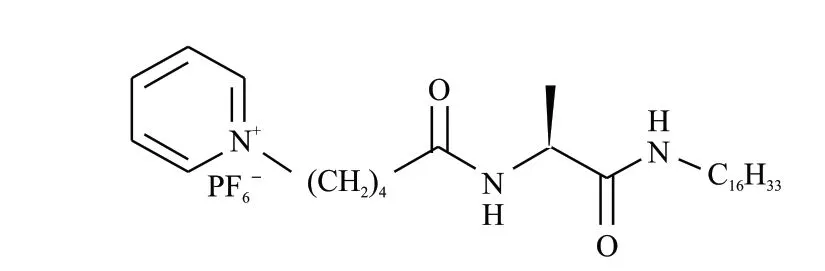
Fig.1 Molecular structure of L-16Ala5PyPF6
The molecular structure of the low-molecularweight gelator (LMWG) L-16Ala5PyPF6is shown in Fig.1.It can cause physical gel inn-propanol, ethyl acetate, butylene oxide, water, benzene, 1,4-dioxane and chloroform.The circular dichroism (CD) and ultraviolet (UV) spectra of the L-16Ala5PyPF6aggregates in pure water were taken in a 0.1 cm sample cell (Fig.2(a)).Though there’s a weak light scattering effect observed in the UV spectrum, no linear dichroism signal is found.One positive cotton effect signal at 204 nm and three negative cotton effect signals at 223, 255.6, 262.5,and 269.4 nm are found with increasing concentration from 0.02 to 0.10 mg·mL-1.The negative cotton effect signals bands at 250-270 nm originated from the π-π interactions between neighbouring pyridine rings.It demonstrates that pyridine rings pack in lefthandedness.The negative cotton effect signal at 223 nm originated from carbonyl groups, indicating that the carbonyl groups stack into left-handedness.The SEM and TEM images show that 12.5 mg of L-16Ala5PyPF6assembled into right-handed twisted nanoribbons in 1.0 mL of deionized H2O (Fig.2(b)).
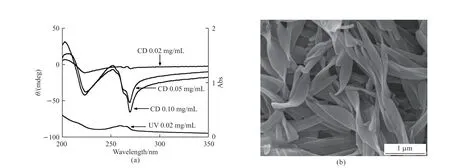
Fig.2 (a) CD and UV absorption spectra of L-16Ala5PyPF6 aggregates in water; (b) SEM image of assemblies of 12.5 mg of L-16Ala5PyPF6 in 1.0 mL H2O
Due to the morphology of the supermolecule self-assemblies of L-16Ala5PyPF6is difficult to be transferred in pure n-propanol, the sol-gel replication was studied in a mixture of water and n-propanol.The surface morphologies of TSTV were observed by a field emission scanning electron microscopy (FESEM).The specimen was sputtered with 10 nm Pt before taking SEM pictures.As shown in Fig.3(a), TSTV is righthanded twisted nanorods with uniform morphology.The lengths of them are about several microns.The high magnification TEM images indicated twisted tubular structures (Figs.3(b)-3(c)).The outside and inside diameters of the tubular nanoribbons are about 50-110 and 25-60 nm.It should be noted that the pores were found vertical to the walls of the twisted silica tubular nanoribbons.The pore size is about 3.9 nm.The thickness of the twisted tubular nanoribbons is about 24.6 nm.The holes on the surface of this type of pipe are perpendicular to the surface of the pipe.Compared to traditional pipes with open ends, this structure is more conducive to the storage and release of drugs and is not prone to drug blockage.
3.2 Morphology study
The N2adsorption-desorption isothermals and pore size distribution curve of the sample are recorded in Fig.4.The sample shows a mesoporous structure, which exhibits a typical type IV curve.The H3 hysteresis loop atP/P0= 0.4-0.85, 0.85-0.95 and 0.95-1 originated from the vertical pores channels on the walls, the cavities of TSTVs and the voids among TSTVs, respectively.The BET surface area and pore volume of the sample are calculated 531 and 0.8 cm³/g,respectively.The high specific surface area is beneficial for their potential applications in drug release and asymmetric catalysis.The Barrett-Joyner-Halenda(BJH) pore size distribution plot, shows a uniform mesopore at 3.9 nm (Fig.4(b)) corresponding to the diameter of the mesopores in the walls.
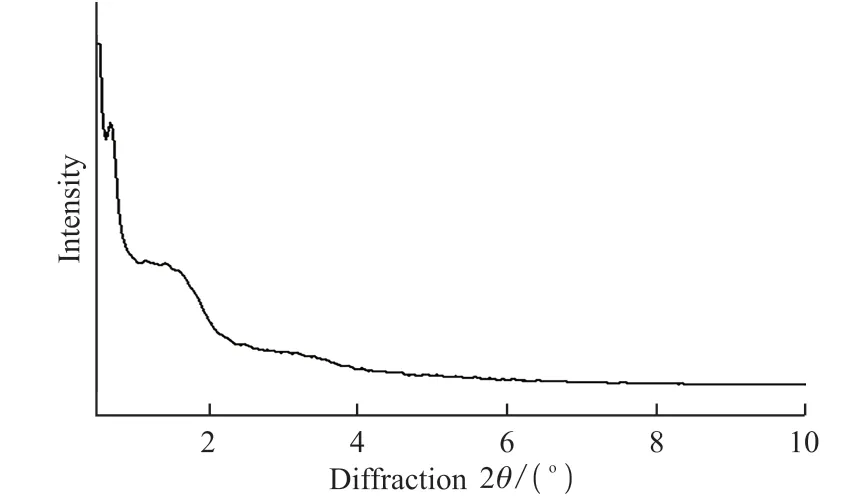
Fig.5 Small-angle X-ray diffraction pattern (Cu Kα radiation λ =0.15418 nm) of TSTV
The XRD pattern of the calcined TSTV is shown in Fig.5.One sharp diffraction peak and two broad diffraction peaks at 2θof TSTV were approximately 0.68°, 1.44°, and 3.16°, indicating an ordered structure.For the right-handed twisted silica tubular nanoribbons with perpendicular pore channels to the walls shown here, the mesopores are proposed to be formed via a structural transition in sol-gel transcription process[16].
3.3 Solvent effect study
Simultaneously, solvent effect was also studied in the reaction system.Different silica nanostructures were obtained by replacingn-propanol with equal amounts of different solvents in the reaction system in Fig.6.If an equal volume of isopropanol(a), ethanol(b),ethyl acetate(c) or 1,4-dioxane(d) was used instead of n-propanol, silica nanosheets were obtained.It can be seen that solvents play an important role in the selfassembly process of chiral small molecules during the chiral replication of silica, resulting in the formation of different silica nanostructures in different solvent environments[21-24].Up to now, the dual-template method is the most common method to fabricate radiation holes[15,17].To the best knowledge of us, this is the first report on preparing single-handed twisted silica tubular nanoribbons with vertical pore channels in the walls through a single-templating approach.Meanwhile, Yang reported 2D or 3D pore architecture prepared using L-ValPyPF6as template[8], it can be seen concluded that PF6-also played a crucial role in the formation of radiation holes.The formation of these radiation channels may also be related to the corrosion of silicon by fluoride ions.
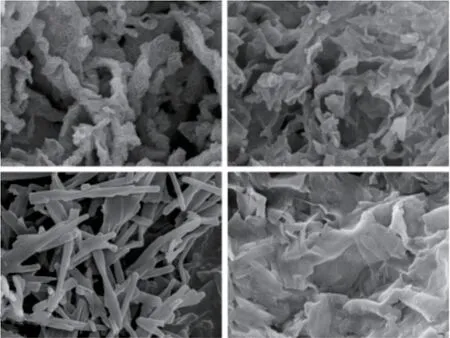
Fig.6 FESEM images of various silica nanostructures
3.4 Mechanism investigation
To investigate the structures evolution of the vertical holes in the walls, the forming process was studied by observing the reaction system at different reaction time intervals using TEM (Fig.7).Similar mechanism studies have been reported in the published literatures[21,24].One drop of the solution was taken out from the reaction system and placed on the supporting films of TEM grids at different reaction time intervals after the dripping of TEOS.The supporting films were quickly blow-dried using nitrogen to terminate the reaction.Before adding TEOS, it was found that the self-assemblies of L-16Ala5PyPF6showed a double twisted ribbon structure in the reaction mixture (Fig.7(a)).However, only fine nanospheres and nanoribbons were identified at 5 s after dropping TEOS (Fig.7(b)).Therefore, it was concluded that L-16Ala5PyPF6re-assembled, when TEOS was added into the reaction mixture.Silica oligomers were adsorbed on the both sides and the surfaces of the nanoribbons through the electrostatic interactions at 15 s (Fig.7(c)).As the reaction proceeded, silica oligomers decreased at 30 s (Fig.7(d)).Due to the silica oligomers concentrated on both the surfaces and the edges of the double twisted nanoribbons, hence tubular nanoribbons were generated (Fig.7(e)).Meanwhile, the silica oligomers could also adsorb at the terminals of the double twisted nanoribbons, thus a close-ended tubular structure was formed with closed ends (Fig.3(b)).There were some holes identified in the walls (Fig.7(e)).When the organic templates were removed, many pores were identified clearly (Fig.3(b)).
The formation of the morphology and pore architectures of TSTV is illustrated in Fig.8.Firstly,the LMWG (Fig.8(a)) assembled into double twisted nanoribbons through hydrogen bondings, hydrocarbon chains and aromatic rings (Fig.8(b)).After dropping TEOS, the silica oligomers and LWMG reassembled into a two-dimensional hexagonal structures caused by the hydrophobic association of the hydrocarbon chains[27].The assemblies of L-16Ala5PyPF6became shorter rod-like micelles vertical to the surface of the twisted nanoribbons[16].The silica oligomers adsorbed on the edges and terminals of the double-twisted nanoribbons (Fig.8(c)).As the reaction proceeded,the silica nanoparticles linked to each other after polycondensation, and thus formed twisted tubular nanoribbons with closed ends.Finally, twisted silica tubular nanoribbons with pore channels vertical to the wall surfaces was formed after removing the organic self-assemblies through calcination (Fig.8(d))[1].The hollow cavity was caused by the gap between the double-twisted nanoribbons.We proposed that the formation of TSTV could be explained via a structural transition from the double twisted nanoribbons to the rod-like micelles, the vertical pores were formed after removing the templates[16].
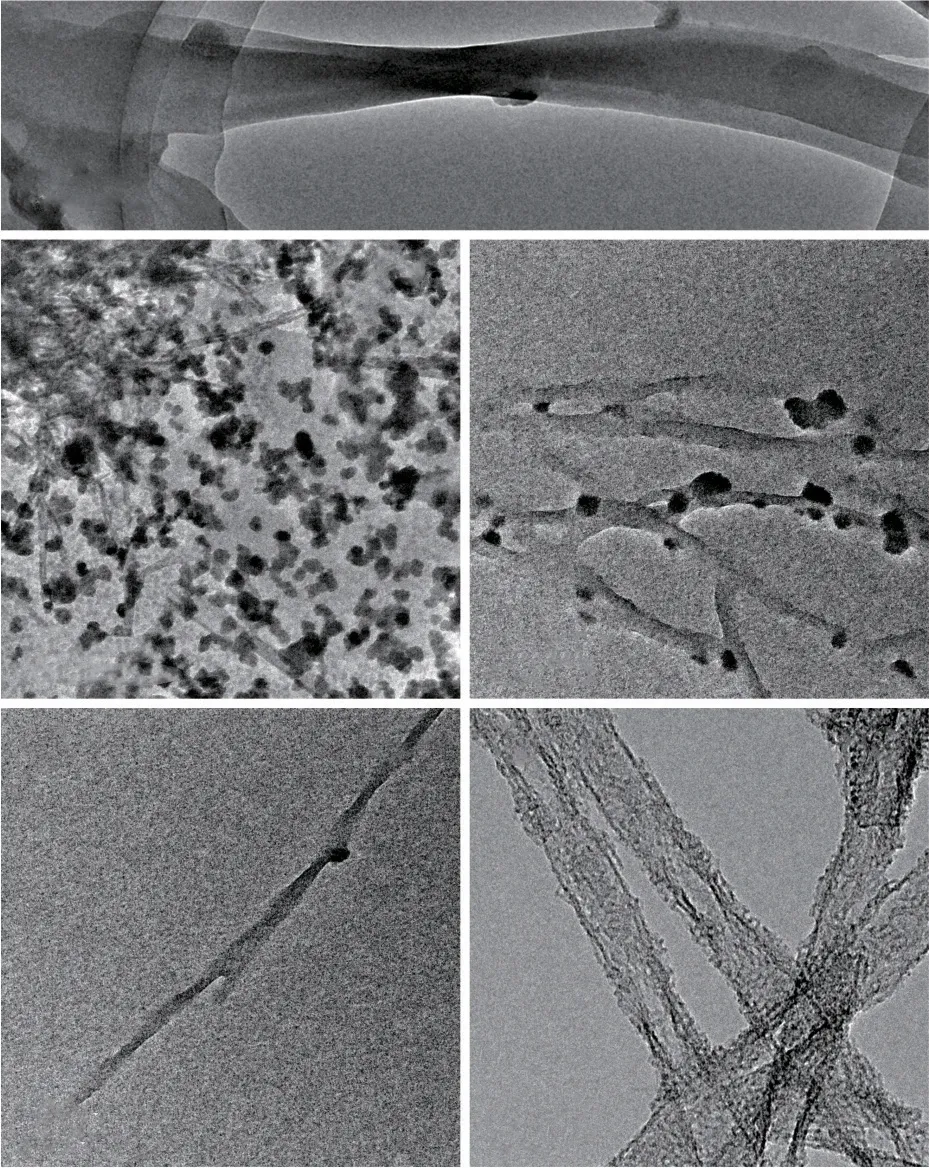
Fig.7 TEM images of the reaction system at different reaction time intervals: (a) 0 s; (b) 5 s; (c) 15 s; (d) 30 s; (e) 4 days
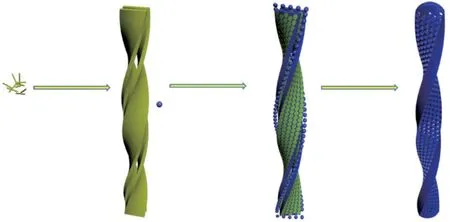
Fig.8 Proposed mechanism for the formation of TSTV: (a) Lowmolecular weight gelators; (b) Double-twisted nanoribbons;(c) Twisted tubular nanoribbons; (d) Twisted silica tubular nanoribbons with pore channels vertical to the wall surfaces(TSTV)
3.5 Drug release study
Drug-controlled release behaviour of this sample was studied by loading ibuprofen in the phosphate buffer solution (PBS) with a PH value of 7.4.The drug release curve of TSTV is shown in Fig.9.The initial drug release in 0.5 h reduced dramatically,about 30.7wt% drug was released.More than 86.7wt%ibuprofen was diffused out within 13 h in the PBS solution.It was indicated that the sample TSTV could be effective in drug delivering and releasing.Due to the large number of radiation holes on the surface of the nanotube and the large surface area, it is more conducive to the loading of ibuprofen drugs.There are a large number of radiation holes on the surface of the silica reported in this paper.Compared with the traditional one-dimensional nanotubes[25,26], there are more drug release holes and drug blockage is not easy to occur.
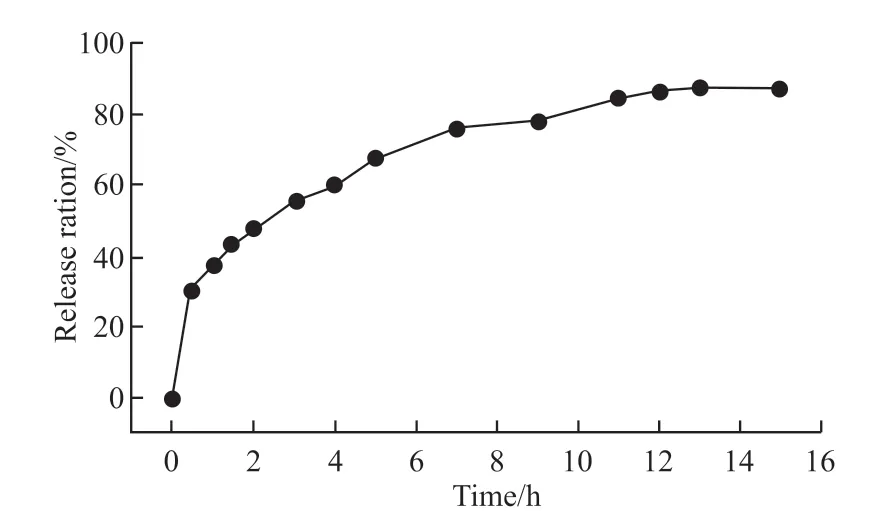
Fig 9 Cumulative release rates of ibuprofen from TSTV in PBS solution
4 Conclusions
In summary, single-handed twisted silica nanostructures with pore channels vertical to the wall surfaces were fabricated.To the best knowledge of us,the single-handed helical silica with radiation holes on the surface prepared by the single-template method is simpler than the traditional double-template method.The vertical pore channels structures were left after removing the self-assemblies of L-16Ala5PyPF6, via a structural transition in the sol-gel transcription process.The high surface areas and chiral radial pore structures can be potentially used in the fields of chiral separation,chiral recognition and asymmetric catalysis.
Conflict of interest
All authors declare that there are no competing interests.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Enhanced Electrochemical Performances of Ni Doped Cr8O21 Cathode Materials for Lithium-ion Batteries
- Design on the Prestressed Concrete Frame Beam-column
- Synthesis and Flocculation of Polyacrylamide with Low Water Absorption for Non-dispersible Underwater Concrete
- Experimental Behavior of Recycled Aggregate Concrete Filled Steel Tubular Columns
- Impact-abrasive Wear Behavior of ZTA and NbC Reinforced Fe60 Matrix Composites
- Synthesis and Characterization of Hollow Strontium Carbonate Pompons by Composite Soft Template Method
