Effects of Co2O3 Addition on Microstructure and Properties of SiC Composite Ceramics for Solar Absorber and Storage
2024-01-03ZHOUYangWUJianfengTIANKezhongXUXiaohongMASitongLIUShaoheng
ZHOU Yang, WU Jianfeng, TIAN Kezhong, XU Xiaohong, MA Sitong, LIU Shaoheng
(State Key Laboratory of Silicate Materials for Architectures, Wuhan University of Technology, Wuhan 430070, China)
Abstract: SiC composite ceramics for solar absorber and storage integration are new concentrating solar power materials.SiC composite ceramics for solar absorber and storage integration were fabricated using SiC,black corundum and kaolin as the raw materials, Co2O3 as the additive via pressureless graphite-buried sintering method in this study.Influences of Co2O3 on the microstructure and properties of SiC composite ceramics for solar absorber and storage integration were studied.The results indicate that sample D2 (5wt% Co2O3) sintered at 1 480 ℃ exhibits optimal performances for 119.91 MPa bending strength, 93% solar absorption, 981.5 kJ/kg(25-800 ℃) thermal storage density.The weight gain ratio is 12.58 mg/cm2 after 100 h oxidation at 1 000 ℃.The Co2O3 can decrease the liquid phase formation temperature and reduce the viscosity of liquid phase during sintering.The liquid with low viscosity not only promotes the elimination of pores to achieve densification,but also increases bending strength, solar absorption, thermal storage density and oxidation resistance.A dense SiO2 layer was formed on the surface of SiC after 100 h oxidation at 1 000 ℃, which protects the sample from further oxidation.However, excessive Co2O3 will make the microstructure loose, which is disadvantageous to the performances of samples.
Key words: SiC composite ceramics; Co2O3; microstructure; solar absorption; thermal storage density
1 Introduction
As the energy crisis and global warming have become global problems, in-depth research on concentrated solar power technology has become an important way to solve the above issues[1].The performance of thermal absorption system and thermal storage system directly affects the power generation efficiency of solar thermal power plant.However, the thermal absorption system and the thermal storage system will produce thermal loss in the process of thermal exchange[2,3].To solve the above issues, absorber and storage integration materials are proposed in this study.The solar absorber and storage integration is a technology combining thermal absorption system and thermal storage system(Fig.1).After absorbing thermal, materials can be directly stored in a hot tank, which can reduce thermal loss in the process of thermal exchange and improve thermal efficiency.
Heat resistant metal mesh knitting was mostly used in early thermal absorber materials, such as TSA heat absorbers in Spain[4].However, the metal knitting exhibited poor oxidation resistance at high temperatures, and was prone to melt or soften if the heat flow was uneven.A large number of studies have shown that the working temperature of metal materials cannot exceed 800 ℃.Ceramics have a higher working temperature than metals[5].Al2O3ceramics can withstand high working temperature without oxidation, thus can be used as thermal absorbing material.However, the color of Al2O3ceramic is white, resulting in the low solar absorption.Concretes have short service life (less than 10 years) and low service temperature (less than 400 ℃)[6],which are not conducive to large-scale application.Many oxide ceramics (eg, Al2O3, ZrO2and cordierite,etc) are considered to be suitable for sensible heat storage materials due to their high refractoriness (more than 1 000 ℃) and high heat capacity[7-9].However, due to the poor thermal conductivity, oxide ceramics are susceptible to thermal shock, and the drastic decrease in mechanical properties after the thermal shock has limited their applications at high temperature.Hence, it is important to explore a new solar absorber and storage integration material.
SiC composite ceramic materials have good mechanical properties and high thermal conductivity at high temperature[10], and they have black color, thus can be used as high temperature thermal absorption and thermal storage material.It is difficult to obtain dense SiC ceramics by pressureless sintering since its covalent bond.Therefore, numerous researchers have made unremitting efforts to lower the sintering temperature and improve its density.It is usually necessary to introduce second phase or additives to promote sintering[11-13].Metal oxide is a kind of additive for SiC liquid phase sintering that has been widely studied[14].At present, studies on preparing thermal absorption and thermal storage materials by using SiC have been reported,but there is no report on preparing thermal absorber and storage integration ceramic materials by using SiC.Xuet al[15]prepared cordierite/SiC composites with a solar absorption of 76.2%-78.8%.Xuet al[16]prepared mullite bonded silicon carbide heat absorbing ceramics with a bending strength of 44.2 MPa and an oxidation weight gain of 25.43 mg/cm2after oxidizing at 1 300℃ for 100 h.Sunet al[17]prepared SiC-diamond ceramics with a specific heat capacity of 0.71 J /(g·K).Most heat storage materials have poor high temperature resistance, leading to a low heat storage density.For example, Belessiotis[18]prepared paraffin/SiO2composite phase change material with latent heat of 156 kJ/kg.Golestani[19]prepared SiO2nanocomposites with phase change latent heat of 123.8 kJ/kg.
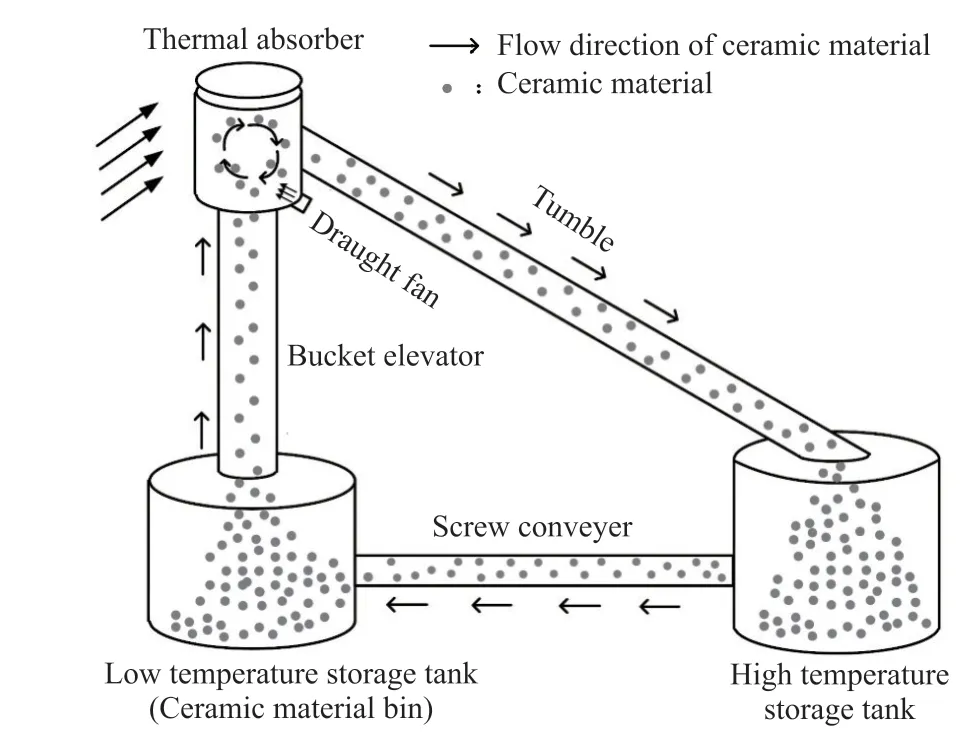
Fig.1 Model of solar absorber and storage integration system
To sum up, the current research on SiC ceramic materials still has problems such as low strength, low solar absorption and poor oxidation resistance, and the current thermal storage materials have the problem of low heat storage density.In this research, Co2O3was introduced as an additive to prepare SiC composite ceramics.Moreover, the effects of Co2O3on the phase composition, microstructure, solar absorption, thermal physical properties and oxidation resistance of SiC composition ceramics were investigated in detail.
2 Experimental
SiC (20-61 μm, Dongguan Baxter Abrasives Co.,Ltd, Guangdong, China), black corundum (Gongyi Wanfeng Abrasives Co., Ltd, Zhengzhou, China) and kaolin (Jingdezhen Taoyuan Mining Co., Ltd, Jiangxi,China) were utilized to fabricate the SiC composite ceramics, and Co2O3(purity ≥ 98.5%, Shanghai Macklin Biochemical Co., Ltd, Shanghai, China) as the additive.The sample formulations are designed as D0, D1,D2, and D3 (Table 1).The mixture was dry-grinded using ball-milling machine, and blending with 5wt%PVA (polyvinyl alcohol).Then the powder mixtures were pressed into the cylindrical bodies (Φ30 mm×5 mm) at a pressure of 50 MPa and rectangular bodies(37 mm×6.5 mm×6.5 mm) at a pressure of 40 MPa.The green bodies were sintered at 1 440, 1 460, 1 480,1 500, and 1 520 ℃ via pressureless graphite-buried sintering method with a heating rate of 5 ℃/min and holding time of 2 h in an electrical furnace.
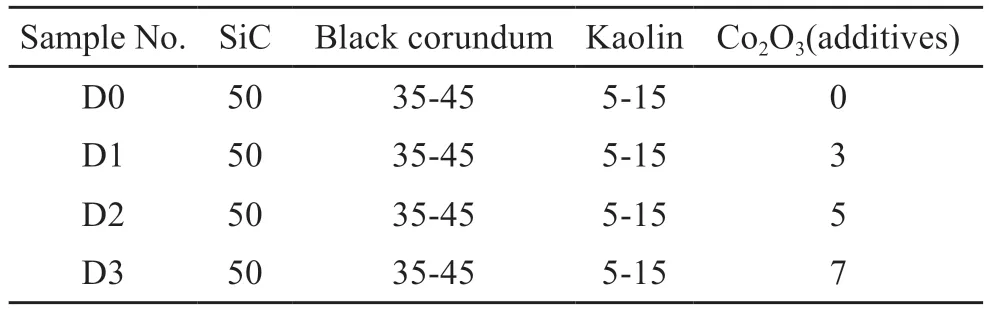
Table 1 The batch formula compositions of samples/wt%
Water absorption (Wa), apparent porosity (Pa)and bulk density (D) were measured by the Archimedes method.An electronic universal testing machine (Model RGM-4100, Reger Instrument Co., Ltd., Shenzhen,China) was employed to determine the bending strength(σ).The span was 28 mm and the loading rate was 1 mm/min.The phase compositions were characterized by X-ray diffraction (XRD) (Model D/Max- IIIA, Rigaku., Japan.) using Cu Kα1 radiation.The samples were tested in the range of 5-80° with a step of 0.02°.The microstructures of the fractured surface of the as-prepared ceramics samples (etched by 5wt% HF for 90 s)were observed by scanning electron microscope (SEM)(Model JSM-5610LV, Jeol., Japan).Solar absorption was tested by a UV-Vis-NIR spectrophotometer (Model UV-3600, Shimadzu, Japan).To investigate the oxidation resistance, the cylindrical samples were heated to 1 000 ℃ and held for 10 h, and then moved to the air for cooling down.Secondly, their quality was measured and the weight was measured and the weight gain rate was calculated according to formula (1):
where,∆Wis the weight gain rate (mg/cm2),m0andmthe quality (mg) before and after oxidation respectively,andSthe superficial area (cm2) of the sample.
3 Results and discussion
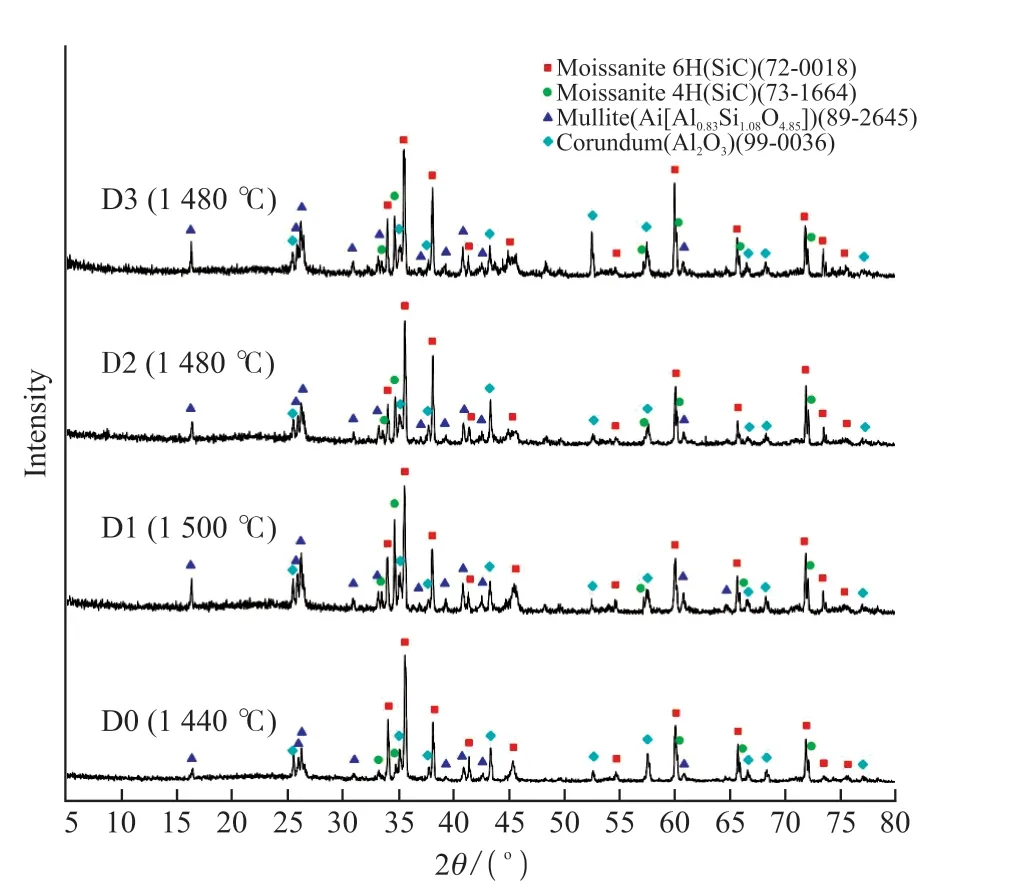
Fig.2 XRD patterns of the samples sintered at different temperatures
3.1 Effect of Co2O3 on phase composition
Fig.2 presents XRD patterns of the samples sintered at optimal temperatures.As seen in Fig.2 the phase compositions of each sample after sintering were SiC, corundum and mullite, indicating that the addition of a small amount of Co2O3has no significant effect on the phase composition of the sample.The mullite content of samples D1-D3 increased compared with that of sample D0.This is because Co2O3 can reduce the high temperature liquid viscosity.The dissolution rate of Al2O3 into liquid phase increased with the decrease of the viscosity of liquid phase, and thus the reaction of liquid silicate with Al2O3 becomes extensive to facilitate the mullite grains to grow up.Mullite is partly transformed from kaolin[20] and partly from the reaction between corundum and quartz from oxidation of SiC grains[21].The combination of in-situ mullite and corundum with SiC particles is beneficial to the improvements of density and physical properties of samples.
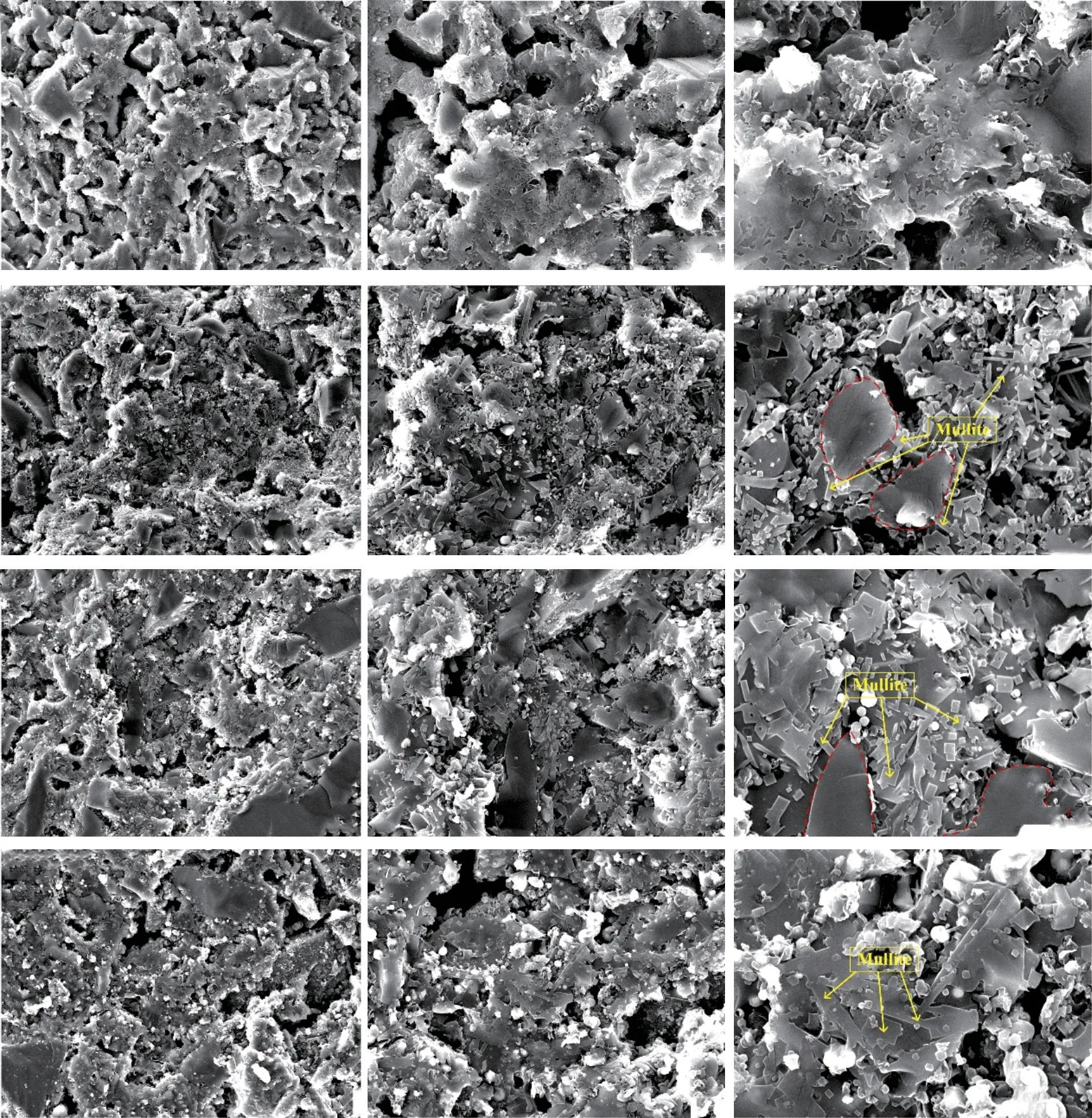
Fig.3 SEM images of fractured surface of the samples sintered at different temperatures (5wt% HF for 90 s; (a-c): D0 1 440 ℃; (d-f): D1 1 500 ℃; (g-i): D2 1 480 ℃; (j-l): D3 1 480 ℃)
3.2 Effect of Co2O3 on microstructure
Fig.3 presents the SEM morphologies of the fracture surface of the samples sintered at optimal temperatures.Sample D0 (Fig.3(c)) without Co2O3has a loose microstructure and only a small amount of mullite grains can be seen, thus the bending strength is as low as 78.01 MPa.As seen in Fig.3(f), Fig.3(i),and Fig.3(l), a large number of mullite grains form in samples D1-D3, indicating that Co2O3can promote the formation of mullite.The mullite grains grow larger with increasing the Co2O3content.Some coarse mullite grains with over size are found in sample D3 sintered at 1 480 ℃ (Fig.3(l)).These coarsening mullite grains may not tightly interlocked with each other as the fine grains[22,23].Therefore, sample D3 can not achieve high bending strength when the temperature rises to 1 500℃.Mullite, corundum and glass phase as a reinforcing phase mainly play two roles: the first is covering SiC grains to prevent its further oxidation at high temperature; the second is to combine the SiC grains together at high temperature to improve the density and bending strength of the samples.
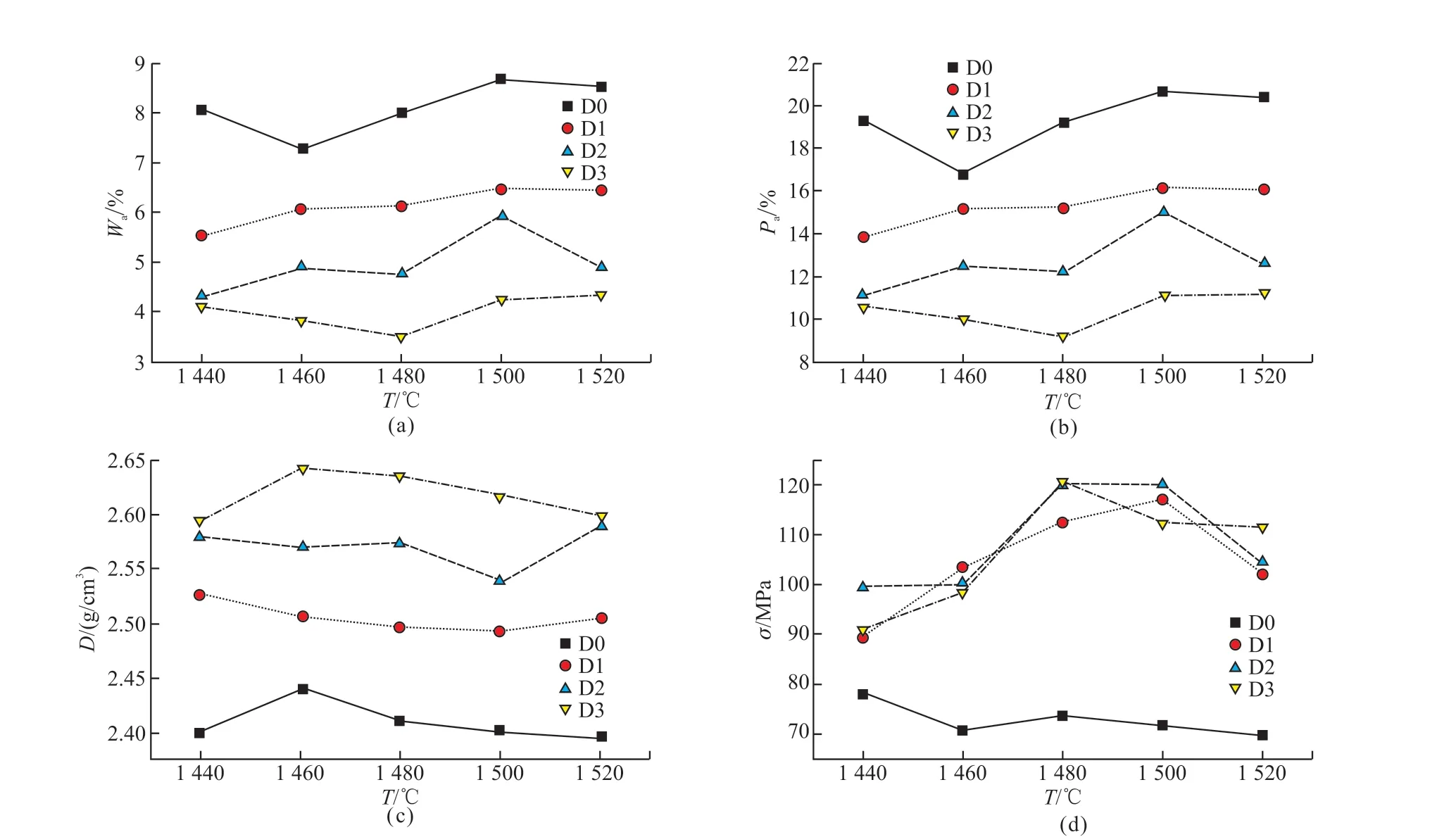
Fig.4 Relationships between physical properties and sintering temperatures of the samples: (a) Wa; (b) Pa; (c) D; (d) σ
3.3 Effect of Co2O3 on physical performance
Fig.4 shows the relationships between physical properties and sintering temperatures of the samples.As shown in Fig.4, Wa and Pa of samples decrease significantly with the Co2O3content increases, while D andσincrease inversely.The results indicate that the addition of Co2O3can promote the formation of liquid phase and reduce the viscosity of liquid phase.The liquid phase with lower viscosity can not only accelerate the mass transfer and crystal growth, but also fill into the pores to allow the samples to achieve densification and improve the bending strength.
Sample D2 with 5% Co2O3sintered at 1 480-1 500 ℃ have high bending strengths of 119.91 and 119.92 MPa, respectively.The bending strength is improved compared with previous studies about SiC composite ceramics (86.2 and 78.71 MPa)[24,25].The bending strengths of samples D1 and D3 reach the maximum value at 1 500 and 1 480 ℃, respectively, and then keep decreasing with sintering temperature increasing since the over-sintering effect (Photos of the samples are shown in Fig.5).The results show that Co2O3can also reduce the formation temperature of liquid phase.The bending strength of the samples is highly related to the quantity, size and distribution of pores[26].Pores can not only reduce the effective area under load of materials but also cause stress concentration, resulting in decreasing bending strength[27].

Fig.5 Photos of the samples sintered at different temperatures
3.4 Effect of Co2O3 on solar absorption
The reflectance spectra of samples are presented in Fig.6, and the solar absorption (αs) results[28](Table 2) are calculated through equation (2), where λ is the wavelength (μm), ρs(λ) the reflectivity (%), andEs(λ)the solar radiation intensity (W), respectively.
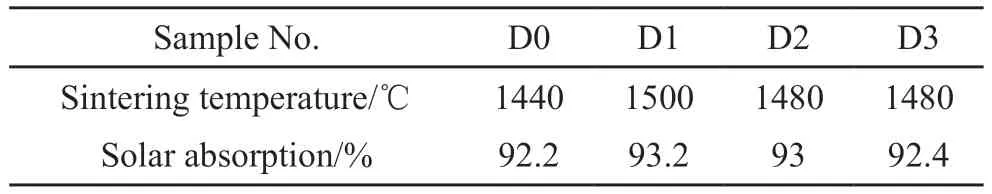
Table 2 Experimental results of the absorption of samples
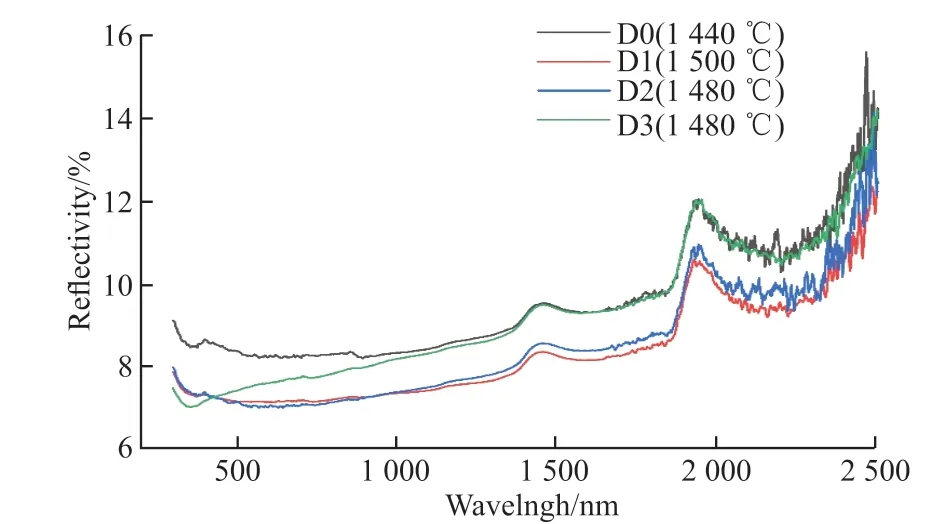
Fig.6 Reflectance spectra of the samples
The material will absorb sunlight through the transition of valence electron and the vibration of atomic when it is irradiated by sunlight, resulting in the rise of temperatures[29].Fig.6 shows that the reflectivity of samples D1-D3 is slightly lower than that of sample D0, and thus the absorptions of samples D1-D3 is slightly higher.The addition of Co2O3can promote densification and uniform distribution of grains, which is beneficial for the vibration of atomic and energy migration, thus the addition of Co2O3can improve the absorption.The solar absorption of sample D3 is lower than that of samples D1 and D2, which is attributed to the uneven distribution of coarse mullite grains in sample D3.Overall, the absorptions of samples D0-D3 range from 92.2% to 93.2%.Since SiC material is a kind of high absorption material[30], the addition of Co2O3has little effect on absorption.With the addition of Co2O3, the SiC composite ceramics for solar absorber and storage integration exhibit a significantly higher solar absorption, comparing with the previous works of cordierite/silicon carbide composite with a solar absorption of 76.2%-78.8%[15].
3.5 Effect of Co2O3 on thermal physical properties
Table 3 displays the experimental results of thermal physical properties of samples sintered at different temperatures.According to the results, the thermal conductivity and thermal diffusivity of the samples decrease with the increase of test temperature, which can be attributed to the increase of phonon scattering resulting from increasing temperature[31,32].The porosity in the ceramics also contributes to the reduction in thermal conductivity since air has much lower thermal conductivity than solid particle, resulting from the scattering occurs when phonons cross the barrier[33-35].Thus thermal diffuses more slowly in sample D0 than in samples D2 and D3.The heat capacity of samples D2 and D3 is higher than that of sample D0, since the addition of Co2O3can improve the bulk density and mullite content.The mullite grains in sample D2 (Fig.3(i)) interlock tightly with each other, resulting in higher heat capacity.The thermal storage density of samples can be calculated by the following equation[36]:
where,Cssis the heat capacity,T0andTsare the initial and final temperature, respectively.The equation (3)shows that the thermal storage density is closely related to the heat capacity and thermal storage temperature.Therefore, the sample D2 sintered at 1 480 ℃ has the highest thermal storage density of 981.5 kJ/kg.Compared with the micro-scale phase change thermal stor-age material prepared by Liu[37]with a thermal storage density of 270.6 kJ/kg, the thermal storage density of the ceramics is higher.Thermal storage ceramic materials can work at high temperature resulting from high temperature resistance and thus have high thermal storage density.Compared with the Al2O3-SiC-ZrO2thermal storage ceramics (heat storage density is 916.91 kJ/kg) prepared by Wu[38], the thermal storage density is higher.
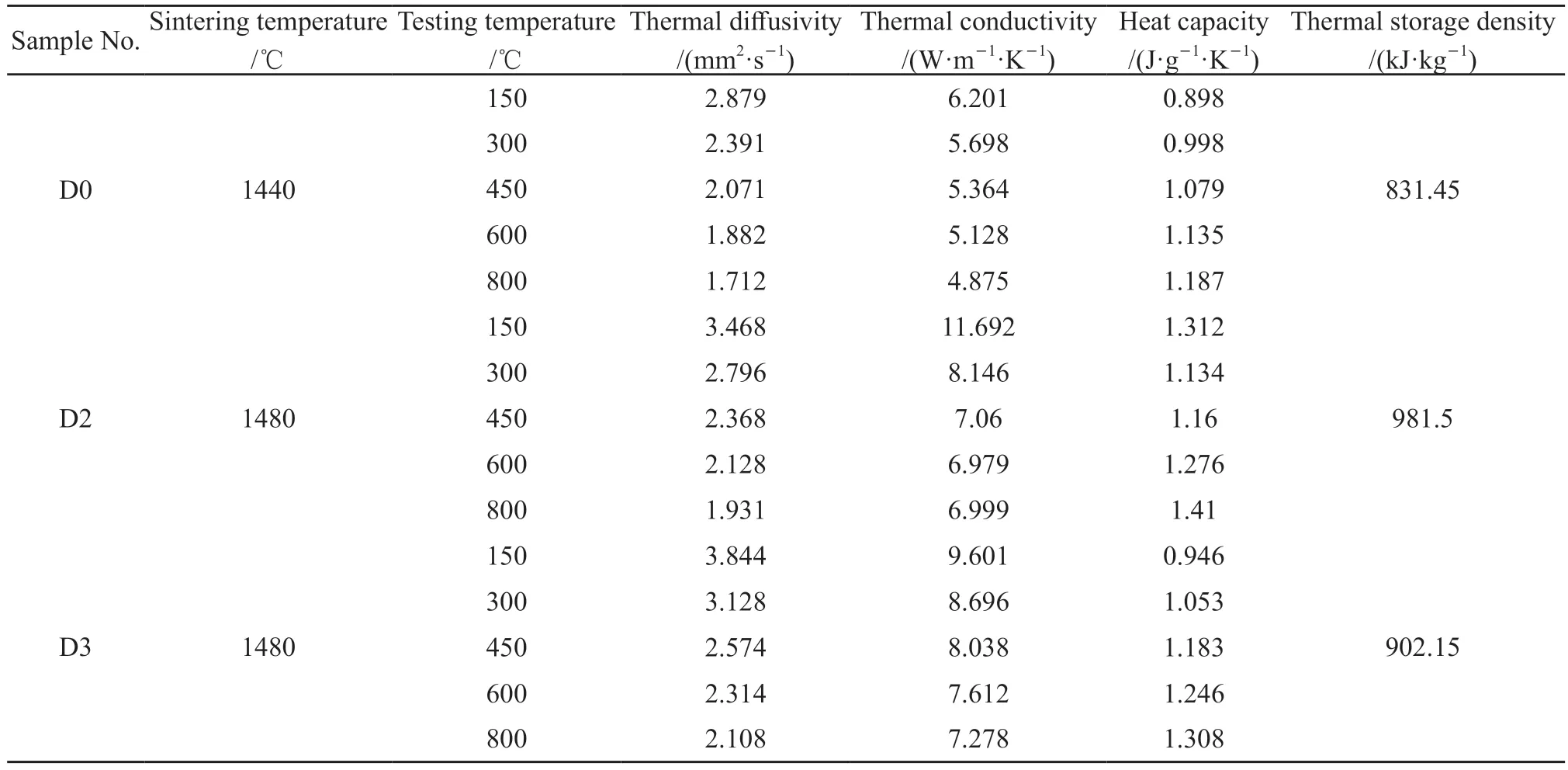
Table 3 Experimental results of thermal physical properties of the samples sintered at different temperatures
3.6 Effect of Co2O3 on oxidation resistance
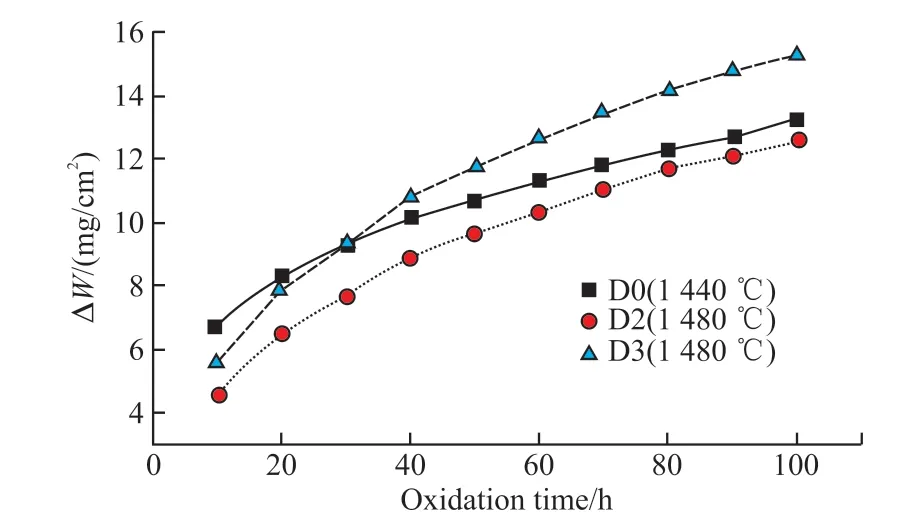
Fig.7 The relationship between oxidation weight gain ratio of samples sintered at different temperatures and oxidizing time
Since the SiC composite ceramics for solar absorber and storage integration work at high temperature for a long time, a good oxidation resistance is essential to resist damage, which significantly affects the service life of the SiC composite ceramics.The oxidation resistance of samples D0, D2 and D3 sintered at optimal temperatures was characterized by the weight gain rate in this study, which is presented in Fig.7.As seen in Fig.7, the weight gain rate of sample D3 with 7wt%Co2O3is lower than that of sample D0 at the initial oxidation stage, while it is higher than that of sample D0 after 30 h oxidation, indicating that excessive content of Co2O3is detrimental to the oxidation resistance.The sample D2 with 5wt% Co2O3has good oxidation resistance with a low weight gain rate of 12.58 mg/cm2after 100 h oxidation.The whole oxidation indicated that the weight gain led by layer formation of oxidized products obeyed the parabolic law.The liquid phase to fill the pores, promote particle rearrangement, and then improve the density during oxidation.A dense SiO2layer was formed on the surface of SiC during the oxidation,which protected the sample from further oxidation.
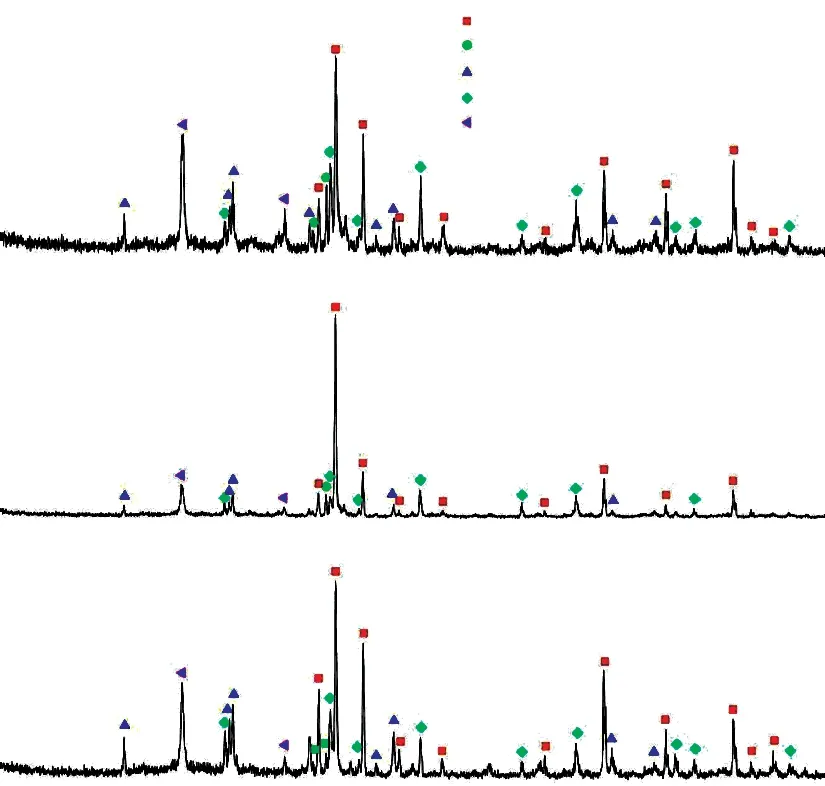
Fig.8 XRD patterns of the samples sintered at different temperatures after 100 h oxidation

Fig.9 SEM images of fractured surface of the samples sintered at different temperatures after 100 h oxidation (etched by 5wt% HF for 90 s;(a-c): D0 1 440 ℃; (d-f): D2 1 480 ℃; (g-i): D3 1 480 ℃)
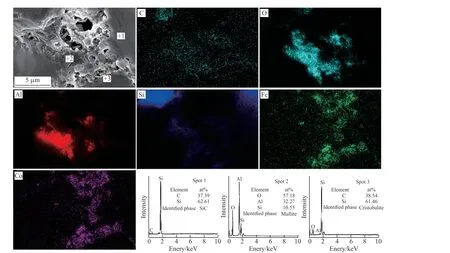
Fig.10 Secondary electron images and element mapping of sample D2 sintered at 1 480 ℃ after 100 h oxidation
Fig.8 presents the XRD patterns of samples after 100 h oxidation.As observed in Fig.8 the cristobalite phase appears after 100 h oxidation, which is derived from the oxidation reaction of SiC according to the reaction Eq.(4)[39].The SiO2generated from the oxidation reaction of SiC forms a dense oxidation layer on the surface of SiC grains, which can reduce the contact of oxygen and SiC particles, and thus reducing the oxidation rate of the sample[40].Fig.8 also shows that the SiO2content of sample D2 is relatively low compared with samples D0 and D3, indicating that only a small amount of SiC particles are oxidized and the oxidation resistance is great.
Fig.9 presents the SEM micrographs of fractured surface of the samples sintered at different temperatures after 100 h oxidation.It is noted in Fig.9(c) that some micro-cracks appear in sample D0 after 100 h oxidation, while no obvious micro-cracks are found in Fig.9(f) and Fig.9(i), indicating that the addition of Co2O3can make more liquid phase fill cracks and act as an oxidation barrier[41].In addition, reaction (4) is accompanied by a certain volume expansion, which can cover the micro-crack and pores, and thus decreasing the weight gain rate[42].
As illustrated in Fig.10, SiC grains are connected by Al2O3-SiO2-Fe2O3-Co2O3eutectic glass.Spot 1 is SiC grain for the distribution of Si and C.Al, Si, and O elements exist in spot 2, which may be mullite.Spot 3 is cristobalite generated by oxidation on the surface of SiC grains, which reduces the effective area of oxidation reaction and protects SiC particles from further oxidation[43].The microstructure of sample D3 (Fig.9)is looser than sample D2.The high content of Co2O3in sample D3 leads to the relatively low viscosity of the liquid phase, causing the coalescence and growth of pores, which will promote the generation of connected pores and increase the channels of oxygen into the samples, and thus increasing the weight gain rate.Therefore, the content of Co2O3should not be too much, and the sample D2 with 5wt% Co2O3have optimal properties in this study.
4 Conclusions
The SiC composite ceramics for solar absorber and storage integration were fabricated with SiC, black corundum and kaolin.
a) The results indicate that sample D2 (5wt%Co2O3) sintered at 1 480 ℃ exhibited optimal performances for 119.91 MPa bending strength, 93% solar absorption, 981.5 kJ/kg (25-800 ℃) thermal storage density.The weight gain ratio was 12.58 mg/cm2after 100 h oxidation at 1 000 ℃.
b) The Co2O3could decrease the liquid phase formation temperature and reduce viscosity of liquid phase during sintering.The low viscosity liquid phase not only promoted the elimination of pores to achieve densification, but also increased bending strength, solar absorption and thermal storage density.
c) A dense SiO2layer was formed on the surface of SiC after 100 h oxidation at 1 000 ℃, which protected the sample from further oxidation.At the same time, the addition of Co2O3could promote the filling of micro-cracks and pores in liquid phase, and thus reduce the oxygen diffusion channel and improve the oxidation resistance of samples.However, too much Co2O3will make the microstructure become loose, and the serious degradation on property of sample.
Conflict of interest
All authors declare that there are no competing interests.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Enhanced Electrochemical Performances of Ni Doped Cr8O21 Cathode Materials for Lithium-ion Batteries
- Design on the Prestressed Concrete Frame Beam-column
- Synthesis and Flocculation of Polyacrylamide with Low Water Absorption for Non-dispersible Underwater Concrete
- Experimental Behavior of Recycled Aggregate Concrete Filled Steel Tubular Columns
- Impact-abrasive Wear Behavior of ZTA and NbC Reinforced Fe60 Matrix Composites
- Synthesis and Characterization of Hollow Strontium Carbonate Pompons by Composite Soft Template Method
