CXCL8在胃肠道间质瘤中的表达及临床意义
2024-01-01周林森张心仪邵伟伟周广军

[摘" "要]" "目的:研究CXC趋化因子配体8(CXC chemokine ligand 8,CXCL8)在胃肠道间质瘤(gastrointestinal stromal tumors,GIST)中的表达以及临床意义。方法:通过生物信息学分析耐药和敏感GIST组织转录组的差异基因,绘制差异基因的韦恩图和火山图,筛查差异最明显的基因。收集4例伊马替尼耐药GIST组织标本和4例敏感GIST组织标本,采用免疫组织化学法检测CXCL8蛋白的表达。将88例GIST患者分为CXCL8高表达组49例和低表达组39例,比较两组患者临床病理参数。绘制Kaplan-Meier生存曲线,比较CXCL8高表达与低表达患者总生存期(overall survival,OS)及无病生存期(disease-free survival,DFS)。采用单因素和多因素Cox回归模型分析影响患者生存期的因素。结果:生物信息学分析显示,耐药GIST组织中CXCL8基因表达高于敏感组织。免疫组织化学结果显示,耐药GIST组织中CXCL8蛋白表达显著高于敏感组织(P<0.05)。CXCL8蛋白表达与肿瘤最大直径、核分裂相计数以及危险度分级有关(P<0.05)。Kaplan-Meier生存曲线显示,CXCL8高表达患者DFS低于低表达患者(P<0.05)。Cox回归模型分析显示,CXCL8高表达是影响GIST患者DFS的独立危险因素。结论:CXCL8促进GIST耐药,与GIST患者的预后相关,可能成为胃肠道间质瘤特异性治疗的新靶点。
[关键词]" "胃肠道间质瘤;CXC趋化因子配体8;免疫组织化学;生存期
[中图分类号]" "R735.2 [文献标志码]" "A [DOI]" "10.19767/j.cnki.32-1412.2024.05.002
The expression and clinical significance of CXCL8 in gastrointestinal stromal tumors
ZHOU Linsen, ZHANG Xinyi, SHAO Weiwei, ZHOU Guangjun
(Department of General Surgery, Yancheng First People’s Hospital, Jiangsu 224000)
[Abstract]" "Objective:To investigate the expression and clinical significance of CXC chemokine ligand 8 (CXCL8) in gastrointestinal stromal tumors (GIST). Methods:The differential genes of drug-resistant and sensitive tissue transcriptomes were analyzed through bioinformatics, and the Venn diagram and volcano diagram of differential genes were drawn to find out the genes with the most obvious differences. Immunohistochemical methods were used to detect the expression of CXCL8 protein in 4 imatinib resistant GIST and 4 sensitive GIST tissues. The Eighty-eight GIST patients were divided into CXCL8 high expression group (49 cases) and low expression group (39 cases), and the clinicopathological parameters were compared between the two groups of patients. Kaplan-Meier survival curve was drawn to compare overall survival (OS) and disease-free survival (DFS) in patients with high and low CXCL8 expression. Univariate and multivariate Cox regression models were used to analyze the factors affecting DFS. Results:Bioinformatics analysis showed that the expression of CXCL8 gene in drug-resistant GIST tissues was higher than that in sensitive tissues. Immunohistochemical results showed that the expression of CXCL8 protein in drug-resistant GIST tissues was significantly higher than that in sensitive tissues, with statistical significance (Plt;0.05). CXCL8 protein expression was correlated with the maximum tumor diameter, mitotic phase count, and risk stratification (Plt;0.05). Kaplan-Meier survival curve showed that DFS in patients with high CXCL8 expression was lower than that in patients with low CXCL8 expression (Plt;0.05). Cox regression model analysis showed that high expression of CXCL8 was an independent risk factor for DFS in GIST patients. Conclusion:CXCL8 promotes drug resistance of GIST and is associated with the prognosis of GIST patients, which may be a new target for specific treatment of gastrointestinal stromal tumors.
[Key words]" "gastrointestinal stromal tumors; CXC chemokine ligand 8; immunohistochemistry; survival period
胃肠道间质瘤(gastrointestinal stromal tumors,GIST)是胃肠道最常见的间叶源性肿瘤[1],以c-kit和血小板源性生长因子受体A(platelet-derived growth factor receptors A,PDGFR-A)突变为其发病的生物学基础,靶向c-kit和PDGFR-A治疗是GIST的最佳选择[2]。酪氨酸激酶抑制剂伊马替尼在问世20余年间一直是胃肠道间质瘤的标准一线治疗,效果令人瞩目,但约50%患者在治疗2年后发生耐药,即便换用舒尼替尼、瑞戈非尼和瑞派替尼等后线药物,效果甚微[3-4]。
CXC趋化因子配体8(CXC chemokine ligand 8,CXCL8)是最强效的中性粒细胞趋化因子,在应对感染和组织损伤中发挥重要作用[5]。CXCL8的血管生成和炎症效应促进肿瘤的生长和转移[6]。在肿瘤微环境中,CXCL8过表达通过浸润免疫细胞、基质细胞和肿瘤细胞本身,促进内皮细胞迁移和肿瘤血管新生。CXCL8还诱导肿瘤细胞上皮-间质转化,促进肿瘤细胞侵袭、转移,增强对免疫细胞的抵抗[7-9]。本研究采用免疫组化方法研究CXCL8在GIST组织中的表达,探讨CXCL8的临床意义及其对预后的影响。
1" "材料与方法
1.1" "生物信息学分析" "登录美国国立生物技术信息中心创建的高通量基因表达数据库Gene Expression Omnibus(https://www.ncbi.nlm.nih.gov/geo/),筛选出GSE 132542和GSE 155800两个数据集,通过R包分析两个数据集GIST耐药和敏感组织转录组的差异基因,绘制差异基因的韦恩图和火山图,筛选差异最明显的基因。
1.2" "GIST肿瘤组织标本" "收集2021年1月—12月在本院手术切除的8例GIST肿瘤组织标本,其中4例为伊马替尼耐药,4例术前未接受过伊马替尼治疗。本研究经医院医学伦理委员会批准同意。
1.3" "免疫组织化学法检测" "肿瘤组织中各基因蛋白表达标本来源于2016年1月—2017年12月在我院手术、并经病理确诊的88例GIST患者。石蜡包埋的组织切片经烤片后使用LEICA BOND-MAX全自动免疫组化仪行免疫组化检测,所用一抗分别为CXCL8(鼠单抗,1∶100,Abcam-89336)、GPAT2(兔多抗,1∶100,Abcam-122373)、COL12A1(兔多抗,1∶100,Abcam-121304)和OGN(兔多抗,1∶200,华美生物CSB-PA016314LA01HU)。SP试剂盒购自Abcam公司。实验步骤按说明书进行,用PBS代替一抗处理的切片作为阴性对照片,已知阳性片作为阳性对照片。由两位病理科主任医师采用双盲法判读结果,若胞质或胞膜有棕黄至黄褐色染色颗粒判为阳性。阳性细胞率0~10%计0分,11%~50%计1分,51%~80%计2分,81%~100%计3分。染色强度计分:无着色为0分,淡黄色为1分,黄褐色为2分,棕褐色为3分。阳性细胞率计分与染色强度计分之和0~2分为低表达,3~6分为高表达。
1.4" "生存期观察" "88例GIST患者术后每6~12个月进行CT扫描,随访至2022年12月,记录患者生存情况。总生存期(overall survival,OS)定义:首次诊断至死亡时间或末次随访时间;无病生存期(disease-free survival,DFS)定义:自手术后第1天至肿瘤复发、转移或末次随访时间。
1.5" "统计学处理" "应用SPSS 26.0统计学软件对数据进行分析处理。计数资料以频数表示,组间比较采用χ2检验;绘制Kaplan-Meier生存曲线,采用Log-Rank检验比较两组患者的生存期;采用单因素Cox比例风险模型筛选影响DFS的危险因素,将单因素Plt;0.05的变量纳入多因素Cox比例风险模型中,筛选影响DFS的独立危险因素。Plt;0.05为差异有统计学意义。
2" "结" " " 果
2.1" "生物信息学分析" "生物信息学分析发现,相较于敏感组织,耐药GIST组织中GPAT2、CXCL8、COL12A1和OGN基因高表达。见图1。
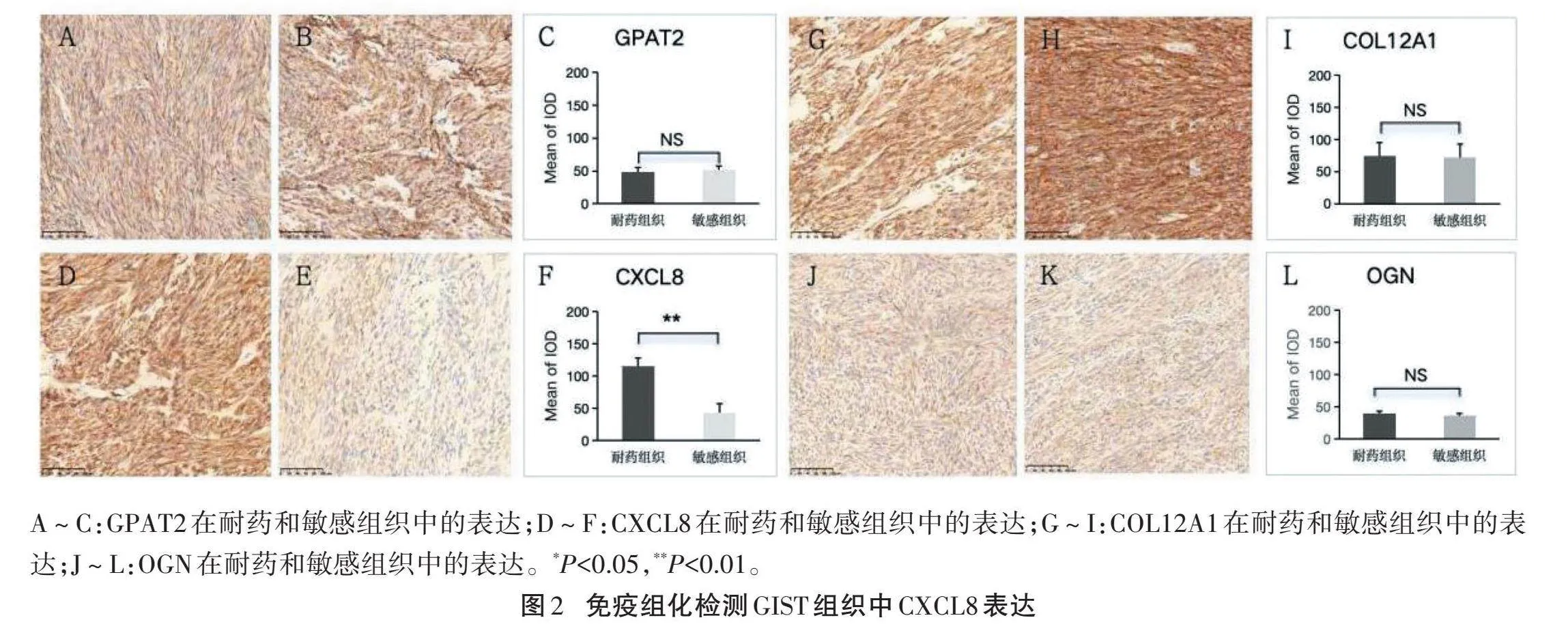
2.2" "GIST组织中CXCL8蛋白表达" "免疫组织化学检测结果显示,耐药GIST组织中CXCL8蛋白表达水平显著高于敏感组织,差异有统计学意义(P<0.05),但耐药组织与敏感组织中GPAT2、COL12A1和OGN蛋白表达水平比较,差异均无统计学意义(P>0.05)。见图2。
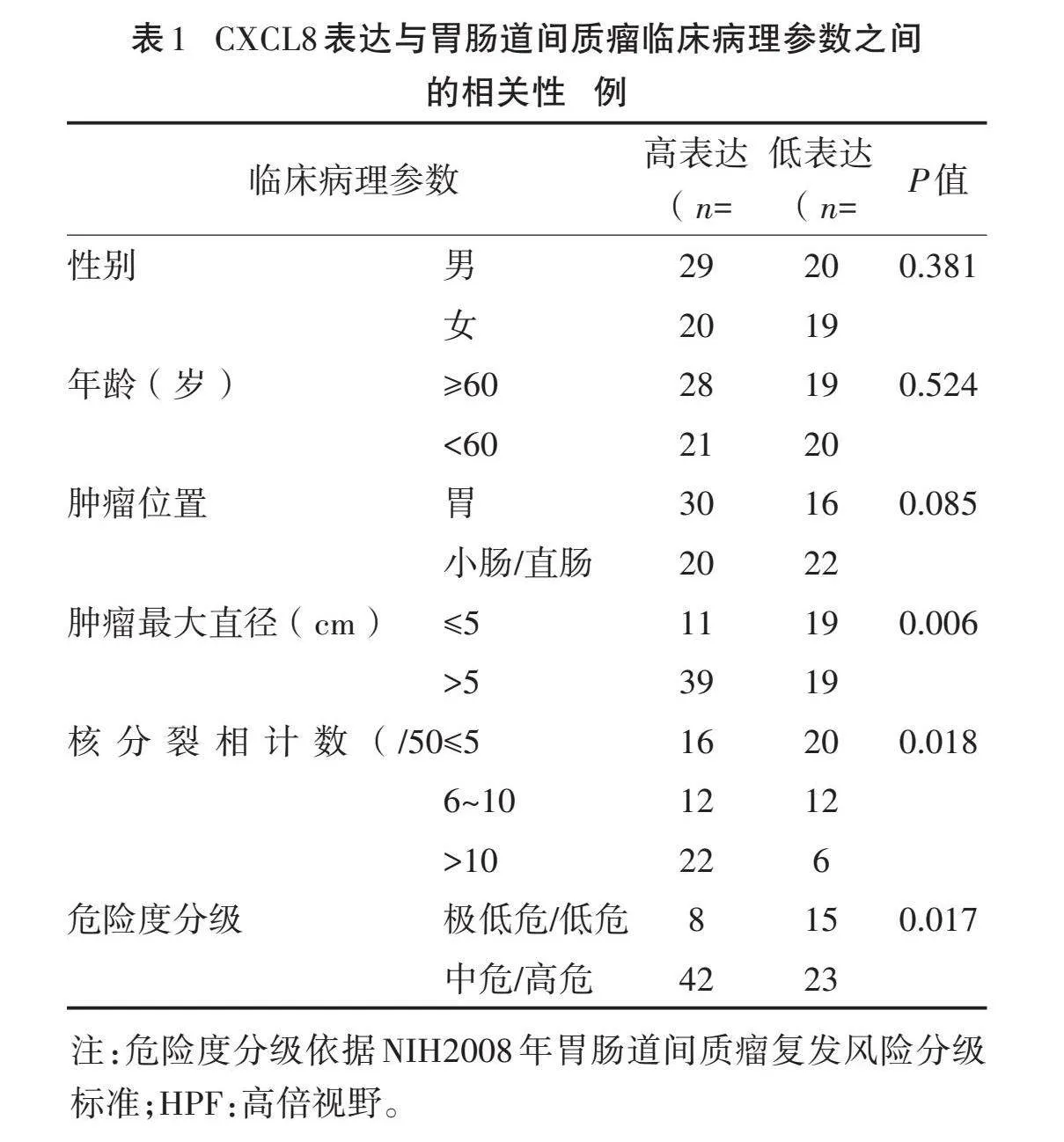
2.3" "CXCL8表达与胃肠道间质瘤患者临床病理参数的相关性" "88例GIST组织标本中CXCL8高表达49例(55.68%),低表达39例(44.32%)。CXCL8蛋白表达与GIST肿瘤最大直径、核分裂相计数及危险度分级有关(Plt;0.05),而与性别、年龄、肿瘤位置无关(Pgt;0.05)。见表1。
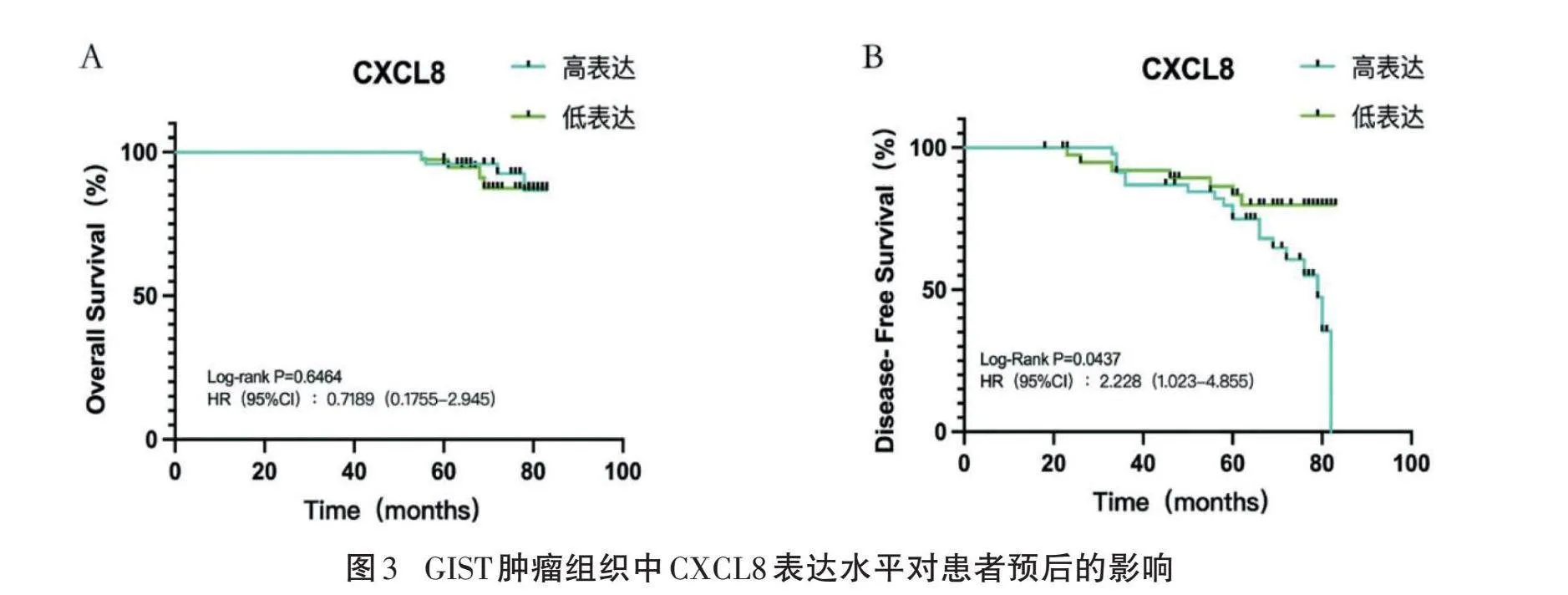
2.4" "CXCL8表达与胃肠道间质瘤患者预后的相关性" "88例GIST患者随访54~89个月,中位随访时间70个月。Kaplan-Meier生存曲线分析显示,CXCL8高表达患者OS低于低表达患者,但差异无统计学意义(P=0.6464)。CXCL8高表达患者DFS低于低表达患者,差异有统计学意义(P=0.0437),见图3。
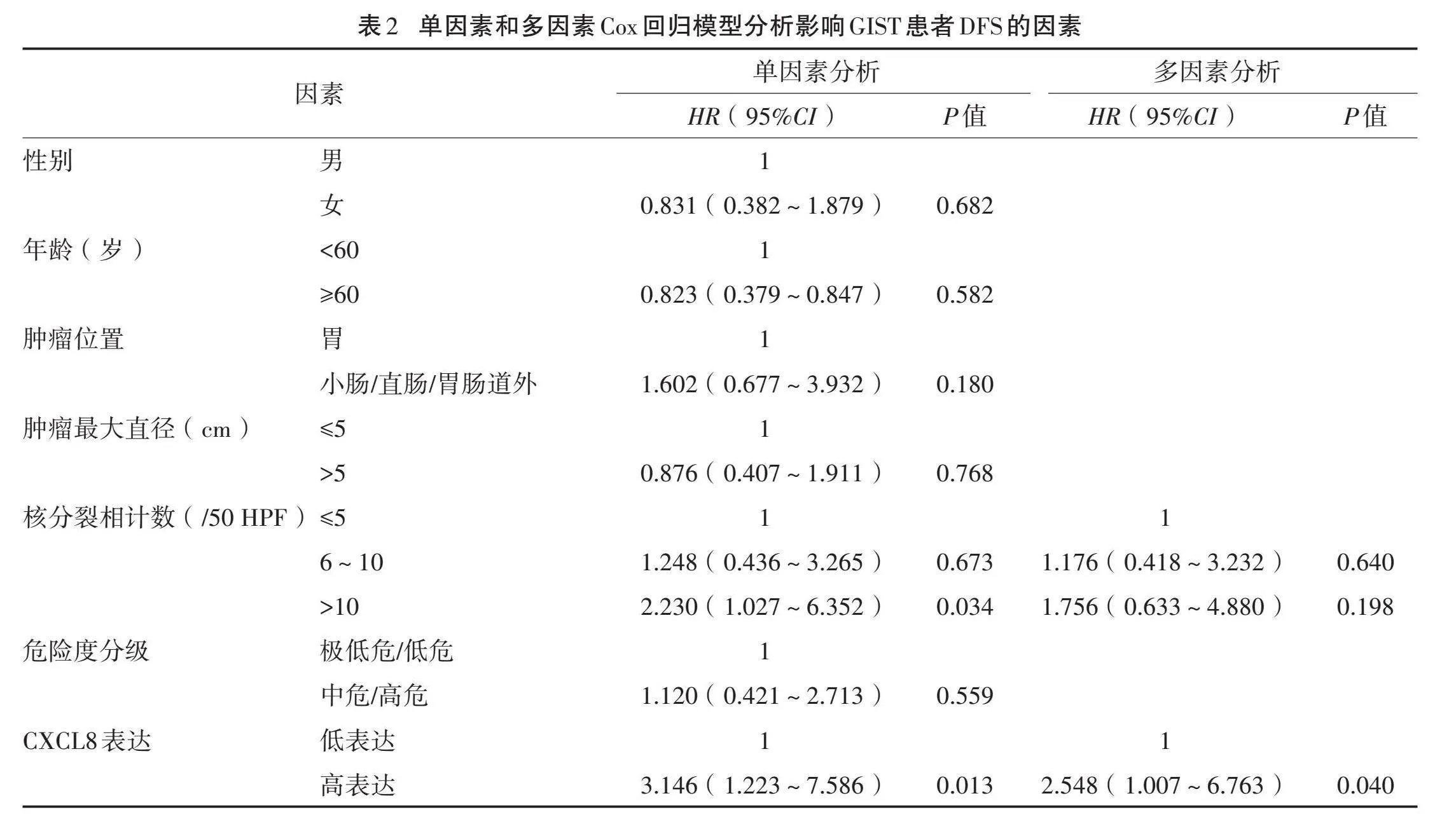
单因素Cox回归模型分析结果显示,核分裂相计数、CXCL8蛋白表达是影响患者预后的危险因素(Plt;0.05),多因素Cox回归模型分析显示,CXCL8蛋白高表达是影响GIST患者DFS的独立危险因素(P=0.047)。见表2。
3" "讨" " " 论
GIST耐药分为原发性耐药和继发性耐药,耐药过程中大量原癌基因激活和抑癌基因失活,筛选耐药相关癌基因,寻找有效的治疗方法对晚期耐药GIST患者至关重要[10]。研究显示,CXCL8与乳腺癌、肺癌、前列腺癌、肝细胞癌和胰腺癌的恶性进展相关,靶向CXCL8并联合其他抗肿瘤方法可抑制肿瘤进展[11-14]。我们从GEO数据库中筛选合适的数据集,通过生物信息学分析发现GPAT2、CXCL8、COL12A1和OGN基因在GIST耐药组织中高表达,可能是导致GIST耐药的重要原因。免疫组化检测发现,耐药组织中CXCL8蛋白表达较敏感组织明显升高(P<0.05),而GPAT2、COL12A1和OGN蛋白表达与敏感组织比较,差异均无统计学意义(P>0.05),提示CXCL8表达升高可能与GIST耐药有关。GPAT2、COL12A1和OGN基因虽然在耐药组织中高表达,但是在翻译成蛋白过程中,某种调控机制导致其最终蛋白表达无明显差异,而何种机制导致蛋白表达的降低还需要进一步验证。在正常情况下,CXCL8表达极低,不易被检测到[15]。在化疗、靶向药物治疗、缺氧状态等刺激下,CXCL8表达升高[16]。我们推测伊马替尼持续治疗可能刺激GIST细胞以及肿瘤微环境中其他细胞表达CXCL8,CXCL8表达增加进一步促进GIST对伊马替尼的耐药。
本研究结果显示,CXCL8蛋白表达与GIST肿瘤最大直径、核分裂相计数及危险度分级有关。Kaplan-Meier生存曲线显示,CXCL8高表达患者DFS低于低表达患者(P=0.0437)。多因素Cox回归模型分析显示,CXCL8蛋白高表达是影响GIST患者DFS的独立危险因素,提示CXCL8可作为GIST治疗后复发风险的预测指标,对于CXCL8高表达患者可适当延长伊马替尼的治疗时间,以降低复发风险。
综上所述,CXCL8高表达促进胃肠道间质瘤耐药,与肿瘤复发风险相关,可能成为特异性治疗的新靶点。
[参考文献]
[1] VON MEHREN M,JOENSUU H. Gastrointestinal stromal tumors[J]. J Clin Oncol,2018,36(2):136-143.
[2] JOENSUU H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor[J]. Hum Pathol,2008,39(10):1411-1419.
[3] KELLY C M,GUTIERREZ SAINZ L,CHI P. The management of metastatic GIST:current standard and investigational therapeutics[J]. J Hematol Oncol,2021,14(1):2.
[4] REICHARDT P,DEMETRI G D,GELDERBLOM H,et al. Correlation of KIT and PDGFRA mutational status with cli-nical benefit in patients with gastrointestinal stromal tumor treated with sunitinib in a worldwide treatment-use trial[J]. BMC Cancer,2016,16:22.
[5] RUSSO R C,GARCIA C C,TEIXEIRA M M,et al. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases[J]. Expert Rev Clin Immunol,2014,10(5):593-619.
[6] ROMAGNANI P,LASAGNI L,ANNUNZIATO F,et al. CXC chemokines:the regulatory link between inflammation and angiogenesis[J]. Trends Immunol,2004,25(4):201-209.
[7] HA H,DEBNATH B,NEAMATI N. Role of the CXCL8-CXCR1/2 axis in cancer and inflammatory diseases[J]. Theranostics,2017,7(6):1543-1588.
[8] DAVID J M,DOMINGUEZ C,HAMILTON D H,et al. The IL-8/IL-8R axis:adouble agent in tumor immune resistance[J]. Vaccines,2016,4(3):22.
[9] CAMBIER S,GOUWY M,PROOST P. The chemokines CXCL8 and CXCL12:molecular and functional properties,role in disease and efforts towards pharmacological intervention[J]. Cell Mol Immunol,2023,20(3):217-251.
[10] GIULIANI F,COLUCCI G. Is there something other than imatinibmesilate in therapeutic options for GIST[J]?Expert Opin Ther Targets,2012,16(Suppl 2):S35-S43.
[11] LI P,ROZICH N,WANG J X,et al. Anti-IL-8 antibody activates myeloid cells and potentiates the anti-tumor activity of anti-PD-1 antibody in the humanized pancreatic cancer murine model[J]. Cancer Lett,2022,539:215722.
[12] HORN L A,RISKIN J,HEMPEL H A,et al. Simultaneous inhibition of CXCR1/2,TGF-β,and PD-L1 remodels the tumor and its microenvironment to drive antitumor immunity[J]. J Immunother Cancer,2020,8(1):e000326.
[13] ARMSTRONG C W D,COULTER J A,ONG C W,et al. Clinical and functional characterization of CXCR1/CXCR2 biology in the relapse and radiotherapy resistance of primary PTEN-deficient prostate carcinoma[J]. NAR Cancer,2020,
2(3):zcaa012.
[14] LESLIE J,MACKEY J B G,JAMIESON T,et al. CXCR2 inhibition enables NASH-HCC immunotherapy[J]. Gut,2022,71(10):2093-2106.
[15] HOFFMANN E,DITTRICH-BREIHOLZ O,HOLTMANN H,et al. Multiple control of interleukin-8 gene expression[J]. J Leukoc Biol,2002,72(5):847-855.
[16] WAUGH D J J,WILSON C. The interleukin-8 pathway in cancer[J]. Clin Cancer,2008,14(21):6735-6741.
[收稿日期] 2024-09-23
(本文编辑" "赵喜)
