肺炎克雷伯菌产KPC酶合并膜孔蛋白OmpK36缺失对头孢他啶/阿维巴坦耐药的分子机制研究
2023-08-25张鹏李婕曹若楠
张鹏?李婕?曹若楠
摘要:目的 研究产KPC酶肺炎克雷伯菌(Klebsiella pneumoniae, Kp)对头孢他啶/阿维巴坦耐药的分子机制。方法 临床分离2株对头孢他啶/阿维巴坦耐药的产KPC酶Kp C9和C14,利用胶体金法进行碳青霉烯酶型检测,基质辅助激光解吸飞行时间质谱(MALDI-TOF MS)进行同源性分析,MIC法进行药物最低抑菌浓度检测,聚丙烯酰胺凝胶电泳(SDS-PAGE)及基因测序检测外膜蛋白表达及基因突变,羰基氰化物间氯苯腙(CCCP)抑制试验检测主动外排机制。结果 产KPC酶Kp C9和C14同其他产KPC酶Kp同源性较低,对头孢他啶/阿维巴坦的MIC分别为16和32μg/mL,外膜蛋白SDS-PAGE显示Kp C9和C14均有OmpK36的缺失,外膜蛋白基因序列分析显示Kp C9和C14的OmpK36基因序列均存在个别位点的突变、缺失,CCCP抑制试验显示CCCP不能提高Kp C9和C14对头孢他啶/阿维巴坦的敏感性。结论 KPC酶合并膜孔蛋白OmpK36缺失可引起Kp对头孢他啶/阿维巴坦耐药。
关键词:肺炎克雷伯菌;碳青霉烯酶;膜孔蛋白;頭孢他啶/阿维巴坦
中图分类号: R978.1文献标志码:A
Molecular mechanism of resistance of Klebsiella pneumoniae to ceftazidime/avibactam due to combination of KPC enzyme and the loss of OmpK36 porin
Zhang Peng, Li Jie, and Cao Ruo-nan
(Clinical Laboratory, Yijishan Hospital of Wannan Medical College, Wuhu 241001)
Abstract Objective To study the molecular mechanism of resistance of Klebsiella pneumoniae (KP) producing KPC enzyme against ceftazidime/avibactam. Methods Kp C9 and C14 which producing KPC enzyme resistant to ceftazidime/avibactam were clinically isolated, and the carbapenemase type was detected by the colloidal gold method. Homology analysis was carried with matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS). The minimum inhibitory concentration (MIC) method was used to perform drug resistant tests. Outer membrane protein expressions were examined by SDS-PAGE. Gene mutations were detected by gene sequencing. Inhibition assays were performed by carbonyl cyanide m-chlorophenylhydrazone (CCCP) to detect active efflux system. Results Kp C9 and C14 producing KPC enzyme have low homology with other Kp producing KPC enzymes. MIC of Kp C9 and C14 to ceftazidime/avibactam was 16~32 μg/mL, respectively. SDS-PAGE showed that both Kp C9 and C14 had the loss of OmpK36. The OmpK36 gene sequence of Kp C9 and C14 contained a few point mutations and deletions at individual sites. The MIC of ceftazidime/avibactam did not change in the presence of CCCP. Conclusion Ceftazidime/avibactam resistance in Kp C9 and C14 isolates is attributable to the production of KPC enzyme combined with the loss of OmpK36 porin.
Key words Klebsiella pneumoniae; Carbapenemase; Porin; Ceftazidime/Avibactam
碳青霉烯类耐药肠杆菌目细菌近来成为危害全球公共卫生安全的主要病原体[1-2],碳青霉烯类耐药机制包括细菌产碳青霉烯酶、超广谱β-内酰胺酶、外排系统上调、膜孔蛋白缺失所致外膜渗透性改变等[3]。肺炎克雷伯菌碳青霉烯酶(Klebsiella pneumoniae carbapenemase, KPC)于1996年美国北卡罗来纳州发现并在全世界广泛传播[4]。产KPC酶肺炎克雷伯菌对亚胺培南、美罗培南等碳青霉烯类药物及大多数抗菌药物均耐药,给临床治疗带来了一定的困难。2015年以来,阿维巴坦作为一种新型β-内酰胺酶抑制剂,因其具有长半衰期、低分子量、亲和重要催化基团等优点,与头孢他啶组合后针对产KPC酶肺炎克雷伯菌较其他药物治疗效果更为明显[5]。但经过一段时间的临床应用,肠杆菌目细菌对头孢他啶/阿维巴坦(ceftazidime/avibactam, CZA)耐药率亦有报道,有研究显示世界范围内肠杆菌目细菌对CZA耐药率平均水平低于2.6%[6],而2017年中国报道该耐药率为5.4%[7],高于全球平均水平。2021年2—3月我们于本院分离得到2株Kp C9和C14,经分析两者均产KPC酶,与其他产KPC酶Kp同源性较低,并同时合并膜孔蛋白OmpK36缺失导致对CZA耐药。
1 材料与方法
1.1 材料
1.1.1 菌株来源
Kp C9,于2021年2月分离自皖南医学院弋矶山医院重症医学科1例72岁女性患者痰液标本。该患者患有主动脉瓣狭窄、肺动脉高压、重症肺炎、呼吸衰竭等疾病,入院后行Morrow术+主动脉瓣置换+二尖瓣/三尖瓣成形术,术后转入重症医学科并发多器官功能衰竭、肺部感染加重,使用哌拉西林/他唑巴坦钠3.375 g q8h治疗1周后分离得到Kp。
Kp C14,于2021年3月分离自皖南医学院弋矶山医院血液内科1例51岁女性患者中心静脉导管标本。该患者患有急性髓系白血病、腔隙性梗死、上呼吸道感染等疾病,入院后行同胞全合异基因造血干细胞移植,出现发热,使用利奈唑胺0.6 g q12 h和亚胺培南/西司他丁钠1 g q6 h各治疗5 d和3 d后分离得到Kp。
其他18株对CZA敏感产KPC酶Kp分离自皖南医学院弋矶山医院住院患者微生物送檢标本。
以上菌株经VITEK-2全自动细菌鉴定仪鉴定均为Kp。外膜蛋白分析选择Kp ATCC13883作为对照菌株。
1.1.2 主要仪器与试剂
VITEK-2全自动细菌鉴定与药敏分析系统及配套试剂(法国BioMérieux公司)、CP5-01碳青霉烯酶检测试剂盒(天津一瑞生物科技股份有限公司)、MALDI-TOF MS检测系统及配套试剂(法国BioMérieux公司)、垂直电泳仪及聚丙烯酰胺凝胶(美国Bio-rad公司)、PCR试剂盒(日本Takara公司)、质粒小量提取试剂盒(德国Qiagen公司)、羰基氰化物间氯苯腙(美国Sigma公司)、血平板及巧克力平板(济南百博生物技术股份有限公司)、MH琼脂平板(英国Oxoid公司)。
1.2 方法
1.2.1 药物敏感试验
使用VITEK-2配套的革兰阴性菌药敏卡AST-N334测定临床分离Kp对常见抗菌药物的MIC值,采用微量肉汤稀释法测定Kp对CZA和多黏菌素B的MIC值。检测结果根据美国临床与实验室标准化研究所CLSI M100-S30推荐的折点进行判读。
1.2.2 碳青霉烯酶型鉴定
采用胶体金免疫层析技术,检测样本与金标抗体结合形成抗原抗体免疫复合物,通过毛细作用在纤维素膜上层析并与包被固化的碳青霉烯酶单抗反应,形成双抗体夹心检测条带,固定于KPC、NDM、IMP、VIM和OXA-48相应检测线上。
1.2.3 菌株同源性分析
将临床分离Kp菌株涂布靶板孔位,室温晾干后,加1 μL 70%甲酸覆盖样本,晾干后再加1 μL IVD HCCA基质液,加样完成后将靶板放入质谱仪进行蛋白质指纹峰图谱采集,通过不同位置100次激光点击获得蛋白谱峰信息,经VITEK MS SARAMIS软件分析鉴定结果并利用配套科研软件导入图谱数据库对检测结果进行聚类分析,相似度<70%者为不同质谱型别。
1.2.4 外膜蛋白及基因序列分析
Kp C9、C14和其他Kp及标准菌株分别接种于MH肉汤培养基,37℃摇床过夜,超声破碎细菌,100000×g 4℃离心1 h后收集沉淀,用含2%十二烷基肌酸钠Tris-Mg重悬沉淀,室温放置30 min,重复2次,外膜蛋白与上样缓冲液充分混匀煮沸5 min后进行SDS-PAGE,25 mA恒流电泳70 min后考马斯亮蓝染色,对比临床分离菌株和标准菌株外膜蛋白表达的差异。同时应用参考文献[8]引物PCR扩增OmpK36基因并送交安徽佰欧晶医学科技有限公司进行序列分析。
1.2.5 外排系统机制分析
同时制备含有50 μmol/L CCCP和不含CCCP的CZA浓度梯度为1~32 μg/mL的MH培养基,将Kp C9和C14接种于该MH培养基,测定细菌的MIC值。
2 结果
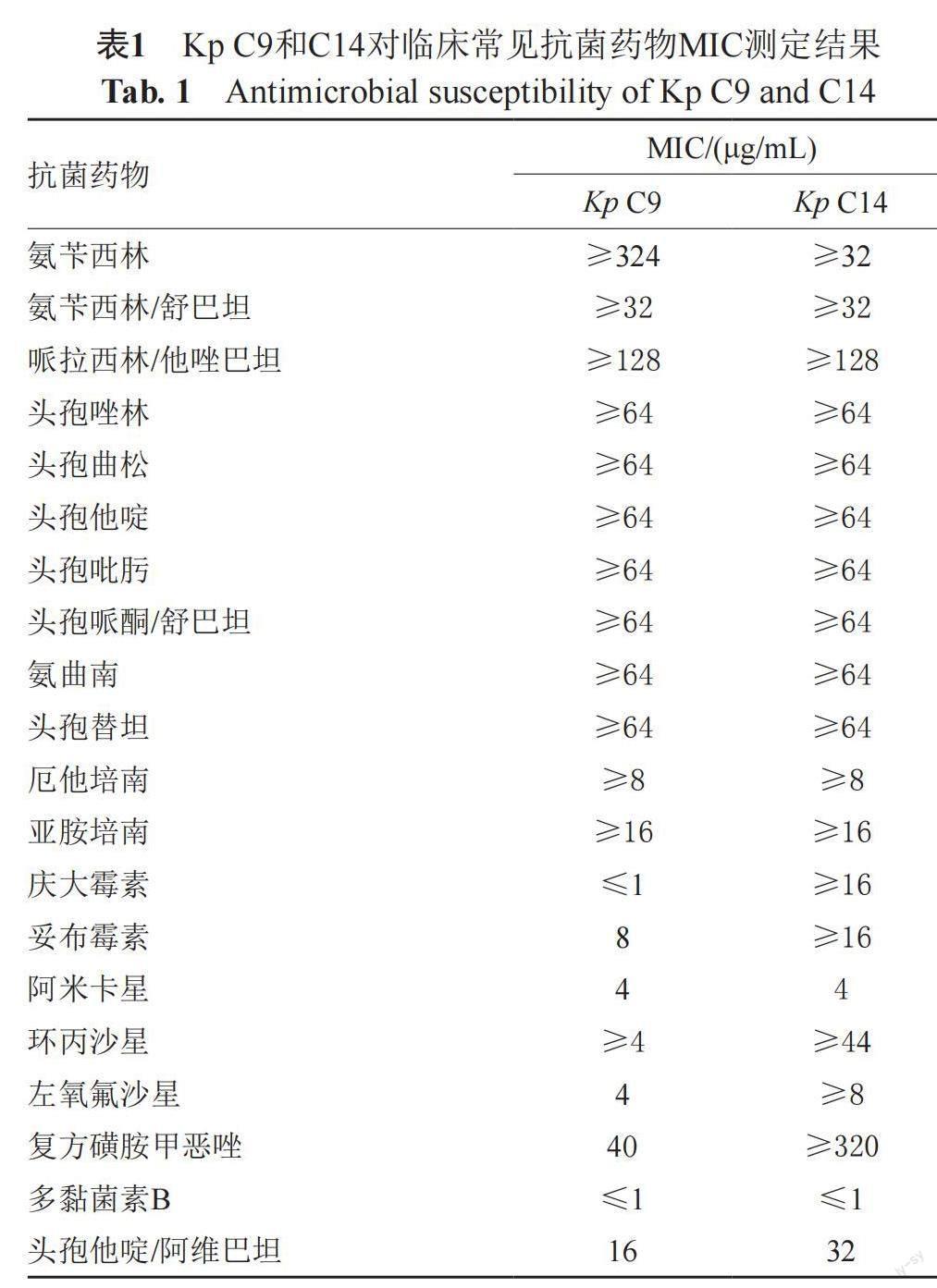
2.1 Kp对抗菌药物的耐药结果
收集临床分离耐碳青霉烯类Kp 20株,其中Kp C9和C14对CZA的MIC值分别为16 μg/mL和32 μg/mL,结果判断为耐药。除CZA外,Kp C9对其他β-内酰胺类抗菌药物均呈现高水平耐药,对多黏菌素B、阿米卡星、复方磺胺甲恶唑敏感,Kp C14对其他β内酰胺类抗菌药物也呈现高水平耐药,仅对多黏菌素B、阿米卡星敏感,见表1。其余18株Kp对CZA的MIC值分别为0.5~2 μg/mL,结果判断为敏感。
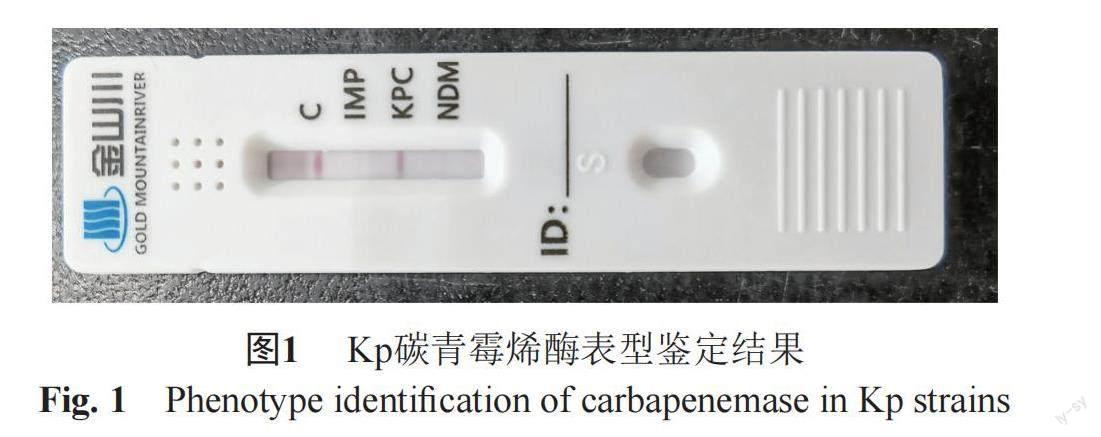
2.2 Kp产碳青霉烯酶表型鉴定及同源性分析
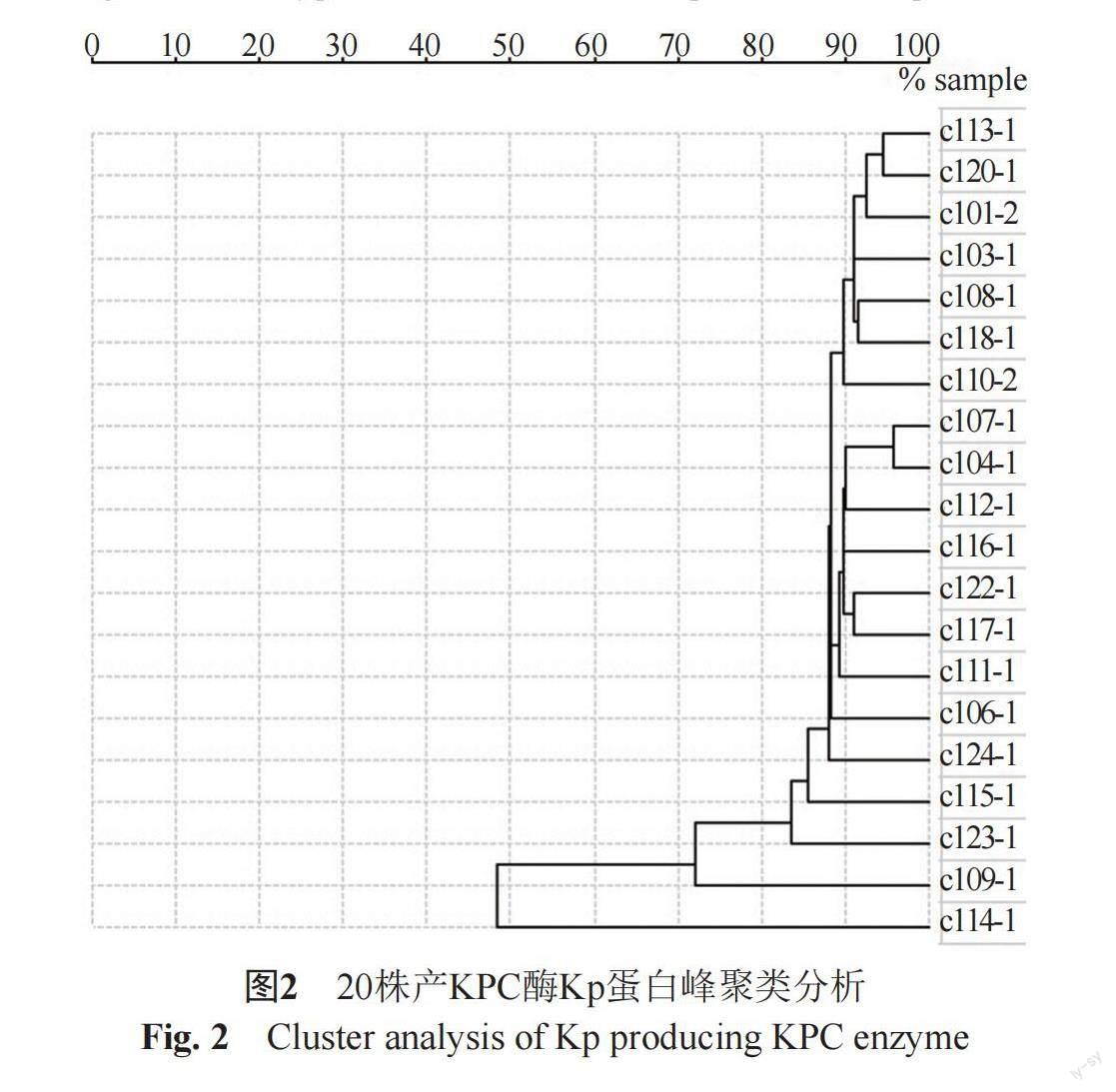
20株耐碳青霉烯类Kp采用胶体金免疫层析技术定性检测碳青霉烯酶型,结果显示所有Kp均为KPC酶阳性,见图1。再使用质谱仪对该20株Kp进行蛋白质指纹峰图谱采集,并对检测结果进行聚类分析并构建发育树,根据不同菌株质谱间相似度>70%划分为同一质谱型别,结果显示Kp C14与其余19株Kp亲缘关系较远,Kp C9与其余18株Kp亲缘关系较远,剩余Kp之间亲缘关系较近,见图2。
2.3 Kp外膜蛋白及基因序列检测结果
Kp ATCC13883和20株Kp的外膜蛋白经SDS-PAGE后,Kp ATCC13883可见4种蛋白,LamB位于相对分子质量46000条带,OmpA位于相对分子质量32000条带,OmpK36位于相对分子质量39000条带,OmpK35位于相对分子质量35000条带。Kp C9和C14都有相对分子质量39000条带的缺失,而其他Kp条带与质控菌株均相同(图3)。Kp C9和C14经OmpK36引物PCR扩增后产物测序与GenBank中Kp C3(登录号:Z33506.1)数据对比发现,Kp C9和C14的OmpK36基因序列存在个别位点的突变、缺失,其中Kp C9点突变74个,缺失碱基数2个,Kp C14点突变79个,缺失碱基数20个。
2.4 主動外排机制检测结果
在含有CCCP的不同浓度CZA MH培养基中,Kp C9对CZA MIC值为16 μg/mL,Kp C14对CZA MIC值为32 μg/mL,与不含CCCP时MIC结果相同,表明Kp C9和C14对CZA不存在主动外排耐药机制。
3 讨论
KPC型丝氨酸酶最常见于耐碳青霉烯类肺炎克雷伯菌,产KPC酶肺炎克雷伯菌对大多数β-内酰胺类抗菌药物呈现高度耐药,且对亚胺培南、美罗培南等碳青霉烯类药物也耐药[9]。随着多重耐药菌在全世界不断增多,临床迫切需求新型抗菌药物[10]。治疗策略之一就是选择β-内酰胺类药物联合β-内酰胺酶抑制剂,然而传统的β-内酰胺酶抑制剂如克拉维酸、他唑巴坦、舒巴坦等对某些β-内酰胺酶缺乏活性,导致对多重耐药菌治疗无效[11]。阿维巴坦作为一种新型β-内酰胺酶抑制剂对Ambler A类、C类甚至某些D类酶具有广泛的活性[12]。有研究表明阿维巴坦联合头孢他啶对产ESBL酶、AmpC酶、KPC酶、OXA-48等肠杆菌目细菌和多重耐药铜绿假单胞菌均有良好的抗菌效果[13-14]。体外试验研究显示头孢他啶/阿维巴坦对肠杆菌目细菌如大肠埃希菌、肺炎克雷伯菌、奇异变形杆菌、肠杆菌属细菌的MIC90为0.5 μg/mL,而单独头孢他啶对肠杆菌目细菌的MIC90为64 μg/mL,美罗培南耐药肠杆菌目细菌有83.5%对头孢他啶/阿维巴坦敏感[15]。对于产KPC酶肠杆菌目细菌,头孢他啶/阿维巴坦敏感率为97.5%[16]。头孢他啶/阿维巴坦对非发酵菌如铜绿假单胞菌的MIC90为8 μg/mL,而单独头孢他啶对铜绿假单胞菌的MIC90为64 μg/mL,美罗培南耐药铜绿假单胞菌有72%对头孢他啶/阿维巴坦敏感[17],对于产KPC酶铜绿假单胞菌,头孢他啶/阿维巴坦敏感率为76%[16]。因此,头孢他啶/阿维巴坦对碳青霉烯类耐药肠杆菌目细菌具有更好的抗菌活性。
尽管头孢他啶/阿维巴坦被批准用于临床时间不长,但一些国家和地区已有该药耐药的报道,需引起广泛关注。目前肠杆菌目细菌对头孢他啶/阿维巴坦总体耐药率为0.6%,其中产ESBL酶菌株对头孢他啶/阿维巴坦耐药率为2.8%[18-19],而产碳青霉烯酶菌株对头孢他啶/阿维巴坦耐药率高达24.7%[20],尤其产金属酶菌株耐药率更高达98.6%[21]。对肺炎克雷伯菌而言,耐碳青霉烯类菌株对头孢他啶/阿维巴坦耐药率可达16.7%~21%[22-23]。究其耐药原因,主要有以下几方面:①膜孔蛋白缺失致外膜渗透性改变,当OmpK36存在时,肺炎克雷伯菌对头孢他啶/阿维巴坦MIC值为0.5~1 μg/mL[24],②外排系统功能增强,有研究表明外排泵抑制剂PAβN并未增加肺炎克雷伯菌对头孢他啶/阿维巴坦的MIC值[25],③靶蛋白突变,PBP3蛋白氨基酸插入可增加大肠埃希菌对头孢他啶/阿维巴坦的MIC值[26]。本研究收集耐碳青霉烯类肺炎克雷伯菌20株,碳青霉烯酶型鉴定均产KPC酶,药敏结果显示C9和C14两株菌对头孢他啶/阿维巴坦耐药,其余肺炎克雷伯菌对头孢他啶/阿维巴坦均敏感,为进一步研究该两株菌耐药机制,经同源性分析发现C9和C14与其他菌株亲缘关系较远,外膜蛋白SDS-PAGE和基因测序结果显示C9和C14均有OmpK36蛋白缺失和OmpK36基因序列个别位点的突变、缺失,而其余菌株外膜蛋白条带均正常,CCCP抑制试验显示C9和C14对头孢他啶/阿维巴坦的MIC值没有变化。这一结果表明,OmpK36缺失在头孢他啶/阿维巴坦耐药中起了重要作用。
综上所述,头孢他啶/阿维巴坦作为新型含β-内酰胺酶抑制剂抗菌药物,自2015年上市针对革兰阴性杆菌尤其肠杆菌目细菌呈现良好的抗菌活性,从一定程度上减轻了多重耐药菌和泛耐药菌的抗生素选择压力。但头孢他啶/阿维巴坦耐药菌株的出现需引起临床和院感的高度重视,针对此类耐药菌应避免单独使用头孢他啶/阿维巴坦,可考虑联合多黏菌素B或氨基糖苷类等药物进行治疗。因此,加强细菌耐药检测和耐药机制研究对临床合理使用抗菌药物和医院感染管理意义重大。
参 考 文 献
McKenna M. Antibiotic resistance: the last resort[J]. Nature, 2013, 499(7459): 394-396.
Kuehn B M. "Nightmare" bacteria on the rise in US hospitals, long-term care facilities[J]. JAMA, 2013, 309(15): 1573-1574.
Brink A J. Epidemiology of carbapenem-resistant Gram-negative infections globally[J]. Curr Opin Infect Dis, 2019, 32(6): 609-616.
Yigit H, Queenan A M, Anderson G J, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae[J]. Antimicrob Agents Chemother, 2001, 45(4): 1151-1161.
Levasseur P, Girard A M, Miossec C, et al. In vitro antibacterial activity of the ceftazidime-avibactam combination against Enterobacteriaceae, including strains with well-characterized β-lactamases[J]. Antimicrob Agents Chemother, 2015, 59(4): 1931-1934.
Kazmierczak K M, Bradford P A, Stone G G, et al. In vitro activity of ceftazidime-avibactam and aztreonam-avibactam against OXA-48-carrying Enterobacteriaceae isolated as part of the International Network for Optimal Resistance Monitoring (INFORM) global surveillance program from 2012 to 2015[J]. Antimicrob Agents Chemother, 2018, 62(12): e00592-18.
Yin D, Wu S, Yang Y, et al. Results from the China Antimicrobial Surveillance Network (CHINET) in 2017 of the in vitro activities of ceftazidime-avibactam and ceftolozane-tazobactam against clinical isolates of Enterobacteriaceae and Pseudomonas aeruginosa[J]. Antimicrob Agents Chemother, 2019, 63(4): e02431-18.
Kaczmarek F M, Dib-Hajj F, Shang W, et al. High-level carbapenem resistance in a Klebsiella pneumoniae clinical isolate is due to the combination of bla(ACT-1) beta-lactamase production, porin OmpK35/36 insertional inactivation, and down-regulation of the phosphate transport porin phoe[J]. Antimicrob Agents Chemother, 2006, 50(10): 3396-3406.
Yang X, Dong N, Chan E W, et al. Carbapenem resistance-encoding and virulence-encoding conjugative plasmids in Klebsiella pneumoniae[J]. Trends Microbiol, 2021, 29(1): 65-83.
Breijyeh Z, Jubeh B, Karaman R. Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it[J]. Molecules, 2020, 25(6): 1340.
Bebrone C, Lassaux P, Vercheval L, et al. Current challenges in antimicrobial chemotherapy: Focus on β-lactamase inhibition[J]. Drugs, 2010, 70(6): 651-679.
Jean S S, Gould I M, Lee W S, et al. New drugs for multidrug-resistant Gram-negative organisms: Time for stewardship[J]. Drugs, 2019, 79(7): 705-714.
Karlowsky J A, Kazmierczak K M, Valente M L N F, et al. In vitro activity of ceftazidime-avibactam against enterobacterales and Pseudomonas aeruginosa isolates collected in Latin America as part of the ATLAS global surveillance program, 2017—2019[J]. Braz J Infect Dis, 2021, 25(6): 101647.
Castanheira M, Doyle T B, Collingsworth T D, et al. Increasing frequency of OXA-48-producing enterobacterales worldwide and activity of ceftazidime/avibactam, meropenem/vaborbactam and comparators against these isolates[J]. J Antimicrob Chemother, 2021, 76(12): 3125-3134.
de Jonge B L M, Karlowsky J A, Kazmierczak K M, et al. In vitro susceptibility to ceftazidime-avibactam of carbapenem-nonsusceptible Enterobacteriaceae isolates collected during the INFORM global surveillance study (2012 to 2014)[J]. Antimicrob Agents Chemother, 2016, 60(5): 3163-3169.
Kazmierczak K M, Biedenbach D J, Hackel M, et al. Global dissemination of blaKPC into bacterial species beyond Klebsiella pneumoniae and in vitro susceptibility to ceftazidime-avibactam and aztreonam-avibactam[J]. Antimicrob Agents Chemother, 2016, 60(8): 4490-4500.
Nichols W W, de Jonge B L M, Kazmierczak KM, et al. In vitro susceptibility of global surveillance isolates of Pseudomonas aeruginosa to ceftazidime-avibactam (INFORM 2012 to 2014)[J]. Antimicrob Agents Chemother, 2016, 60(8): 4743-4749.
Karlowsky J A, Kazmierczak K M, Bouchillon S K, et al. In vitro activity of ceftazidime-avibactam against clinical isolates of Enterobacteriaceae and Pseudomonas aeruginosa collected in Asia-Pacific countries: Results from the INFORM global surveillance program, 2012 to 2015[J]. Antimicrob Agents Chemother, 2018, 62(7): e02569-17.
Castanheira M, Doyle T B, Mendes R E, et al. Comparative activities of ceftazidime-avibactam and ceftolozane-tazobactam against Enterobacteriaceae isolates producing extended-spectrum β-lactamases from U.S. hospitals[J]. Antimicrob Agents Chemother, 2019, 63(7): e00160-19.
Senchyna F, Gaur R L, Sandlund J, et al. Diversity of resistance mechanisms in carbapenem-resistant Enterobacteriaceae at a health care system in Northern California, from 2013 to 2016[J]. Diagn Microbiol Infect Dis, 2019, 93(3): 250-257.
Kazmierczak K M, de Jonge BLM, Stone G G, et al. In vitro activity of ceftazidime/avibactam against isolates of Enterobacteriaceae collected in European countries: INFORM global surveillance 2012-15[J]. J Antimicrob Chemother, 2018, 73(10): 2782-2788.
Flamm R K, Nichols W W, Sader H S, et al. In vitro activity of ceftazidime/avibactam against Gram-negative pathogens isolated from pneumonia in hospitalised patients, including ventilated patients[J]. Int J Antimicrob Agents, 2016, 47(3): 235-242.
Wilson W R, Kline E G, Jones C E, et al. Effects of KPC variant and porin genotype on the in vitro activity of meropenem-vaborbactam against carbapenem-resistant Enterobacteriaceae[J]. Antimicrob Agents Chemother, 2019, 63(3): e02048-18.
Shields R K, Clancy C J, Hao B, et al. Effects of Klebsiella pneumoniae carbapenemase subtypes, extended-spectrum β-lactamases, and porin mutations on the in vitro activity of ceftazidime-avibactam against carbapenem-resistant K. pneumoniae[J]. Antimicrob Agents Chemother, 2015, 59(9): 5793-5797.
Castanheira M, Doyle T B, Hubler C, et al. Ceftazidime-avibactam activity against a challenge set of carbapenem-resistant Enterobacterales: Ompk36 L3 alterations and β-lactamases with ceftazidime hydrolytic activity lead to elevated MIC values[J]. Int J Antimicrob Agents, 2020, 56(1): 106011.
Zhang Y, Kashikar A, Brown C A, et al. Unusual Escherichia coli PBP 3 insertion sequence identified from a collection of carbapenem-resistant Enterobacteriaceae tested in vitro with a combination of ceftazidime-, ceftaroline-, or aztreonam-avibactam[J]. Antimicrob Agents Chemother, 2017, 61(8): e00389-17.
