红树林植物内生真菌次级代谢产物研究进展(2018—2019年)
2023-08-25徐志勇熊伟吴磊刘云飞谢传奇徐静
徐志勇?熊伟?吴磊?刘云飞?谢传奇?徐静
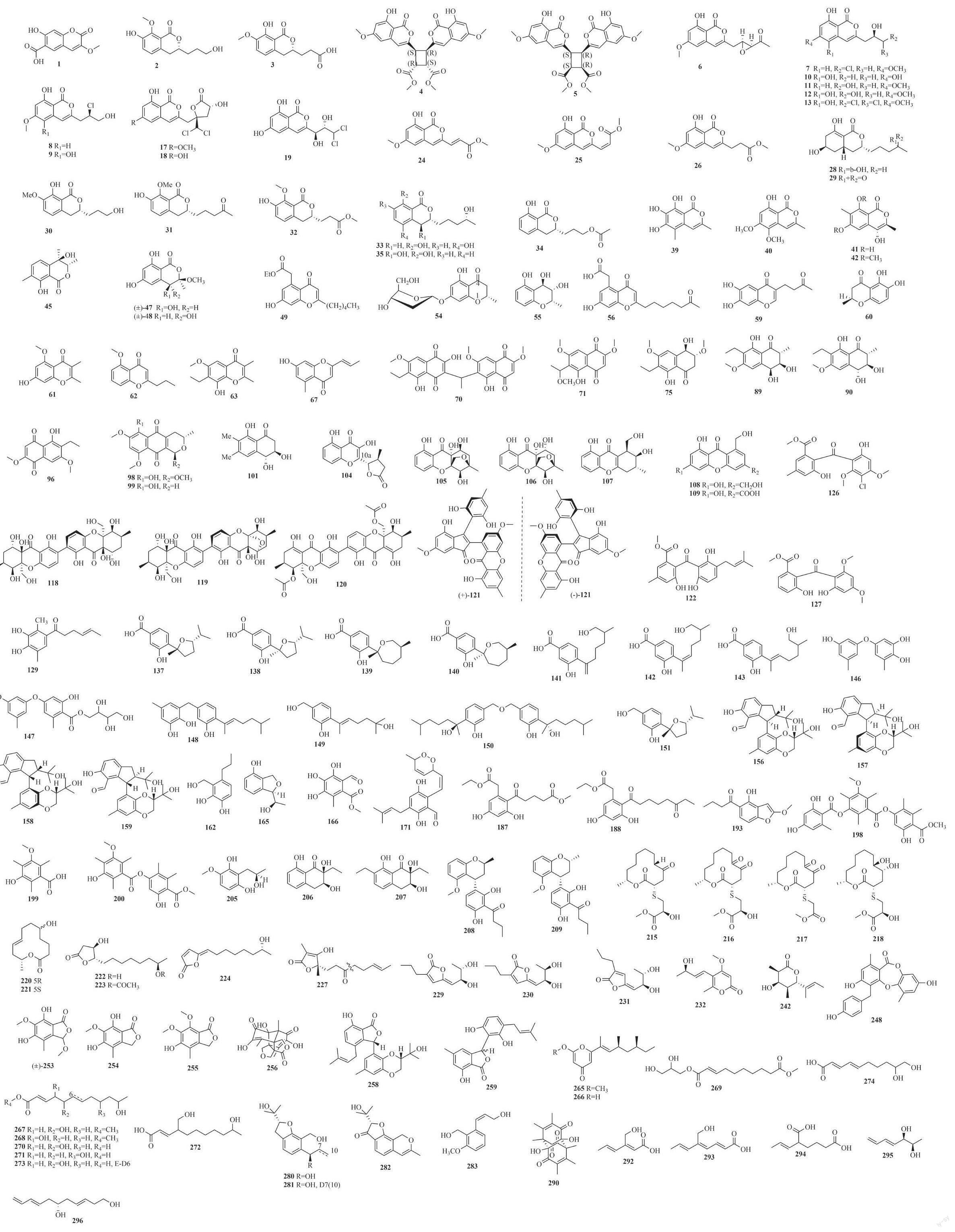
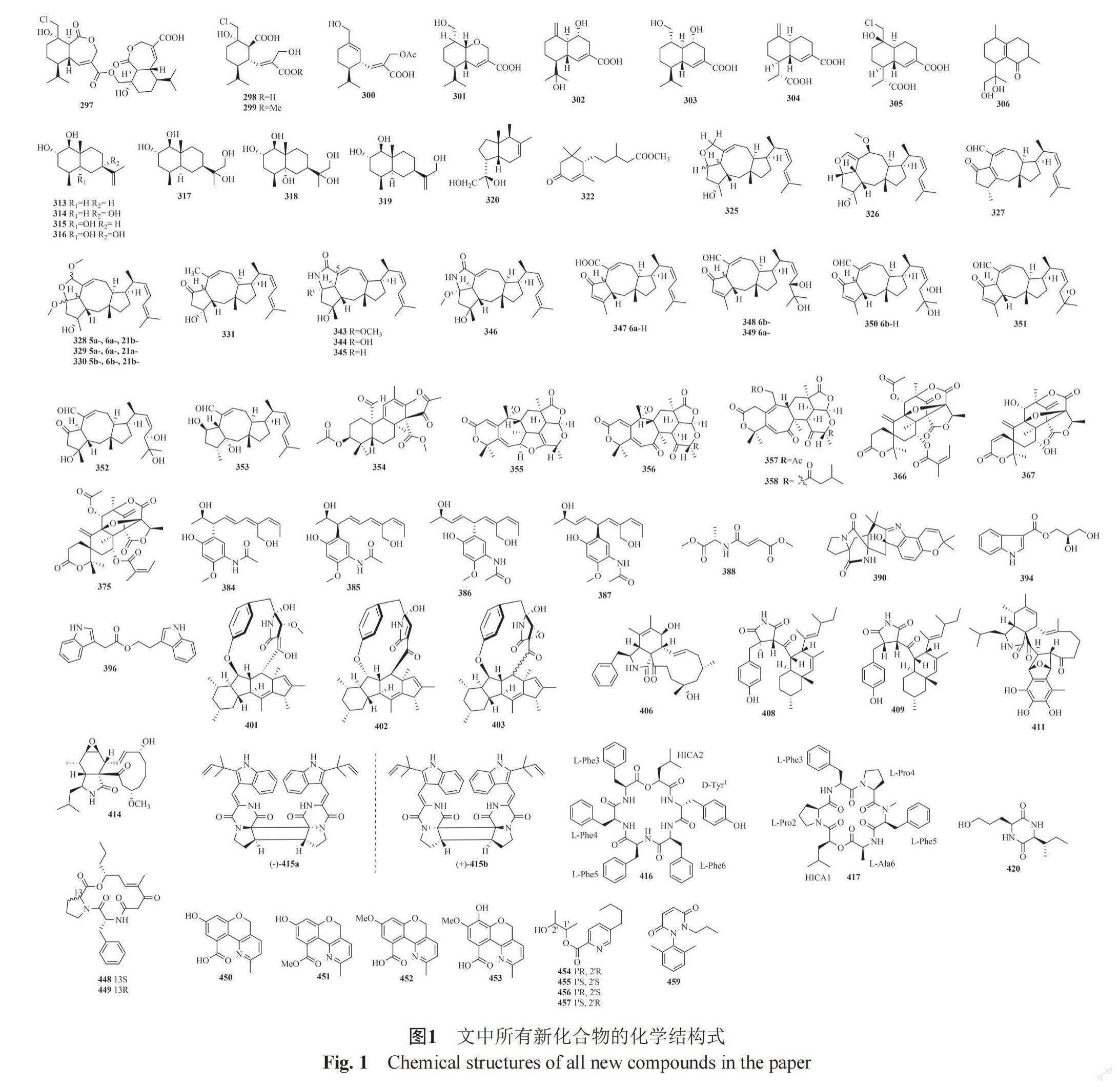
摘要:红树林植物长期处于高盐、缺氧、频繁潮汐等独特环境,其内生真菌与宿主植物共同进化,从中挖掘出了许多结构新颖的功能分子,是天然产物先导化合物发现的重要来源。本文综述了2018年1月—2019年12月发现的来源于红树林植物内生真菌的次级代谢产物,重点总结了它们的化学结构类型、宿主来源。描述化合物460个,其中新化合物217个,出新率达47.2%;结构类型涉及聚酮类、萜类、生物碱;研究较多的菌属为曲霉属Aspergillus、间座壳属Diaporthe、青霉属Penicillium、枝孢属Cladosporium;研究较多的宿主植物为秋茄、红海榄、海莲、角果木。本文旨在总结本领域的研究成果与存在问题,为今后研究提供参考。
关键词:红树林内生真菌;次级代谢产物;化学结构
中图分类号:Q936文献标志码:A
Research progress on the secondary metabolites produced by endophytic fungi isolated from mangrove plants (2018—2019)
Xu Zhi-yong1, Xiong Wei1, Wu Lei1, Liu Yun-fei1, Xie Chuan-qi1, and Xu Jing2
(1 Institute of Applied Chemistry, Jiangxi Academy of Sciences, Nanchang 330096;
2 School of Chemical Engineering and Technology, Hainan University, Haikou 570228)
Abstract Mangrove plants have long been in a unique environment such as high salinity, hypoxia, and frequent tides. Their endophytic fungi co-evolved with their host plants, and many functional molecules with novel structures have been unearthed. They are important source of lead compounds of natural products. This paper reviews the secondary metabolites produced by endophytic fungi derived from mangrove plants from the beginning of 2018 to the end of 2019, with emphasis on compounds structural types and host sources. 460 compounds are described, including 217 new compounds (47.2%). Structural types involve polyketides, terpenes, and alkaloids. The main producing strains are affiliated to genera such as Aspergillus, Diaporthe, Penicillium, and Cladosporium. The main host plants are Kandelia candel, Rhizophora stylosa, Bruguiera sexangula, and Ceriops tagal. Research progress and open questions are summarized and discussed to provide references for future research in the paper.
Key words Mangrove endophytic fungi; Secondary metabolites; Chemical structure
红树林(Mangrove)是一类生长在热带、亚热带海岸或者河口潮间带的耐盐植物群落,是陆地和海洋环境之间动态过渡的重要生态系统。红树林植物生境特殊,包括高盐、高温潮湿、厌氧的土壤、潮汐活动和高强度的微生物和动物竞争[1-3],其内生真菌与宿主长期进化,从中已挖掘出许多结构新颖的功能分子。事实证明,红树林来源微生物是挖掘天然产物的巨大宝藏,是小分子药物的重要来源。迄今为止,很多研究者们对天然产物来源微生物和内生真菌代谢产物进行了综述,Xu等[4-5]在Curr Med Chem和Rsc Adv杂志上对2011年之前和2011—2013年报道的红树林微生物来源的功能分子和活性天然产物进行了评述,在2011年之前发现了348个功能分子,2013年之前共464个新代谢物,Wang等[6]综述了2008年到2013年中段,在中国南海起源的红树林真菌产生的110个化合物;Proksch教授课题组对2000—2010年间红树林内共生真菌作为生物活性天然产物来源的开发潜力及研究進展进行了概述[3,7],Blunt和Carroll等[8-11]对海洋天然产物进行综述,其中一节包括2014—2017年红树林来源真菌产生的新化合物(共486个)。本文将对2018年1月—2019年12月文献报道的红树林植物内生真菌次级代谢产物按化合物的结构分类进行评述,所有新化合物的化学结构式如图1。首次出现的宿主植物均在正文里标注了拉丁名和样品来源,凡是后续没有提供来源的菌株均为前述提到过的同一菌株。
1 聚酮类化合物
1. 1 香豆素及异香豆素类化合物
Yan等[12]从红树林植物老鼠簕(Acanthus ilicifolius Linn.,中国广东)新鲜果实中的1株内生真菌Epicoccum nigrum SCNU-F0002中分离得到1个新的香豆素类化合物(1)和两个新的异香豆素类化合物(2~3)。10个新的异香豆素peniisocoumarins A~J (4~13)及3个已知类似物(14~16)分离自红树林植物秋茄(Kandelia candel,中国珠海)果实内生真菌Penicillium commune QQF-3[13]。Chen等[14]从半红树林植物阔苞菊(Pluchea indica,中国广西)健康树枝内生菌Ascomycota sp. CYSK-4中分离获得3个新二氯异香豆素(17~19)及6个已知类似物(11, 14, 20~23)。Wu等[15]从老鼠簕(中国海南)生鲜叶内生菌Aspergillus sp.HN15-5D中分离获得3个新异香豆素衍生物aspergisocoumrins A~C(24~26)及两个已知类似物(14, 27)。8个新的异香豆素peniciisocoumarins A~H(28~35)及3个已知类似物(36~38)分离自红树林植物尖瓣海莲(Bruguiera sexangula var. rhynchopetala,中国南海)内生真菌Penicillium sp. TGM112[16]。4个新的异香豆素botryospyrones A-D(39~42)分离自半红树植物苦槛蓝(Myoporum bontioides (Siebold & Zucc.) A. Gray,中国雷州半岛)叶内生真菌Botryosphaeria ramosa L29[17]。3个已知的异香豆素(16, 43~44)分离自尖瓣海莲(中国南海)茎内生真菌D. eschscholtzii HJ004[18]。Xu等[19]从红树林植物红海榄(Rhizophora stylosa,中国海南)树枝来源内生真菌Pestalotiopsis sp.101里分离获得1个新的异香豆素类化合物pestalotiopisorin B(45)和1个已知物(46)。两对新的异香豆素异构体penicoffrazins B~C(47~48)被Cao等[20]从红树林植物使君子科拉关木(Laguncularia racemosa,中国海南)叶内生菌Penicillium co?eae MA-314中分离获得。
1.2 色酮类化合物
Luo等[21]从红海榄(中国海南)内生真菌Diaporthe sp. SCSIO 41011分离得到1个新的色酮衍生物pestalotiopsone H(49)和4个已知的色酮类化合物(50~53)。同年,两个新色酮衍生物(2S)-7-O-α-D-呋喃型核糖基-5-羟基-2-甲基苯并吡喃-4-酮(54)和(2S, 3S, 4R)-2-甲基苯并吡喃-3,4,5-三醇(55)被分离自红树林植物海漆(Excoecaria agallocha,中国海南)茎的内生真菌Cladosporium sp. OUCMDZ-302,并且通过X单晶衍射和酸性水解物的糖分析首次确定了54的绝对构型 [22]。Zheng等[23]从红树林植被木果楝(Xylocarpus granatum,中国南海)茎中的1株内生真菌Dothiorella sp. ML002分离得到1个新的cytosporone类化合物(56)和两个已知的类似物(57~58)。新化合物chaetochromone D(59)分离自秋茄(中国广西)枝内生真菌Phoma sp. SYSU-SK-7[24]。新色酮类化合物(2S)-2,3-dihydro-5,6-dihydroxy- -2-methyl-4H-1-benzopyran-4-one(60)分离自红树林植物角果木(Ceriops tagal,中国海南)的1株内生真菌Colletotrichum gloeosporioides[25]。3个新的色酮:7-羟基-5-甲氧基-2,3-二甲基色酮(61)、5-甲氧基-2-丙基色酮(62)和7-乙基-8-羟基-6-甲氧基-2,3-二甲基色酮(63)及4个已知色酮(53, 64~66)分离自尖瓣海莲(中国南海)内生真菌D. eschscholtzii HJ004[26]。新吡喃酮衍生物clapone(67)和两个已知的色酮衍生物(68~69)分别分离自角果木(中国海南)根内生真菌Cladosporium sp. HNWSW-1和角果木(中国南海)茎块真菌Cladosporium sp. JJM22[27-28]。
1.3 醌类化合物
Cui等[29]在生物活性测试指导下,对1株分离自秋茄 (中国广西)枝内生真菌Neofusicoccum austral SYSU-SKS024二氯甲烷段浸膏化学成分进行分析,得到3个新的乙基萘醌衍生物:neofusnaphthoquinone A(70)、6-(1-methoxylethy1)-2,7- dimethoxy juglone(71)和(3R,4R)-3-methoxyl-botryo-sphaerone D(75)以及6个已知的类似物(72~74和76~78),化合物70为不对称萘醌二聚体结构。Yang等[30]从红海榄叶的1株内生真菌Aspergillus nidulans MA143分离获得6个醌类化合物,包括新天然产物isoversicolorin C(79)和5个类似物(80~84)。1个已知的蒽醌chrysophanol(85)分离自秋茄(中国广西)叶内生真菌Ascomycota sp. SK2YWS-L[31-32]。3个已知的醌类化合物(86~88)分离自角果木(中国海南)内生真菌Cladosporium sp. JS1-2。两个新的四氢萘酮daldiniones C-D(89~90)和5个已知的醌类化合物(91~95)被分离自之前描述的真菌D. eschscholtzii HJ004[18] 。新化合物6-乙基-5-羟基-2,7-二甲氧基萘-1,4-二酮(96)分离自红海榄树皮内生真菌Phomopsis sp.33#[33]。已知蒽醌化合物anthraquinone(97)获得自之前描述的Cladosporium sp. HNWSW-1[27]。兩个新萘醌6-羟基-astropaquinone B(98)和astropaquinone D(99)以及1个已知萘醌衍生物(100)分离自红树林植物红茄苳(Rhizophora mucronata,印度尼西亚南苏拉威西)枝内生菌Fusarium napiforme[34]。3个四氢萘酮,包括已知化合物(102~103)和反式(3R,4R)-3,4,8-三羟基-6,7-二甲基-3,4-二氢萘(101)(新化合物)分离自前文所述Cladosporium sp. JJM22[28]。
1.4 氧杂蒽酮类化合物
Hu等[35]从泰国木果楝叶的内生真菌Phomopsis sp.xy21分离得到6个新的氧杂蒽酮类化合物phomoxanthones F-K(104~109)和3个已知聚酮类化合物(110~112),脱羧苯并吡喃酮母核在10a处被4-甲基二氢呋喃取代得到化合物104、化合物105和106为高度氧化的氧杂蒽酮类衍生物,含有新颖的5-甲基-6-氧杂双环部分。3个氧杂蒽酮类似物(113~115)分离自前文所述的Diaporthe sp. SCSIO 41011,同时从菌株Phomopsis sp.33#也分离到化合物113和114[21,33]。前文所述的Aspergillus nidulans MA143產生新天然产物isosecosterigmatocystin(116)和已知化合物sterigmatocystin(117);Phomopsis sp. xy21产生3个新的黄酮二聚体phomoxanthones C~E(118~120),化合物118和119具有高度氧化六氢蒽酮骨架;Ascomycota sp. SK2YWSL产生1对新颖的对映体(±)-ascomindone D(121)、1个新的烯丙基化聚酮类化合物ascomarugosin A(122)和两个已知物(123~124),其中121通过手性HPLC分离纯化得到,是在天然产物中拆分二芳基茚酮异构体的第一个实例。Cladosporium sp. JS1-2产生已知物secalonic acid D(125)[30-32,35]。Zheng等[36]从尖瓣海莲(中国南海)茎中的1株内生真菌Penicillium citrinum HL-5126分离得到两个新化合物penibenzophenones A-B(126~127)和1个已知化合物sulochrin(128)。
1.5 酚和酚酸类化合物
红树林植物木榄(Bruguiera gymnorrhiza,中国湛江)茎块中的一株内生菌Penicillium sp. GD6中分离得到一个新的sorbicillin衍生物(129)和一个已知的类似物(130),130也曾从苦槛蓝内生真菌Penicillium chrysogenum V11中分离得到[37-38]。近两年酚衍生倍半萜在曲霉属(Aspergillus)中被多次发现,并拥有多种生物活性,如Cui等[39]从海漆(中国广西)树枝中的Aspergillus versicolor SYSU-SKS025分离得到已知化合物(131~136)。Wang等[40]从泰国红树植物木果楝叶内生菌Aspergillus sp. xy02中分离得到7个新化合物(137~143)及已知化合物(132~134,144~145)。Wu等[41]从红树林植物秋茄(中国惠州)枝中的Aspergillus flavus QQSG-3分离得到11个酚类化合物(136,146~155),其中146~151为新化合物,148~151为酚类衍生倍半萜。Cui等[42]报道了海漆(中国珠海)枝内生菌Diaporthe sp. SYSU-HQ3的6个酚类化合物(156~161),其中Diaporindenes A-D(156~159)为新化合物,具有2,3-二氢-1氢-茚环兼1,4-苯并二恶烷的新颖骨架。Cai等[43]从秋茄(中国湛江)叶中的Aspergillus sp. ZJ-68分离得到一个新的苯丙烷衍生物asperpanoid A(162)、已知物2-(羟甲基)-3-丙基苯酚(163)和isochroman A(164)。Qiu等[44]从木榄(中国海南)果实的内生菌Mycosphaerella sp. SYSU- DZG01分离得到两个新的epicoccine衍生物(165~166)及已知物(167~170)。Elissawy等[45]从红树林植物白骨壤(Avicennia marina,埃及)叶内生菌Aspergillus sp. AV-2分离得到1个新的烯丙基苯甲醛衍生物dioxoauroglaucin(171)。前文所述的Diaporthe sp. SCSIO 41011产生已知酚衍生长支链酮(172~185)[21];Cladosporium sp. OUCMDZ-302 产生化合物186 [22];Dothiorella sp. ML002产生两个新的cytosporone衍生物dothiorelones K~L(187~188)及4个已知类似物(189~192)[23];Epicoccum nigrum SCNU-F0002产1个新的苯并呋喃酮193及5个已知物(194~197, 170)[46]。Phoma sp. SYSU-SK-7产生新化合物(198~200)和已知化合物(201~204)[24];Colletotrichum gloeosporioides产生具有潜在抗菌活性的新化合物(2'R)-2-(2'-羟丙基)-4-甲氧基-1,3-苯二醇(205)[25];D. eschscholtzii HJ004产生4个新化合物(206~209),206~207为两个四氢萘酮类化合物[18];Penicillium citrinum产生两个已知酚类(210~211)[36];Pleosporales sp. SK7产生4个已知酚类(210, 212~214) [47]。
1.6 内酯类化合物
Zhang等[48]从木榄叶内生菌Cladosporium cladosporioides MA-299分离得到4个新的十二元大环内酯类化合物thiocladospolides A~D(215-218)、已知的同源物pandangolide 3(219)、5个新内酯化合物(220~224)和两个已知物(225~226),其中215~219在C-2处都有硫取代基,通过X衍射单晶首次确定了含硫十二元大环内酯215的绝对构型,222~225为呋喃环内酯。Supratman等[49]利用单菌株多化合物(OSMAC)方法,对分离自印度尼西亚木榄枝的内生真菌Clonostachys rosea B5-2培养发酵时加入苹果汁,研究化学成分得到新单环呋喃类内酯化合物(-)-dihydro-vertinolide(227)及已知同类物228。3个新的单环呋喃类内酯化合物penicilactones A~C(229~231)分离自前文所述真菌Penicillium sp. TGM112[50]。Fan等[51]发现红树林植物正红树(Rhizophora apiculata,中国海南)根来源的Penicillium camemberti OUCMDZ-1492产生的单环α-吡喃酮内酯化合物(232~236),232为新化合物。单环α-吡喃酮内酯化合物不断在多种红树林真菌中发现,如分离自白骨壤共生真菌Sarocladium kiliense HDN11-112的化合物(237~239)[52];分离自木果楝茎内生真菌Xylaria sp. HNWSW-2的化合物astropyrone(240)[53];秋茄内生真菌Colletotrichum tropicale SCSIO 41022产生化合物acropyrone(241)[54];前文所述Penicillium coffeae MA-314和D. eschscholtzii HJ004分别产生新内酯penicoffeazine A(242)[20]、化合物(243~246)[18]。前文所述Colletotrichum gloeosporioides产生4-乙基-3-羟基-6-丙烯基-2H-吡喃-2-酮(247)[25]。红树林内生真菌Trichoderma sp. 307与Acinetobacter johnsonii B2共培养产生了新化合物botryorhodine H(248)及3个类似物(249~251)[55]。Liu等[56]从红树林植物Rhizophora racemosa内生真菌Annulohypoxylon sp.中分离得到10元大环内酯hypoxylide(252)。前文所述的Epicoccum nigrum SCNU-F0002产生4个新的异苯并呋喃酮单体(253~256)及已知物257,分别为(±)-epicoccone C、epicoccone D、epicoccone E、epicolactone A和epicolactone[12];Diaporthe sp. SYSU-HQ3产生新颖化合物isoprenylisobenzofuran A(258)[42];Ascomycota sp. SK2YWS-L产生新烯丙基聚酮ascomfurans C(259)[31];Diaporthe sp. SCSIO 41011产生4个已知苯并呋喃衍生物(260~263)及一个α-吡喃酮单环衍生物(264)[21]。
1.7 其它聚酮类化合物
Zhou等[57]从正红树根内生菌Fusarium solani HDN15-410分离得到两个之前没有描述过的γ-吡喃酮衍生物fusolanones A~B(265~266)。Peng等[58]从红海榄根内生耐盐真菌Cladosporium cladosporioides OUCMDZ-187(10%盐度培养下)分离得到3种新的脂肪酸酯cladosporesters A~C(267~269)和5种新的脂肪酸cladosporacids A~E(270~274)。前文所述真菌Diaporthe sp. SCSIO 41011产生氧化氯杂苯甲酮衍生物(275~279)[59];Aspergillus sp. ZJ-68产生3个新的苯并呋喃类化合物asperfuranoids A~C(280~282)、1个新的苯基丙烷类衍生物(283)和6个已知的类似物(284~289)[43];Mycosphaerella sp. SYSU-DZG01产生新化合物dibefurin B(290)[44];Cladosporium sp. JS1-2产生戊烯酸衍生物1,1'-dioxine-2,2'-dipropionic acid(291)[32];Clonostachys rosea B5-2产生新的clonostach酸(292~294)[49];Cladosporium sp. OUCMDZ-302产生两个新其它类型聚酮化合物(295~296)[22]。
2 萜类化合物
2.1 倍半萜类化合物
Liu等[60]从红树林卤蕨(Acrostichums aureum,喀麦隆杜阿拉)叶来源真菌Rhinocladiella similis分离得到10个新倍半萜衍生物rhinomilisins A-J(297-306)及6个已知类似物(307~312)。Qiu等[61]从角果木(Ceriops tagal,中国海南)叶内生真菌Penicillium sp. J-54分离得到4种新的常春藤型倍半萜penicieudesmol A~D(313~316)。Chen等[62]继续对Penicillium sp. J-54化学成分研究得到3个新的杜鹃花型倍半萜penicieudesmols E-G(317~319)。Deng等[63]从角果木内生真菌Cytospora sp.中得到1个新双环倍半萜seiricardine D(320)。前文所述的Pleosporales sp. SK7产生1个脱落酸型倍半萜(321)[47];Diaporthe sp. SCSIO 41011产生1个新倍半萜1-methoxypestabacillin B(322)和1个已知倍半萜323[64];Xylaria sp. HNWSW-2[53]产生1个已知倍半萜guaidiol(324)。
2.2 二倍半萜类化合物
Zhu等[65]从木榄来源真菌Aspergillus ustus 094102分离得到7个新蛇孢菌素(ophiobolins)型二倍半萜衍生物(325~331)及11个已知类似物(332~342)。Cai等[66]从秋茄叶内生真菌Aspergillus sp. ZJ-68分离得到11个新蛇孢菌素型二倍半萜衍生物asperophiobolins A~K(343~353)及上述报道的已知类似物(325、332~337、339~342),化合物343~346是第一个被发现在C-5和C-21之间具有五元内酰胺单元的ophiobolin衍生物。
2.3 混元萜类化合物
Li等[67]从尖瓣海莲来源内生菌Penicillium simplicissimum MA-332分离得到1个新骨架混元萜simpterpenoid A(354),其具有高度官能化的环己二烯部分,同时环己二烯同一个碳上有-1,2-二酮和甲酸甲酯基团的取代。Chen等[68]从红树林内生真菌Talaromyces amestolkiae YX1分离得到4个新混元萜amestolkolides A~D(355~358)及3个已知的混元萜(359~361)。Xu等[69]从红树林海莲胚内生真菌P. capitalensis分离得到4个已知混元萜(362~365)。前文所述的Penicillium sp. TGM112产两个新混元萜(366~367)及7个已知类似物(368~374)[16];Liu等[70]从秋茄内生真菌Aspergillus terreus H010中分离得到一个新混元萜1,2-dehydro-terredehydroaustin(375)及两个已知的类似物(376~377)。Diaporthe sp. SCSIO 41011产生6个已知的chrodrimanin型混元萜(378~383)[64]。
3 生物碱
3.1 胺与酰胺类化合物
Zhou等[71]从角果木内生菌Penicillium herquei JX4分離得到两对新颖的差向异构化合物penicilquei A-D(384~387)。前文所述的Penicillium chrysogenum V11产生一个新的酰胺类化合物N-fumaryl-L-alanine dimethyl ester(388)及一个新天然产物N,N-bis[(S)-1-methoxycarbonylethyl]fumaric diamide(389)[38]。
3.2 吲哚衍生物
Li等[72-73]从红树林植物桐棉(Thespesia populnea,中国广东)共生菌Aspergillus versicolor HDN 1184分离得到1个新吲哚生物碱taichunamide H(390)。3个吲哚二萜penicilindoles A~C(391~393)分离自木果楝内生真菌Eupenicillium sp. HJ002 。Chang等[74]从红树林植物秋茄来源共生菌Diaporthe arecae中分离到1个新indoleglycerol,命名为arecine(394)。前文所述的内生菌Botryosphaeria ramosa L29,在利用来自宿主成分的抑制性应激策略(UISCH)之后产生一个新天然色胺(3aS, 8aS)-1-acetyl-1, 2, 3, 3a, 8, 8a-hexahydro pyrrolo [2,3b] indol-3a-ol(395)[17];Colletotrichum tropicale SCSIO 41022产生1个新吲哚衍生物colletoindole A(396)及两个新天然产物(397~398)[54];Penicillium chrysogenum V11产生两个已知的吲哚生物碱chaetoglobosin C(399)和chaetoglobosin F (400) [38]。
3.3 吡咯衍生物
Chen等[75]从半红树林植物阔苞菊(中国广西)健康树枝内生菌Didymella sp. CYSK-4中分离得到3个新的十二和十三元环大环生物碱ascomylactams A~C(401~403)及2个已知类似物phomapyrrolidone C(404)与pyrrolidone A(405),化合物401和402的绝对构型被单晶X射线衍射实验所决定,初次描述了(6/5/6/5)四环碳骨架稠合十二或十三元环部分的单晶结构。Yang等[76]从尖瓣海莲内生真菌D. eschscholtzii HJ001分离得到1个新的细胞松弛素[11]-cytochalasa-5(6),13-diene-1,21-dione-7,18-dihydroxy-16,18- dimethyl-10-phenyl-(7S*,13E,16S*,18R*)(406)和1个已知的类似物(407)。前文所述的内生菌Cladosporium sp. HNWSW-1产生2个新的含琥珀酰亚胺的衍生物cladosporitins A~B(408~409)及1个已知吡咯衍生物talaroconvolutin A(410)[27];Mycosphaerella sp. SYSU-DZG01产生1个新的细胞松弛素衍生物asperchalasine I(411)及2个已知的类似物(412~413)[44];Xylaria sp. HNWSW-2产生1个新的细胞松弛素衍生物xylarisin B(414)[53]。
3.4 肽类
Cai等[77]从秋茄叶内生真菌Aspergillus sp. SK-28中分离得到1对具有6/5/4/5/6五环骨架的对映体哌嗪(含吲哚二酮)二聚体(±)-asperginulin A(415),对映二聚体通过手性HPLC实现了分离。Zhu等[78]从苦槛蓝根内生菌Fusarium sp. R5中分离得到2个新环状六肽fusarihexins A~B(416~417)及2个已知的环肽cyclo-[(L)Leu-(L)Leu-(D)Leu-(L)Leu-(L)Val)(418)和cyclo-[(L)Leu-(L)Leu-(D)Leu-(L)Leu-(L)Ile)(419)。前文所述的内生菌Penicillium sp. GD6產生1个新二酮哌嗪化合物5(S-hydroxynorvaline-S-Ile)(420)、2个新天然产物3(S-hydroxylcyclo, S-Pro-S-Phe)(421)、cyclo(S-Phe-S-Gln)(422)以及2个已知的生物碱(423~424)[37];Diaporthe arecae产生23个已知的二酮哌嗪(425~447)[74];Sarocladium kiliense HDN11-112产2个新的十肽(448~449)[52]。
3.5 其它含氮化合物
Chen等[79]从红树林内生真菌Phomopsis sp. 33#分离得到4个新的色酮吡啶衍生物phochrodines A~D(450~53)。前文所述的内生菌Fusarium solani HDN15-410产生4个新的吡啶衍生物fusaricates H-K(454~457)及1个已知的类似物fusaric acid(458)[57];Aspergillus sp. AV-2产生1个新的含氮化合物(459)[45];Cladosporium sp. JS1-2产生1个含氮的新天然产物2-methylacetate-3,5,6 -trimethylpyrazine(460)[32]。
4 分析与讨论
红树林的特殊生境决定了植物内生菌代谢产物的多样性和特殊性。本文系统总结了2018—2019年报道的红树林植物内生真菌次级代谢产物,收录的460个化合物经分类,主要的结构类型为酚类、内酯类、异香豆素,其次是醌类、肽类,其他聚酮类、倍半萜类、二倍半萜类、混元萜类,出新率相对较高的代谢产物结构类型为萜类中的倍半萜类、二倍半萜类,其他聚酮以及其他含氮生物碱。
统计2018—2019年报道的460个代谢产物在各属真菌中的分布发现,已鉴定的菌属有27个,化合物在各属之间分布差异较为明显,曲霉属Aspergillus、间座壳属Diaporthe、青霉属Penicillium和枝孢属Cladosporium代谢产物较丰富,次级代谢产物数量均超过60个;来源于Annulohypoxylon属、Eupenicillium属、Clonostachys属等的次级代谢产物报道较少,但出新率较高。另有大约3%代谢产物由尚不确定菌属的菌株产生,提示还有很多尚未被研究的菌种有待开发,挖掘新的菌种可能是微生物药物研究领域中一条较有效的途径,同时仅有少量报道的产物来自内生真菌的表观遗传调控、共培养[30,55],这提示激活在实验室普通培养条件下未表达的“沉默基因”,将进一步丰富真菌次级代谢产物的种类和数量。
仅就本论文统计结果而言,产生次级代谢产物及新化合物最多的真菌宿主主要有秋茄树属、红树属、木榄属、角果木属、海漆属、老鼠簕属、白骨壤属、木果楝属和卤蕨属等真红树植物,其中研究最多的4种宿主为秋茄、红海榄、海莲以及角果木。但关于半红树植物和伴生植物如阔苞菊属、苦槛蓝属等鲜有报道,这一统计结果为今后红树林内生真菌多样性及代谢化学成分研究的宿主选择,提供了参考,具有一定的指导意义。
5 总结
红树林植物内生真菌是一个化学结构多样性的宝库,2018—2019年报道的红树林植物内生真菌次级代谢产物,共有460个,其中新化合物217个,出新率达47.2%,其中通过模拟红树林特殊生态环境、或真菌-细菌共培养或真菌-真菌共培养、添加表观遗传修饰剂、genome mining技术,OSMAC策略来激活沉默次级代谢产物生物合成基因,进一步挖掘次级代谢产物潜力的研究不多,因此可以加大这些方面的研究力度;红树林内生真菌的分离鉴定,仍集中在部分真红树的植物科属宿主,以及在实验“标准”条件下易于生长和培养的少数可培养真菌类群中,无论宿主植物还是活性菌株都尚未得到充分研究,对新颖性高的菌种开展研究能提高获得新化合物的几率。分离筛选技术的进步与多学科交叉融合的兴起,红树林内生真菌将为新型海洋药物先导化合物的发现提供一个庞大并有待开发的微生物资源宝库。
參 考 文 献
Sridhar K R. Mangrove fungi in India[J]. Curr Sci, 2004, 86(12): 1586-1587.
Jones G, Stanley S J, Pinruan U. Marine endophyte sources of new chemical natural products: A review[J]. Bot Mar, 2008, 51(3): 163-170.
Debbab A, Aly A H, Proksch P. Mangrove derived fungal endophytes- a chemical and biological perception[J]. Fungal Divers, 2013, 61(1): 1-27.
Xu J. Biomolecules produced by mangrove-associated microbes[J]. Curr Med Chem, 2011, 18(34): 5224-5266.
Xu J. Bioactive natural products derived from mangrove-associated microbes[J]. Rsc Adv, 2015, 5(2): 841-892.
Wang X, Mao Z G, Song B B, et al. Advances in the study of the structures and bioactivities of metabolites isolated from mangrove-derived fungi in the South China Sea[J]. Mar Drugs, 2013, 16(10): 3601-3616.
Debbab A, Aly A H, Proksch P. Bioactive secondary metabolites from endophytes and associated marine derived fungi[J]. Fungal Divers, 2011, 49(1): 1-12.
Blunt J W, Copp B R, Keyzers R A, et al. Marine natural products[J]. Nat Prod Rep, 2017, 34(3): 235-294.
Blunt J W, Carroll A R, Copp B R, et al. Marine natural products[J]. Nat Prod Rep, 2018, 35(1): 8-53.
Carroll A R, Copp B R, Davis R A, et al. Marine natural products[J]. Nat Prod Rep, 2019, 36(1): 122-173.
Carroll A R, Copp B R, Davis R A, et al. Marine natural products[J]. Nat Prod Rep, 2020, 37(2): 175-223.
Yan Z, Huang C, Guo H, et al. Isobenzofuranone monomer and dimer derivatives from the mangrove endophytic fungus Epicoccum nigrum SCNU-F0002 possess α-glucosidase inhibitory and antioxidant activity[J]. Bioorg Chem, 2019, 94: 103407.
Cai R, Wu Y, Chen S, et al. Peniisocoumarins A-J: Isocoumarins from Penicillium commune QQF-3, an endophytic fungus of the mangrove plant Kandelia candel[J]. J Nat Prod, 2018, 81(6): 1376-1383.
Chen Y, Liu Z, Liu H, et al. Dichloroisocoumarins with potential anti-inflammatory activity from the mangrove endophytic fungus Ascomycota sp. CYSK-4[J]. Mar Drugs, 2018, 16(2): 54.
Wu Y, Chen S, Liu H, et al. Cytotoxic isocoumarin derivatives from the mangrove endophytic fungus Aspergillus sp. HN15-5D[J]. Arch Pharm Res, 2019, 42(4): 326-331.
Bai M, Zheng C J, Huang G L, et al. Bioactive meroterpenoids and isocoumarins from the mangrove-derived fungus Penicillium sp. TGM112[J]. J Nat Prod, 2019, 82(5): 1155-1164.
Wu Z, Chen J, Zhang X, et al. Four new isocoumarins and a new natural tryptamine with antifungal activities from a mangrove endophytic fungus Botryosphaeria ramosa L29[J]. Mar Drugs, 2019, 17(2): 88.
Liao H X, Zheng C J, Huang G L, et al. Bioactive polyketide derivatives from the mangrove-derived fungus Daldinia eschscholtzii HJ004[J]. J Nat Prod, 2019, 82(8): 2211-2219.
Xu Z, Wu X, Li G, et al. Pestalotiopisorin B, a new isocoumarin derivative from the mangrove endophytic fungus Pestalotiopsis sp. HHL101[J]. Nat Prod Res, 2020, 34(7): 1002-1007.
Cao J, Li X M, Li X, et al. New lactone and isocoumarin derivatives from the marine mangrove-derived endophytic fungus Penicillium coffeae MA-314[J]. Phytochem Lett, 2019, 32: 1-5.
Luo X, Yang J, Chen F, et al. Structurally diverse polyketides from the mangrove-derived fungus Diaporthe sp. SCSIO 41011 with their anti-influenza A virus activities[J]. Front Chem, 2018, 6: 282.
Wang L, Han X, Zhu G, et al. Polyketides from the endophytic fungus Cladosporium sp. isolated from the mangrove plant Excoecaria agallocha[J]. Front Chem, 2018, 6: 344.
Zheng C J, Huang G L, Liao H X, et al. Bioactive cytosporone derivatives isolated from the mangrove-derived fungus Dothiorella sp. ML002[J]. Bioorg Chem, 2019, 85: 382-385.
Chen Y, Yang W, Zou G, et al. Bioactive polyketides from the mangrove endophytic fungi Phoma sp. SYSU-SK-7[J]. Fitoterapia, 2019, 139: 104369.
Luo Y P, Zheng C J, Chen G Y, et al. Three new polyketides from a mangrove-derived fungus Colletotrichum gloeosporioides[J]. J Antibiot, 2019, 72(7): 513-517.
Liao H X, Shao T M, Mei R Q, et al. Bioactive secondary metabolites from the culture of the mangrove-derived fungus Daldinia eschscholtzii HJ004[J]. Mar Drugs, 2019, 17(12): 710.
Wang P, Cui Y, Cai C, et al. Two new succinimide derivatives cladosporitins A and B from the mangrove-derived fungus Cladosporium sp. HNWSW-1[J]. Mar Drugs, 2019, 17(1): 4.
Wu J T, Zheng C J, Zhang B, et al. Two new secondary metabolites from a mangrove-derived fungus Cladosporium sp. JJM22[J]. Nat Prod Res, 2019, 33(1): 34-40.
Cui H, Zhang H, Liu Y, et al. Ethylnaphthoquinone derivatives as inhibitors of indoleamine-2, 3-dioxygenase from the mangrove endophytic fungus Neofusicoccum austral SYSU-SKS024[J]. Fitoterapia, 2018, 125: 281-285.
Yang S Q, Li X M, X G M, et al. Antibacterial anthraquinone derivatives isolated from a mangrove-derived endophytic fungus Aspergillus nidulans by ethanol stress strategy[J]. J Antibiot, 2018, 71(9): 778-784.
Liu Z, Qiu P, Li J, et al. Anti-inflammatory polyketides from the mangrove-derived fungus Ascomycota sp. SK2YWS-L[J]. Tetrahedron, 2018, 74(7): 746-751.
Bai M, Zheng C J, Tang D Q, et al. Two new secondary metabolites from a mangrove-derived fungus Cladosporium sp. JS1-2[J]. J Antibiot, 2019, 72(10): 779-782.
Wang Y, Chen J H, Guo D X, et al. Two novel chromone derivatives: crystal structures and anti-lymphoma activity[J]. Main Group Chem, 2019, 18(2): 101-106.
Supratman U, Hirai N, Sato S, et al. New naphthoquinone derivatives from Fusarium napiforme of a mangrove plant[J]. Nat Prod Res, 2019, 35(9): 1406-1412.
Hu H B, Luo Y F, Wang P, et al. Xanthone-derived polyketides from the Thai mangrove endophytic fungus Phomopsis sp. xy21[J]. Fitoterapia, 2018, 131: 265-271.
Zheng C J, Liao H X, Mei R Q, et al. Two new benzophenones and one new natural amide alkaloid isolated from a mangrove-derived fungus Penicillium citrinum[J]. Nat Prod Res, 2019, 33(8): 1127-1134.
Jiang C S, Zhou Z F, Yang X H, et al. Antibacterial sorbicillin and diketopiperazines from the endogenous fungus Penicillium sp. GD6 associated chinese mangrove Bruguiera gymnorrhiza[J]. Chin J of Nat Medicines, 2018, 16(5): 358-365.
Zhu X, Wu Z, Liang F, et al. A new L-alanine derivative from the mangrove fungus Penicillium chrysogenum V11[J]. Chem Nat Compd, 2018, 54(3): 520-522.
Cui H, Liu Y, Li T, et al. 3-Arylisoindolinone and sesquiterpene derivatives from the mangrove endophytic fungi Aspergillus. versicolor SYSU-SKS025[J]. Fitoterapia, 2018, 124: 177-181.
Wang P, Yu J H, Zhu K, et al. Phenolic bisabolane sesquiterpenoids from a Thai mangrove endophytic fungus, Aspergillus sp. xy02[J]. Fitoterapia, 2018, 127: 322-327.
Wu Y, Chen Y, Huang X, et al. α-Glucosidase Inhibitors: Diphenyl ethers and phenolic bisabolane sesquiterpenoids from the mangrove endophytic fungus Aspergillus flavus QQSG-3[J]. Mar Drugs, 2018, 16(9): 307.
Cui H, Liu Y, Li J, et al. Diaporindenes A-D: Four unusual 2, 3-dihydro1Hindene analogues with anti-inflammatory activities from the mangrove endophytic fungus Diaporthe sp. SYSU-HQ3[J]. J Org Chem, 2018, 83(19): 11804-11813.
Cai R, Jiang H, Zang Z, et al. New benzofuranoids and phenylpropanoids from the mangrove endophytic fungus Aspergillus sp. ZJ-68 [J]. Mar Drugs, 2019, 17(8): 478.
Qiu P, Liu Z, Chen Y, et al. Secondary metabolites with α-glucosidase inhibitory activity from the mangrove fungus Mycosphaerella sp. SYSU-DZG01[J]. Mar Drugs, 2019, 17(8): 483.
Elissawy A M, Ebada S S, Ashour M L, et al. New secondary metabolites from the mangrove-derived fungus Aspergillus sp. AV-2[J]. Phytochem Lett, 2019, 29: 1-5.
Yan Z, Wen S, Ding M, et al. The purification, characterization, and biological activity of new polyketides from mangrove-derived endophytic fungus Epicoccum nigrum SCNU-F0002[J]. Mar Drugs, 2019, 17(7): 414.
Wen S, Fan W, Guo H, et al. Two new secondary metabolites from the mangrove endophytic fungus Pleosporales sp. SK7[J]. Nat Prod Res, 2020, 83(19): 11804-11813.
Zhang F Z, Li X M, Yang S Q, et al. Thiocladospolides A-D, 12-membered macrolides from the mangrove-derived endophytic fungus Cladosporium cladosporioides MA-299 and structure revision of pandangolide 3[J]. J Nat Prod, 2019, 82(6): 1535-1541.
Supratman U, Suzuki T, Nakamura T, et al. New metabolites produced by endophyte Clonostachys rosea B52[J]. Nat Prod Res, 2021, 35(9): 1525-1531.
Bai M, Huang G L, Mei R Q, et al. Bioactive lactones from the mangrove-derived fungus Penicillium sp. TGM112[J]. Mar Drugs, 2019, 17(8): 433.
Fan Y, Zhu G, Wang Y, et al. α-Pyronoids with quorum sensing inhibitory activity from the mangrove fungus Penicillium camemberti OUCMDZ-1492[J]. Chinese J Org Chem, 2018, 38(10): 2798-2813.
Guo W, Wang S, Li N, et al. Saroclides A and B, cyclic depsipeptides from the mangrove-derived fungus Sarocladium kiliense HDN11-112[J]. J Nat Prod, 2018, 81(4): 1050-1054.
Wang P, Cui Y, Cai C H, et al. A new cytochalasin derivative from the mangrove-derived endophytic fungus Xylaria sp. HNWSW-2[J]. J Asian Nat Prod Res, 2018, 20 (10): 1002-1007.
Lin X, Ai W, Li M, et al. Colletoindole A from the mangrove plant endophytic fungus Colletotrichum tropicale SCSIO 41022[J]. Chem Biodivers, 2019, 17(2): e1900040.
Zhang L, Niaz S, Wang Z, et al. α-Glucosidase inhibitory and cytotoxic botryorhodines from mangrove endophytic fungus Trichoderma sp. 307[J]. Nat Prod Res, 2018, 32(24): 2887-2892.
Liu Y, Kurtan T, Mandi A, et al. A novel 10-membered macrocyclic lactone from the mangrove-derived endophytic fungus Annulohypoxylon sp[J]. Tetrahedron Lett, 2018, 59(7): 632-636.
Zhou G, Qiao L, Zhang X, et al. Fusaricates H-K and fusolanones A-B from a mangrove endophytic fungus Fusarium solani HDN15-410[J]. Phytochemistry, 2019, 158: 13-19.
Peng X, Wang Y, Zhu G, et al. Fatty acid derivatives from the halotolerant fungus Cladosporium cladosporioides[J]. Magn Reson in Chem, 2018, 56(1): 18-24.
Luo X, Lin X, Tao H, et al. Isochromophilones A-F, Cytotoxic chloroazaphilones from the marine mangrove endophytic fungus Diaporthe sp. SCSIO 41011[J]. J Nat Prod, 2018, 81(4): 934-941.
Liu S, Zhao Y, Heering C, et al. Sesquiterpenoids from the endophytic fungus Rhinocladiella similis[J]. J Nat Prod, 2019, 82(5): 1055-1062.
Qiu L, Wang P, Liao G, et al. New eudesmane-type sesquiterpenoids from the mangrove-derived endophytic fungus Penicillium sp. J-54[J]. Mar Drugs, 2018, 16(4): 108.
Chen H, Qiu L, Wang P, et al. Three new eudesmane-type sesquiterpenoids from the mangrove-derived endophytic fungus Penicillium sp. J-54[J]. Phytochem Lett, 2019, 33: 36-38.
Deng Q, Li G, Sun M, et al. A new antimicrobial sesquiterpene isolated from endophytic fungus Cytospora sp. from the Chinese mangrove plant Ceriops tagal[J]. Nat Prod Res, 2020, 34(10): 1404-1408.
Luo X W, Chen C M, Li K L, et al. Sesquiterpenoids and meroterpenoids from a mangrove derived fungus Diaporthe sp. SCSIO 41011 [J]. Nat Prod Res, 2021, 35(2): 282-288.
Zhu T, Lu Z, Fan J, et al. Ophiobolins from the mangrove fungus Aspergillus ustus[J]. J Nat Prod, 2018, 81(1): 2-9.
Cai R, Jiang H, Mo Y, et al. Ophiobolin-type sesterterpenoids from the mangrove endophytic fungus Aspergillus sp. ZJ-68[J]. J Nat Prod, 2019, 82(8): 2268-2278.
Li H L, Xu R, Li X M, et al. Simpterpenoid A, a meroterpenoid with a highly functionalized cyclohexadiene moiety featuring gem-propane-1, 2-dione and methylformate groups, from the mangrove-derived Penicillium simplicissimum MA-332[J]. Org Lett, 2018, 20(5): 1465-1468.
Chen S, Ding M, Liu W, et al. Anti-inflammatory meroterpenoids from the mangrove endophytic fungus Talaromyces amestolkiae YX1[J]. Phytochemistry, 2018, 146: 8-15.
Xu Z, Xiong B, Xu J, et al. Chemical investigation of secondary metabolites produced by mangrove endophytic fungus Phyllosticta Capitalensis[J]. Nat Prod Res, 2021, 35(9): 1561-1565.
Liu Z, Liu H, Chen Y, et al. A new anti-inflammatory meroterpenoid from the fungus Aspergillus terreus H010[J]. Nat Prod Res, 2018, 32(22): 2652-2656.
Zhou X M, Zheng C J, Song X M, et al. Bioactive acetaminophen derivatives from Penicillum herquei JX4[J]. Fitoterapia, 2019, 139: 104400.
Li F, Zhang Z, Zhang G, et al. Determination of taichunamide H and structural revision of taichunamide A[J]. Org Lett, 2018, 20(4): 1138-1141.
Zheng C J, Bai M, Zhou X M, et al. Penicilindoles A-C, cytotoxic indole diterpenes from the mangrove-derived fungus Eupenicillium sp. HJ002[J]. J Nat Prod, 2018, 81(4): 1045-1049.
Chang F R, Wang S W, Li C Y, et al. Natural products from Diaporthe arecae with anti‐angiogenic activity[J]. Israel J Chem, 2019, 59(5): 439-445.
Chen Y, Liu Z, Huang Y, et al. Ascomylactams A-C, Cytotoxic 12- or 13-membered-ring macrocyclic alkaloids isolated from the mangrove endophytic fungus Didymella sp. CYSK-4, and structure revisions of Phomapyrrolidones A and C [J]. J Nat Prod, 2019, 82(7): 1752-1758.
Yang L J, Liao H X, Bai M, et al. One new cytochalasin metabolite isolated from a mangrove-derived fungus Daldinia eschscholtzii HJ001[J]. Nat Prod Res, 2018, 32(2): 208-213.
Cai R, Jiang H, Xiao Z, et al. (?)- and (+)-Asperginulin A, a pair of indole diketopiperazine alkaloid dimers with a 6/5/4/5/6 pentacyclic skeleton from the mangrove endophytic fungus Aspergillus sp. SK-28[J]. Org Lett, 2019, 21(23): 9633-9636.
Zhu X, Zhong Y, Xie Z, et al. Fusarihexins A and B: Novel cyclic hexadepsipeptides from the mangrove endophytic fungus Fusarium sp. R5 with antifungal activities[J]. Planta Med, 2018, 84(18): 1355-1362.
Chen H, Huang M, Li X, et al. Phochrodines A-D, first naturally occurring new chromenopyridines from mangrove entophytic fungus Phomopsis sp. 33#[J]. Fitoterapia, 2018, 124: 103-107.
