荧光素酶生物发光法在快速药敏试验中的研究与应用
2023-03-13向杰崔晓莉李璐一周同喜方娟郑业焕
向杰?崔晓莉?李璐一?周同喜?方娟?郑业焕
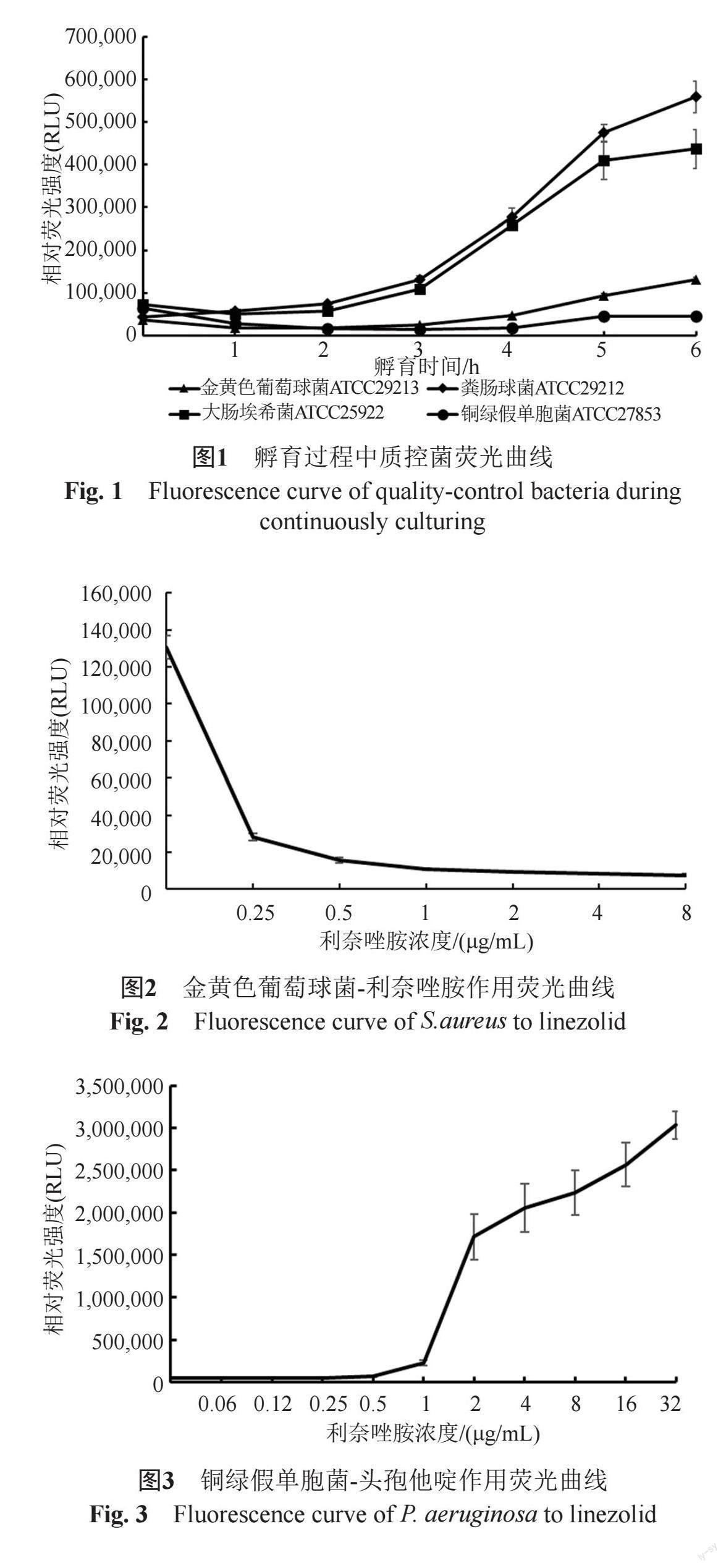
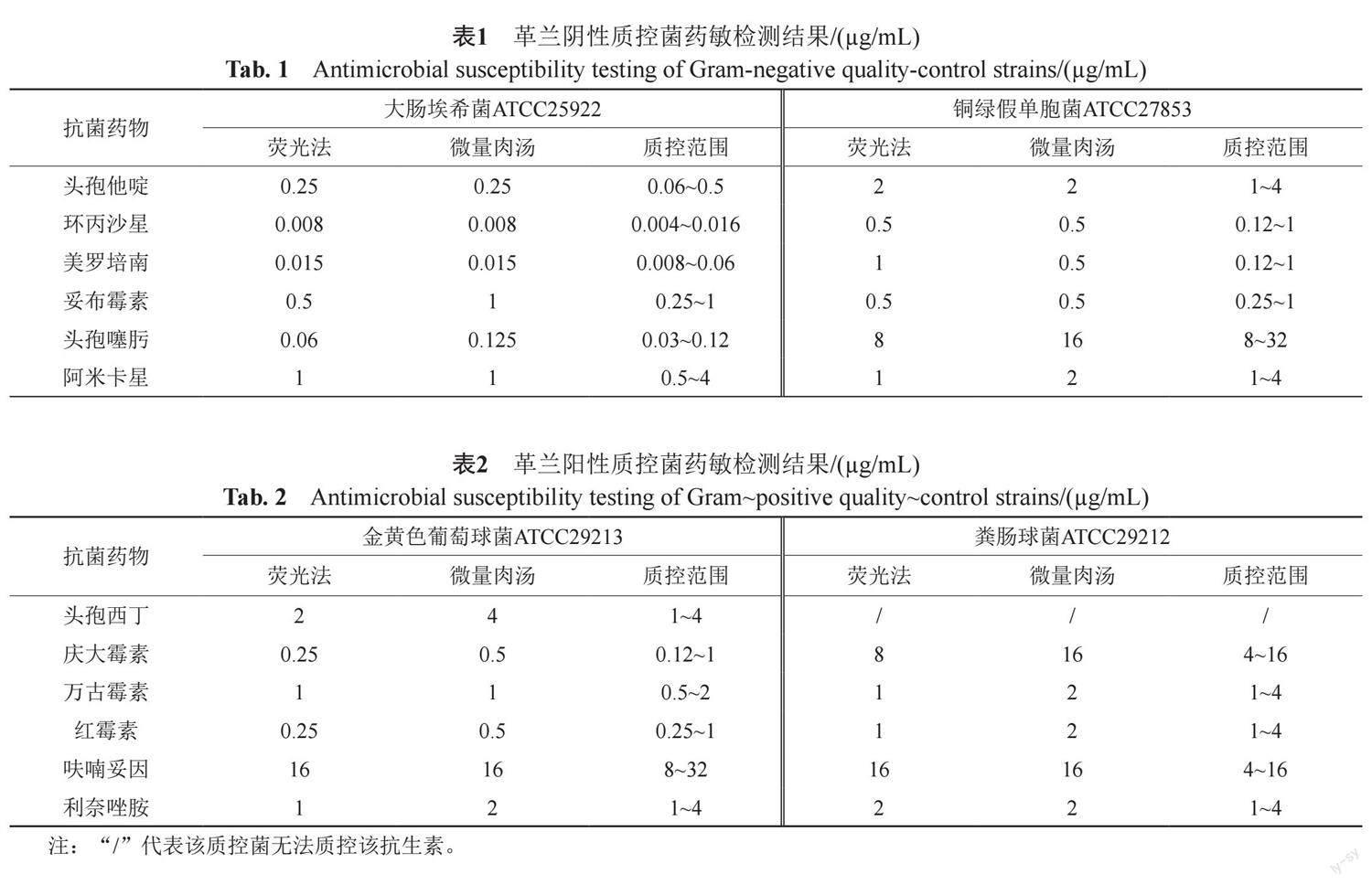
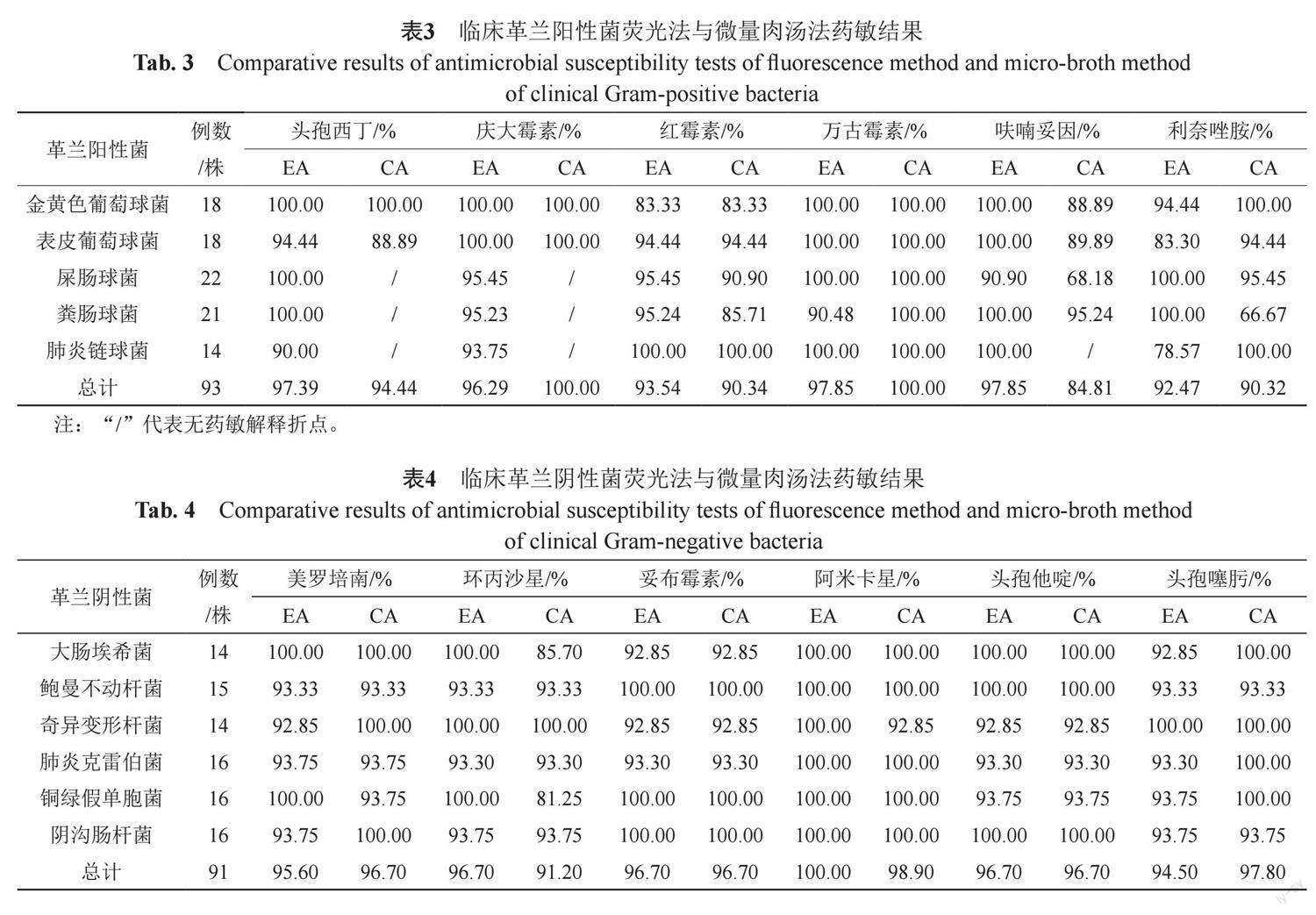
摘要:目的 通过荧光素酶生物发光法与微量肉汤稀释法对质控菌株和临床收集菌株的最低抑菌浓度(minimum inhibitory concentration,MIC)进行测定,探讨荧光素酶生物发光法在病原微生物快速药物敏感性试验中的应用价值。方法 以D-荧光素钠、Mg2+、O2和待测菌株为荧光素酶催化反应底物,连续检测待测菌与抗生素孵育过程中相对光强度(relative light unit, RLU)变化,计算发光率并确定MIC,分析与微量肉汤稀释法的基本一致性(essential agreement,EA)和分类一致性(categorical agreement,CA)。结果 荧光素酶生物发光法快速检测大肠埃希菌、金黄色葡萄球菌等质控菌株MIC与微量肉汤稀释法检测MIC结果CA和EA值均为100%;并应用该检测体系对91株临床革兰阴性菌样本、93株革兰阳性菌样本进行MIC测定,革兰阴性菌检测结果中的6种药物EA和CA分别大于90%和84%,革兰阳性菌检测结果中的6种药物EA和CA分别大于90%和80%,并使检测周期由常规藥敏实验的16~24 h缩短至5~6 h。结论 荧光素酶生物发光法快速检测药敏结果与微量肉汤稀释法结果具有较高一致性,且更快速、敏感,可为今后的临床检验工作提供全新的快速药敏检测方法。
关键词:荧光素酶;发光率;一致性;快速药敏
中图分类号:R9文献标志码:A
Research and application of luciferase bioluminescence in rapid antimicrobial susceptibility testing systems
Xiang Jie, Cui Xiaoli, Li Luyi, Zhou Tongxi, Fang Juan, and Zheng Yehuan
(Autobio Diagnostics Co., Ltd, Zhengzhou 450016)
Abstract Objective The minimum inhibitory concentration (MIC) of quality-control bacteria and clinical strains was determined by the luciferase bioluminescence method and the broth microdilution method to explore the application value of the luciferase bioluminescence method in rapid antimicrobial susceptibility tests of pathogenic microorganisms. Methods Using D-luciferin sodium, Mg2+, O2 and the tested strain as substrates of the luciferase catalytic reaction, continuously detect the change of relative light unit (RLU) during the incubation of the tested bacterium with antibiotics, calculate the luminosity factor and determine the MIC, compare the results that were obtained using the broth micro-dilution (BMD) method, and then evaluate the essential agreement and categorical agreement. Results The CA and EA values of rapid MIC detection of quality control strains, such as E. coli and S. aureus, by the luciferase bioluminescence method and the MIC detection by the broth microdilution method were 100%. In addition, the detection system was applied to perform MIC detection on 91 clinical Gram-negative bacterial samples and 93 Gram-positive bacterial samples. Among the Gram-negative bacterial detection results, the EA and CA values of the 6 drugs were greater than 90% and 84%, respectively. The EA and CA values of the 6 drugs in the Gram-positive bacterial detection results were greater than 90% and 80%, respectively, and the detection duration was shortened from 16~24 h in conventional drug sensitivity experiments to 5~6 h. Conclusion The results of luciferase bioluminescence rapid antimicrobial susceptibility testing (RAST) were highly consistent with those obtained by the broth microdilution method, which was more rapid and sensitive than traditional methods. It could provide a new rapid drug sensitivity detection method for clinical testing in the future.
Khameneh B, Diab R, Ghazvini K, et al. Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them[J]. Microb Pathog, 2016, 95: 32-42.
Trotter A J, Aydin A, Strinden M J, et al. Recent and emerging technologies for the rapid diagnosis of infection and antimicrobial resistance[J]. Curr Opin Microbiol, 2019, 51: 39-45.
Safavieh M, Pandya H J, Venkataraman M, et al. Rapid real-time antimicrobial susceptibility testing with electrical sensing on plastic microchips with printed electrodes[J]. ACS Appl Mater. Interf, 2017, 9(14): 12832-12840.
Espinel-Ingroff A. Comparison of three commercial assays and a modified disk diffusion assay with two broth microdilution reference assays for testing zygomycetes, Aspergillus spp., Candida spp., and Cryptococcus neoformans with posaconazole and amphotericin B[J]. J Clin Microbiol, 2006, 44(10): 3616-3622.
Nolte F S, Metchock B, Williams T, et al. Detection of penicillin-resistant Streptococcus pneumoniae with commercially available broth microdilution panels[J]. J Clin Microbiol, 1995, 33(7): 1804-1806.
Waites K B, Duffy L B, Bebea C M, et al. Standardized methods and quality control limits for agar and broth microdilution susceptibility testing of Mycoplasma pneumoniae, Mycoplasma hominis, and Ureaplasma urealyticum[J]. J Clin Microbiol, 2012, 50(11): 3542-3547.
胡敬志. 螢火虫荧光素酶的性质和应用的研究[D]. 上海: 华东师范大学, 2007.
de Wet J R, Wood K V, Helinski D R, et al. Cloning of firefly luciferase cDNA and the expression of active luciferase in Escherichia coli[J]. Proc Natl Acad Sci, 1985, 82(23): 7860-7869.
常超, 王琨, 王凌, 等. 基于萤火虫荧光素酶ATP生物发光法灵敏度的研究进展[J]. 安徽农业科学, 2013, 41(24): 9879-9881.
Blair J, Webber M A, Baylay A J, et al. Molecular mechanisms of antibiotic resistance[J]. Nat Rev Microbiol, 2015, 13(1): 42-51.
Clinical and Laboratory Standards Institute(CLSI). Performance standards for antimicrobial susceptibility testing. 31st ed. CLSI supplement M100[S]. Clinical and Laboratory Standards Institute, 2021.
Clinical and Laboratory Standards Institute(CLSI). verification of commercial microbial identification and antimicrobial susceptibility testing systems. 1st ed. CLSI guideline M52[S]. Clinical and Laboratory Standards Institute, 2015.
周宁, 张建新, 樊明涛, 等. 细菌药物敏感性实验方法研究进展[J]. 食品工业科技, 2012, 33(9): 459-464.
刘艳杰. 重组萤火虫荧光素酶及其稳定性研究[D]. 天津: 天津大学, 2010.
侯玉柱, 田雨, 柯润辉, 等. ATP生物发光法快速测定物体表面的菌落总数[J]. 食品与发酵工业, 2015, 41(2): 217-220.
刘君, 裴豪, 徐志勤, 等. 三磷酸腺苷发光法与其他方法在异烟肼耐药结核分枝杆菌检测中的比较[J]. 中华医院感染学杂志, 2014, 24(5): 1296-1300.
Belkum A V, Burnham C, Rossen J, et al. Innovative and rapid antimicrobial susceptibility testing systems[J]. Nat Rev Microbiol, 2020, 18(5): 299-311.
Merli D, Pretali L, Fasani E, et al. Analytical determination and electrochemical characterization of the oxazolidinone antibiotic linezolid[J]. Electroanalysis, 2011, 23(10): 2364-2372.
