冷喷涂辅助原位合成CuNiCoFeCrAl2.3高熵合金涂层组织与性能研究
2022-11-08冯力马凯杨伟杰王宁袁昱东李文生
冯力,马凯,杨伟杰,王宁,袁昱东,李文生
热喷涂与冷喷涂技术
冷喷涂辅助原位合成CuNiCoFeCrAl2.3高熵合金涂层组织与性能研究
冯力1,2,马凯1,杨伟杰1,王宁1,袁昱东1,李文生1,2
(1.兰州理工大学 材料科学与工程学院,兰州 730050;2.有色金属先进加工与再利用国家重点实验室,兰州 730050)
为了提升普通金属材料的表面耐腐蚀和耐磨性能,提出了一种在普通金属材料表面制备性能良好的CuNiCoFeCrAl2.3高熵合金涂层的技术工艺。利用冷喷涂技术在45#钢基体上制备混合金属涂层,再经过感应重熔技术将混合金属涂层原位合成为CuNiCoFeCrAl2.3高熵合金涂层。通过采用扫描电子显微镜(SEM)、X射线衍射仪(XRD)、能谱仪(EDS)、显微硬度计、磨料磨损试验机等,对涂层的相组成、显微组织、硬度、耐磨性进行分析。原位合成CuNiCoFeCrAl2.3高熵合金涂层组织致密,元素均匀分布,合金涂层由简单的BCC相构成,涂层的微观组织呈现出典型的枝晶结构。内枝晶区主要富含Co、Cr、Fe和Ni,枝晶间区则富含Cu和Al。CuNiCoFeCrAl2.3高熵合金涂层的显微硬度是45#钢基体的3倍,在干摩擦条件下,CuNiCoFeCrAl2.3高熵合金涂层在摩擦过程中以磨粒磨损为主,涂层在干滑动条件下的磨损率比45#钢基体的磨损率低59%,摩擦因数为0.38,约为45#钢基体的56%,CuNiCoFeCrAl2.3高熵合金涂层的磨损率为2.95×10‒5mm3/(N·m)。使用冷喷涂辅助原位合成CuNiCoFeCrAl2.3高熵合金涂层具有很高的硬度和良好的耐磨性能。
高熵合金;冷喷涂;感应重熔;微观组织;耐磨性
2004年叶均蔚等[1]、Cantor等[2]提出一种新的合金设计的理论,称为“多主元高熵合金”。高熵合金一般由5种或5种以上的元素按照近似等原子比或等原子比合金化后,而主要元素的原子数分数为5%~35%,次要元素的原子数分数小于5%,所得合金的混合熵高于熔化熵,形成简单固溶体一类的合金[3]。由于高熵合金独特的相结构,使得高熵合金具有高强度、良好的耐磨性、高稳定性[4-6]等优异的性能,是具有广泛应用前景的涂层材料。众多学者采用不同的方法将高熵合金制备成为涂层,并推广应用于不同的工业领域。
目前,高熵合金涂层的制备工艺包括冷喷涂激光熔覆[7]、电化学沉积[8]、磁控溅射[9]、冷喷涂[10]等。Ye等[11]通过激光熔覆法制备了AlFeCoNiCuCr高熵合金涂层,获得了纳米结构的固溶体,研究结果表明,随着Al元素的增加,涂层由FCC+BCC向BCC相转变,涂层的显微硬度也会提高。Soare等[12]通过电化学沉积法制备了AlCrFeMnNi和AlCrCuFeMnNi高熵合金涂层。涂层由球形、片状颗粒和直径约500 nm的颗粒簇组成。在873 K高温下热处理2 h可得到BCC固溶结构,对基材有很高的附着力。但是,用这种方法制备的涂层有很多裂纹而且涂层太薄。Huang等[13]使用热喷涂技术制备了AlCrFeMo0.5NiSiTi高熵合金涂层和AlCoCrFeMo0.5NiSiTi高熵合金涂层,涂层主要由BCC和FCC相构成,涂层的厚度约为200 μm,合金的硬度为700~900HV。Ang等[14]通过机械合金化方法研磨制备高熵合金纳米粉末,再通过等离子喷涂方法将高熵合金粉末制备成AlCoCrFeNi和MnCoCrFeNi高熵合金涂层,涂层由FCC和BCC相组成,硬度可达4.42 Gpa。冷喷涂[15-16]属于热喷涂技术的一种,之所以叫冷喷涂,是因为其喷涂时的气流温度远低于传统热喷涂技术。冷喷涂与传统热喷涂技术相比,工作温度低,不需要高温氧化,涂层基体能保持材料颗粒的结构和成分,减少材料的氧化或相变。Yin等[15]采用冷喷涂技术在45#钢基体上用预制的高熵合金粉末成功制备了FeCoNiCrMn高熵合金涂层,由于冷喷涂的特点,涂层没有发生相变,涂层保留了高熵合金粉末的相结构和高熵合金优良的力学性能。Anupam等[16]利用冷喷涂技术制备AlCoCrFeNi高熵合金涂层并研究高温氧化机理,结果表明:在1 100 ℃的环境下,该涂层的表面形成一层氧化物可以保护基体25 h,而通过优化工艺参数可以最大限度提高涂层质量并提高涂层抗氧化性。冷喷涂技术制备的涂层,粉末颗粒之间存在太多的界面,降低了涂层中的附着力,因此有学者采用涂层重熔的方法,来提高冷喷涂层的性能[17]。
本文采用冷喷涂辅助原位合成的方法[18-19]制备厚度为400 μm的CuNiCoFeCrAl2.3高熵合金涂层。这种方法能有效消除冷喷涂涂层中粉末颗粒之间的界面,提高涂层的附着力与内聚力,涂层的附着力可以达到120 Mpa。使用这种方法,不需要预制高熵合金粉末,以混合金属单质粉末为喷涂原料在基体上制备预制混合金属涂层,再利用感应重熔技术原位合成高熵合金涂层。因此,这种方法制备涂层具有制备成本低、周期短、易于调整涂层成分的优点。
1 试验
1.1 涂层的制备
本试验所用原料是根据制备涂层材料要求的商用金属单质粉末(纯度>99.5%),将金属单质粉末机械混合4 h后作为冷喷涂预制原料,冷喷涂原料的微观形貌如图1所示。图1中Cu、Ni、Fe粉末为树枝状的电解粉,Al、Co粉末为球状的雾化粉,Cr粉末为不规则形貌的破碎粉。不同的金属粉末,在相同的冷喷涂工艺下有不同的沉积率,冷喷涂原料的各种金属粉末含量,按照各种金属粉末的沉积率和涂层中的元素成分比率来计算。通过试验,得到不同金属元素上粉率的比值,按照原子比表征为Cu∶Ni∶Co∶Cr∶Fe∶Al2.3=1.7∶1.6∶1.6∶1.4∶1.3∶1.1。最终制备的涂层的金属元素的质量分数见表1。基体材料选用45#钢,喷涂前用丙酮超声清洗基体表面的油污等杂质,然后喷砂粗化处理基体表面。

图1 冷喷涂预制粉末微观形貌
表1 冷喷涂前设计的金属粉末各元素质量分数

Tab.1 the mass percentage of each element of metal powder designed before cold spraying wt.%
1.2 涂层的微观组织及性能检测
采用白俄罗斯国立技术大学设计制造的低压冷喷涂设备(GDU-3-15)在45#钢基体上预制混合金属涂层,冷喷涂设备工艺参数见表2。对冷喷涂制备的混合金属涂层进行感应重熔处理,原位合成高熵合金涂层。感应重熔加热功率选用1.5~2.2 kW,加热时间为10~15 s。
采用D/MAX2500PC型X射线衍射仪(XRD)对冷喷涂混合金属涂层与原位合成高熵合金涂层表面进行相结构分析,扫描速度为4 (°)/min,扫描步长为0.02°,加速电压为40 kV,电流为40 mA,衍射角为20°~90°。采用QuantaFEG450场发射扫描电子显微镜(SEM)、能谱仪(EDS)对高熵合金涂层表面微观形貌结构和微区成分进行分析。使用HV1000型显微硬度计测量试样的硬度,选取维氏硬度,在试样表面选取5个点测量,然后求其平均值。采用美国BRUKER公司生产的UMT-Tribolab型摩擦设备,以直径为6 mm的氧化铝小球作为对磨件,在室温干滑动条件下测试合金涂层的摩擦学性能,试验前需要将涂层和对磨件小球用酒精擦拭干净,摩擦方式为往复式。摩擦试验参数设置为:加载载荷7.5 N,摩擦行程3 mm,频率3 Hz,摩擦时间20 min。涂层的磨损率可以用公式(1)计算[20]。

式中:为磨损率,mm3/(N·m);为磨损的横截面积;为磨损痕长度,mm;为累积摩擦功,N·m;为施加的载荷力,N;为滑动频率,Hz;为摩擦时间,min。
表2 冷喷涂设备工艺参数

Tab.2 Process parameters of cold spraying equipment
2 结果与讨论
2.1 冷喷涂CuNiCoFeCrAl2.3混合金属涂层微观组织及相组成
图2是冷喷涂混合金属涂层横截面的微观形貌和表面XRD图谱。由图2a可看出,冷喷涂涂层组织致密,孔隙小且分散,通过Image软件测得涂层的孔隙率为0.45%±0.13%。从横截面形貌图中可以看出,基体与涂层之间以机械咬合的方式结合在一起,结合界面存在明显的不平整。从图2b可以看出,在冷喷过程中各个金属粒子均未发生相变,涂层中的各种元素均以金属单质相的方式存在。图3为图2a对应的EDS图,各元素比较均匀地分布在涂层中,这为下一步感应重熔原位合成CuNiCoFeCrAl2.3高熵合金涂层奠定了良好的基础。
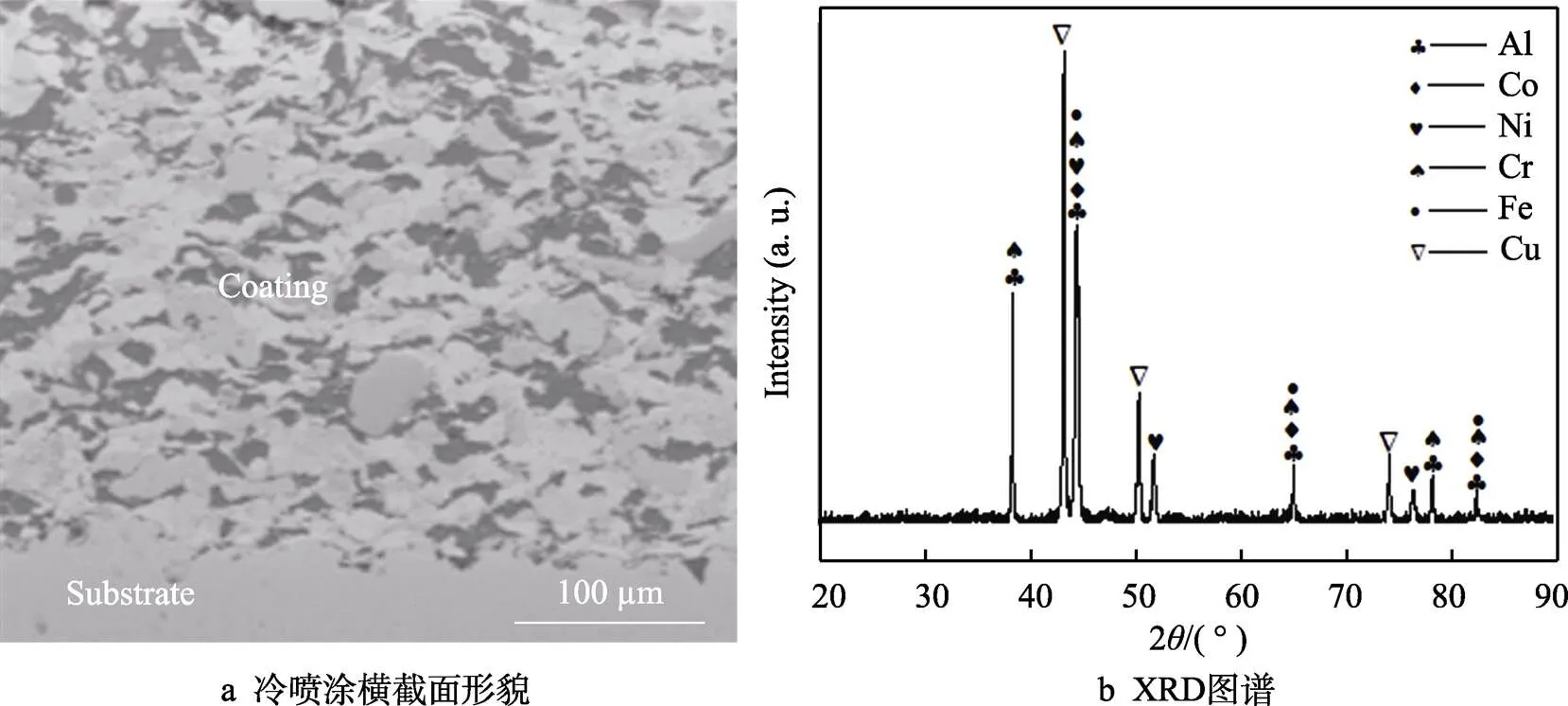
图2 冷喷涂预制混合粉末涂层
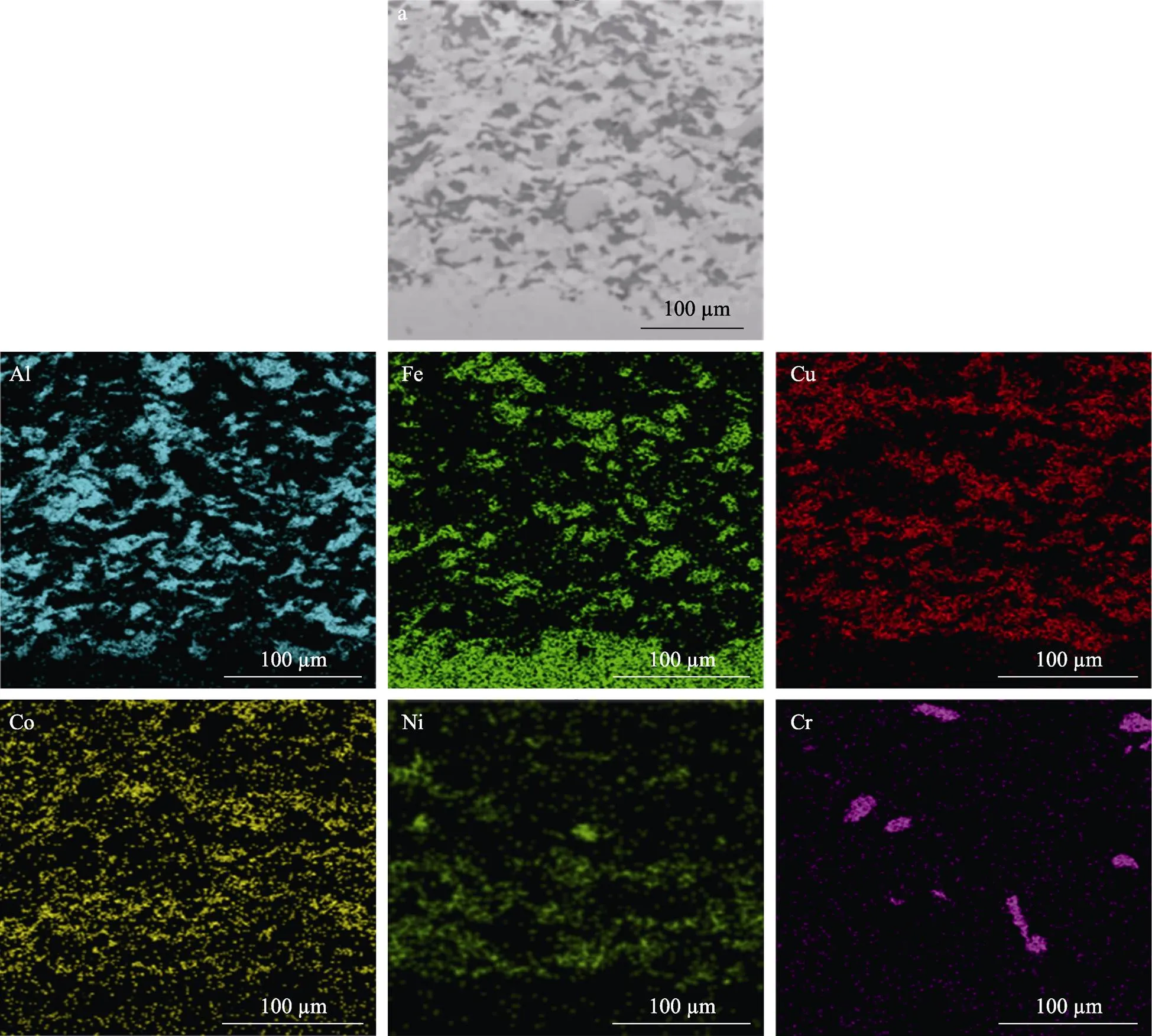
图3 冷喷涂涂层的EDS分析
2.2 冷喷涂辅助原位合成CuNiCoFeCrAl2.3高熵合金涂层的相结构
图4a是冷喷涂金属混合涂层经过感应重熔原位合成CuNiCoFeCrAl2.3高熵合金涂层表面宏观照片及XRD衍射图谱。由图4a可以看出,经过感应重熔表面光滑平整,没有明显的裂纹。说明本试验采用感应重熔工艺适合制备高熵合金涂层。由图4b可以看出,涂层由单一BCC结构固溶体组成。由布拉格方程2sin=可得BCC的晶格常数为0.372 1 nm,衍射峰的位置2大约为44°、65°和82°,与α-Fe的位置接近。根据图4中的XRD分析,在CuNiCoFeCrAl2.3高熵合金涂层中没有金属间化合物出现。简单固溶的高熵合金涂层可以根据以下参数确定[21-23],即混合熵Δmix,混合熵参数,是元素原子尺寸的均方差。这些参数的表达式如下:


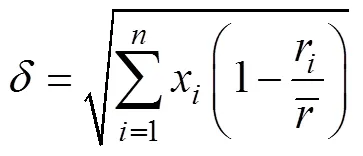
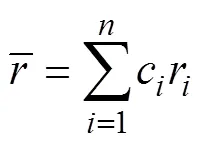

当高熵合金系统中满足Δmix≥1.61、≥1.1、≤6.6,高熵合金易于形成稳定的固溶体结构。针对本研究中的高熵合金涂层系统分析这些参数,计算结果见表3。CuNiCoFeCrAl2.3多组元合金形成了稳定的固溶体结构,与XRD和微观组织分析结果一致。根据高熵合金的概念和高熵合金固溶体形成条件,可以确定本文原位合成的涂层为简单的BCC结构的CuNiCoFeCrAl2.3高熵合金涂层。
2.3 冷喷涂辅助原位合成CuNiCoFeCrAl2.3高熵合金涂层的微观组织
高熵合金涂层的优异性能主要来自于高熵合金晶格畸变效应。根据Hume-Rothery准则[24]可以通过CuNiCoFeCrAl2.3高熵合金中的原子尺寸差异计算出合金的晶格应变,CuNiCoFeCrAl2.3高熵合金涂层的晶格应变是4.13%。从晶体学角度讲,BCC结构相对较为松散,致密度仅为68%[25],BCC相更容易发生变形以释放晶格畸变能[26]。CuNiCoFeCrAl2.3高熵合金涂层的晶格应变属于较大的晶格变形。因此,该涂层倾向形成BCC结构的组织,体现出更好的固溶强化效果。
图5是冷喷涂辅助原位合成CuNiCoFeCrAl2.3高熵合金涂层的SEM图。截面上涂层组织细密,涂层与基体间有较明显的界面。由图5b可知,涂层主要以树枝晶的方式生长。在图5b中有一些黑色斑点,这是因为冷喷涂CuNiCoFeCrAl2.3混合金属涂层中存在少量的孔隙,在感应重熔过程中,孔隙中的空气会氧化涂层中的金属元素,产生少量的Al2O3金属氧化物。图6是图5b的面扫描分析结果,发现各元素较均匀地分布在涂层中。

图4 感应重熔CuNiCoFeCrAl2.3高熵合金涂层的宏观照片及XRD图谱
表3 CuNiCoFeCrAl2.3高熵合金固溶体形成标准条件参数值
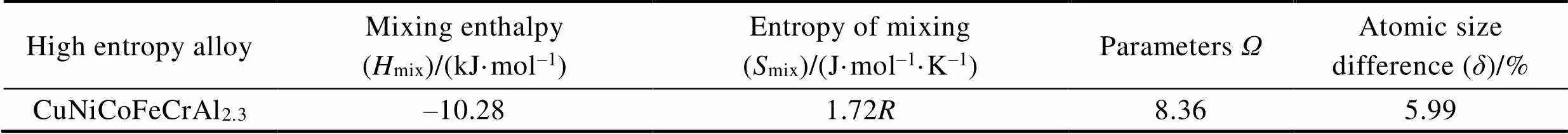
Tab.3 Parameter values of standard conditions for the formation of CuNiCoFeCrAl2.3 high-entropy alloy solid solution
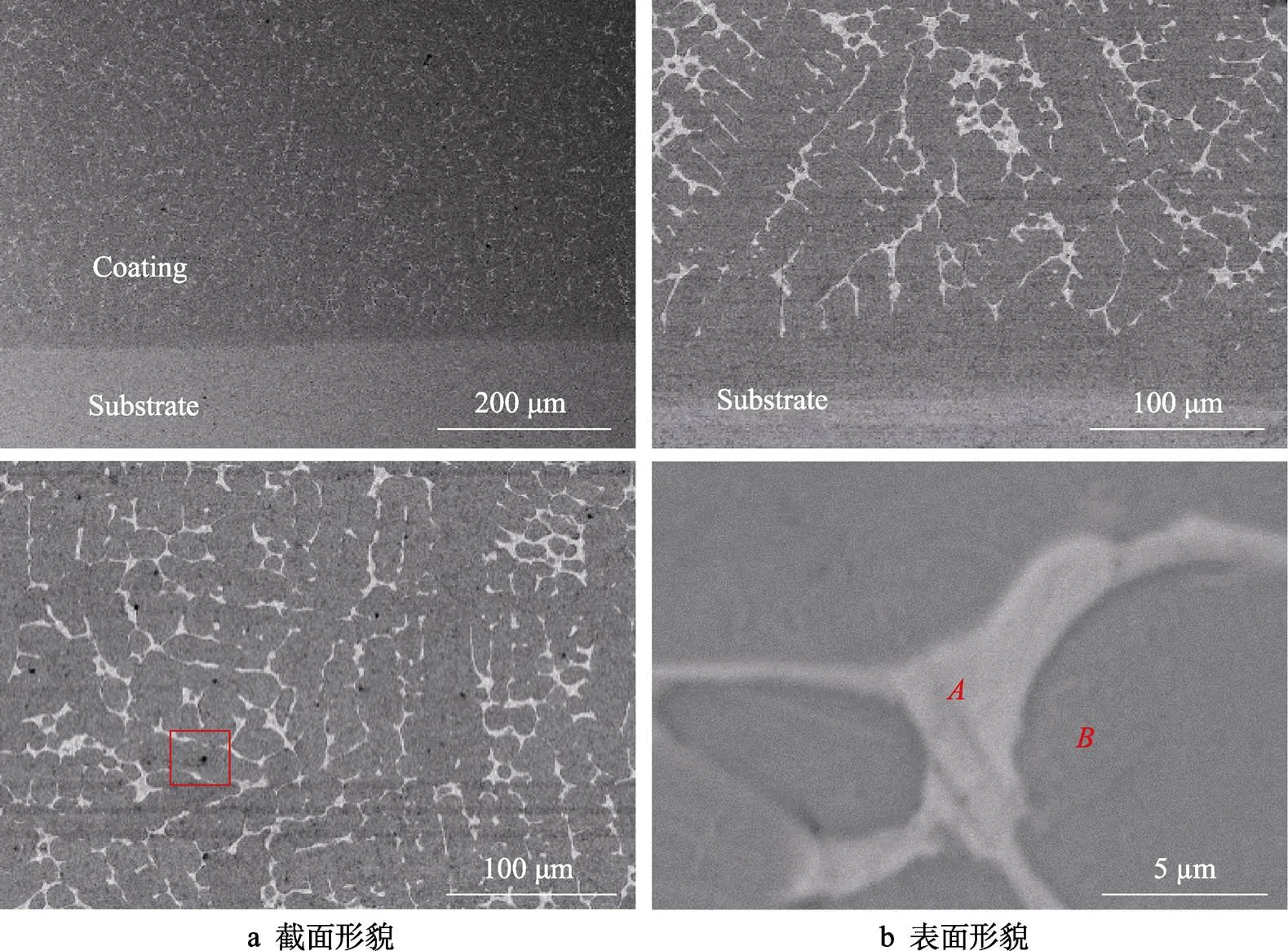
图5 CuNiCoFeCrAl2.3高熵合金涂层的截面和表面形貌
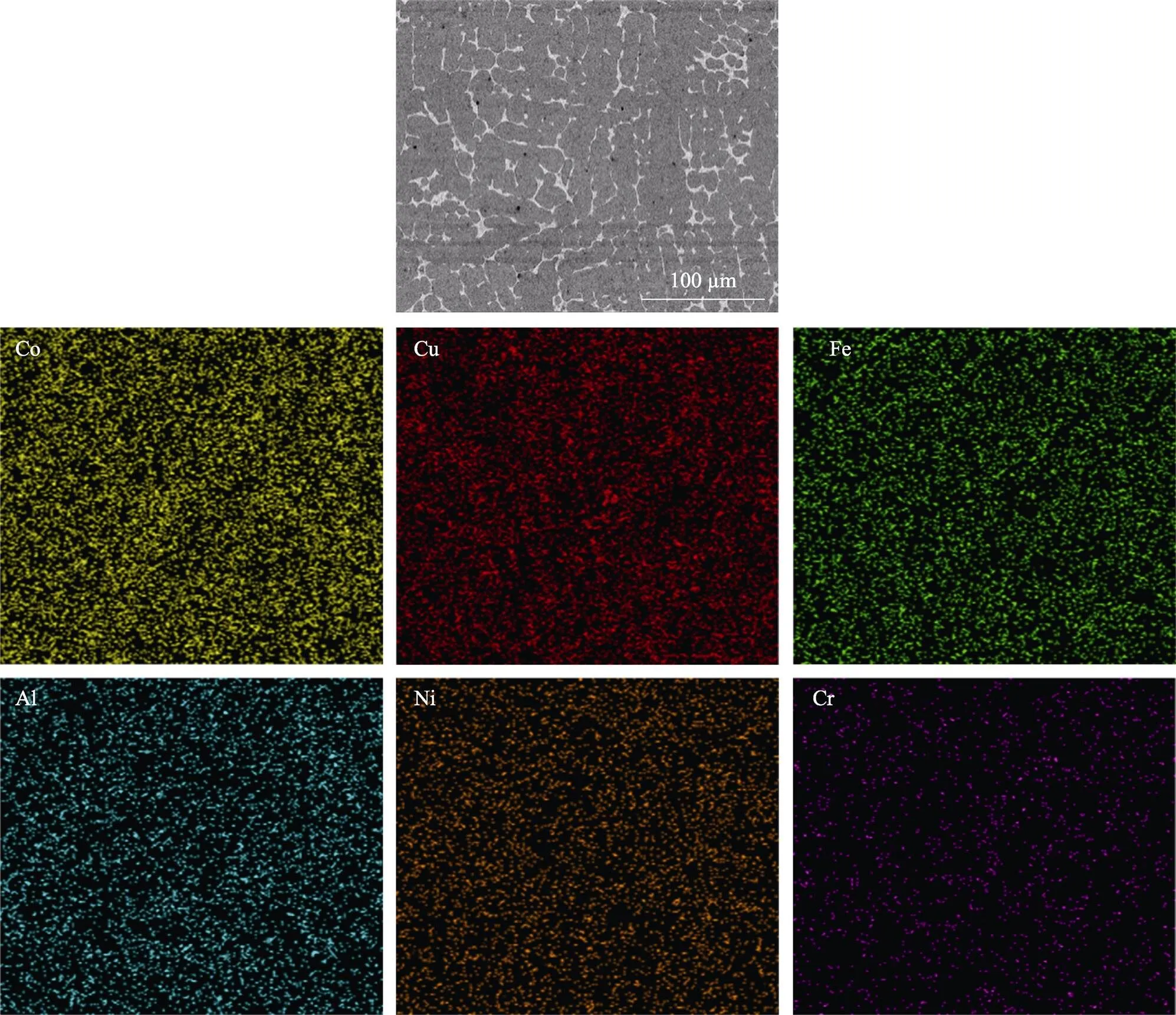
图6 图5b中显微组织SEM及相应的EDS成分分析
将图5b中的枝晶区域定义为区域,枝晶间区域定义为区域,然后EDS成分分析(分别见图7a和图7b)。枝晶内区域主要富含Co、Cr、Fe和Ni,而枝晶间区域则富含Cu和Al。这是因为Cu与Fe、Cr、Co、Ni元素间的混合焓均为正(见表4),导致Cu元素与这些元素的结合能力、互溶性差,阻止了Cu元素进入枝晶区域;Cu与Al的混合焓低,并且易于结合。根据非平衡凝固理论,初次凝固区域(枝晶区域)富含高熔点元素(Fe、Co、Cr、Ni),而枝晶间区域(即最终的凝固区域)通常富含低熔点(Cu)元素。EDS成分分析发现,枝晶区域和晶间区域的Al浓度非常接近,这与Wu等[27-28]的研究结果一致,即Al原子比的增加有利于BCC相形成。
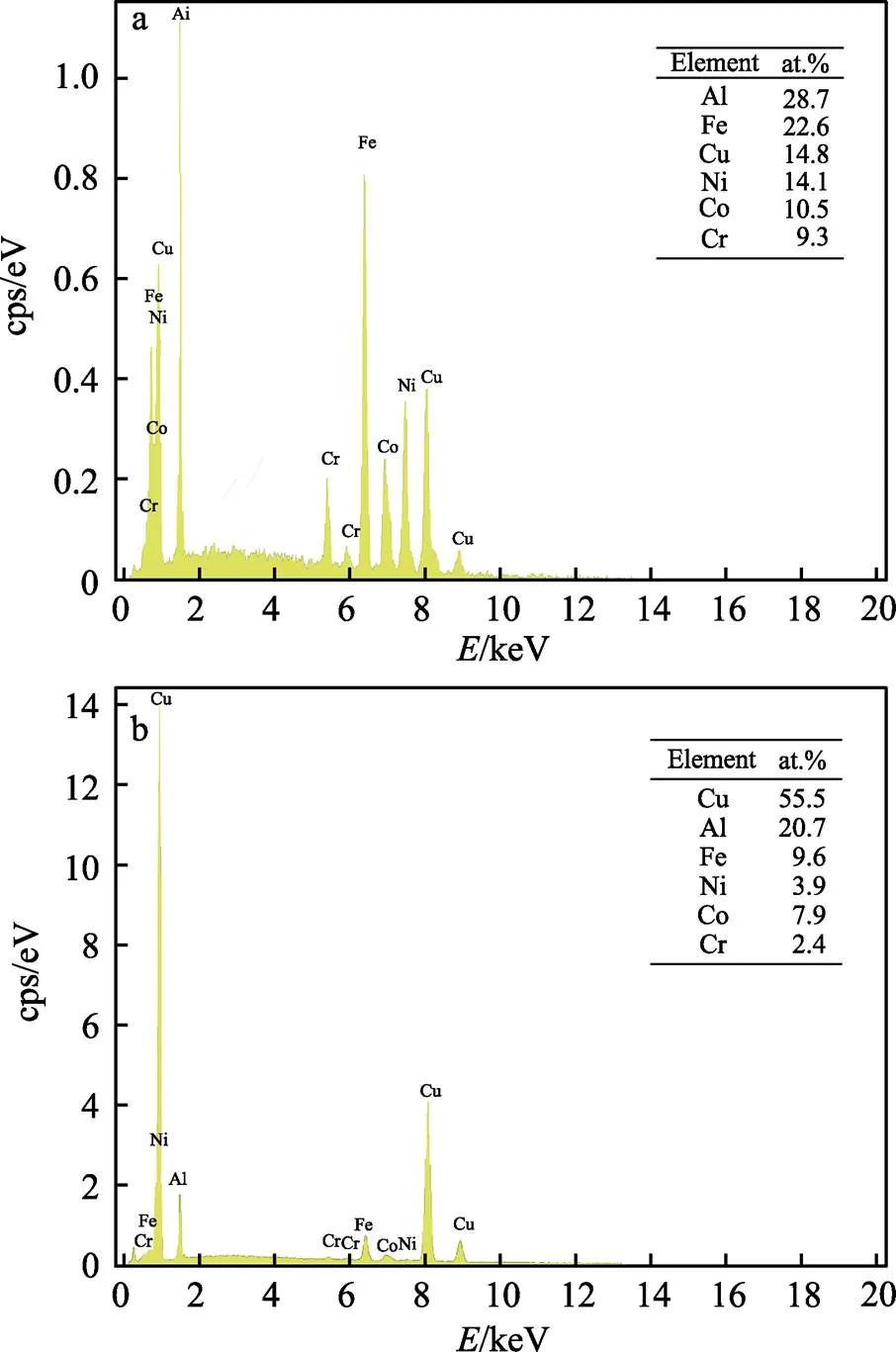
图7 a、b区域 SEM形貌和相应的面谱总图
表4 元素间混合焓

Tab.4 Enthalpy of mixing between elements kJ/mol
2.4 冷喷涂辅助原位合成CuNiCoFeCrAl2.3高熵合金涂层的硬度和摩擦性能
图8a是对冷喷涂辅助原位合成的CuNiCoFeCrAl2.3高熵合金涂层硬度及摩擦性能的检测结果。其中图8a的横坐标是CuNiCoFeCrAl2.3高熵合金涂层表面随机取5个点进行显微硬度测试,涂层表面硬度均匀,不同测试点上的硬度数值波动较小,CuNiCoFeCrAl2.3高熵合金涂层的平均硬度为576HV左右。45#钢基体的显微硬度为170HV左右,CuNiCoFeCrAl2.3高熵合金涂层硬度高的原因是感应重熔后涂层组织由简单的BCC结构固溶体组成。George等[29]研究认为,高熵合金的高硬度是固溶强化和晶界强化综合作用的结果。固溶强化主要是由于合金元素原子半径相差较大引起的晶格畸变,而晶界强化则是由于感应重熔过程中的快速凝固,即快速凝固速率会抑制晶粒长大,导致晶界数目增加,从而导致高熵合金涂层的硬度增加。由文献报道[30-31]可知,冷喷涂辅助感应重熔CuNiCoFeCrAl2.3高熵合金涂层与铸块的CuNiCoFeCrAl2.3高熵合金涂层的硬度几乎相同。图8b是45#基体和CuNiCoFeCrAl2.3高熵合金涂层的摩擦曲线图,基体45#钢的摩擦因数为0.68,CuNiCoFeCrAl2.3高熵合金涂层的摩擦因数为0.38。

图8 CuNiCoFeCrAl2.3高熵合金涂45#钢基体发显微硬度和摩擦因数
图9是磨损表面的形貌。如图9a所示,对45#钢基体进行摩擦试验后,表面有材料撕裂、剥落的痕迹,没有明显的磨料切割痕迹,由此可以推断45#钢基体的磨损主要是黏着磨损。这是因为45#钢基体硬度低、塑性好,在摩擦过程中容易发生塑性变形。当磨损剪切点在基体材料内部时,基体材料会发生撕裂和转移。图9b是CuNiCoFeCrAl2.3高熵合金涂层摩擦试验后的表面形貌,在磨损表面上出现了犁沟和少量材料剥落痕迹,这说明CuNiCoFeCrAl2.3高熵合金涂层的磨损以磨粒磨损为主。材料的硬度越高,耐磨性越好[32]。表5是45#钢基体和CuNiCoFeCrAl2.3高熵合金涂层的磨损率,45#钢基体的磨损率为6.44× 10‒5mm3/(N·m),CuNiCoFeCrAl2.3高熵合金涂层的磨损率2.95×10‒5mm3/(N·m)。
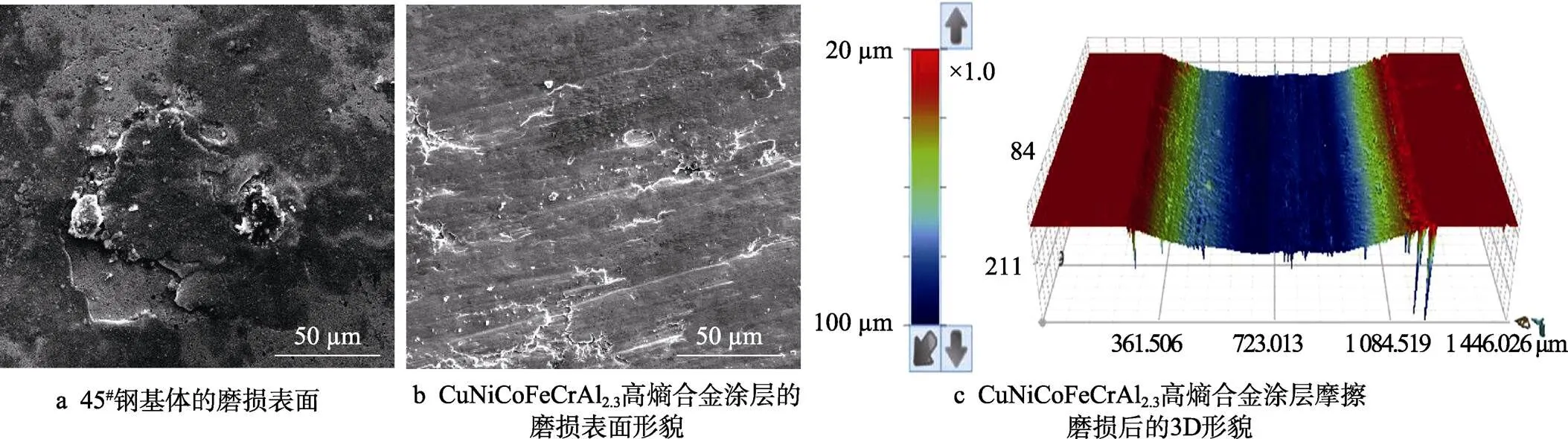
图9 磨损表面的形貌
表5 是45#钢基体和CuNiCoFeCrAl2.3高熵合金涂层的磨损率

Tab.5 shows the wear rate of 45# steel matrix and CuNiCoFeCrAl2.3 high entropy alloy coating
3 结论
1)冷喷涂辅助原位合成CuNiCoFeCrAl2.3高熵合金涂层主要由单一的BCC固溶相组成,其微观结构主要由树枝晶组织,Cu在枝晶间区域富集,而Fe、Co、Cr、Ni分布在枝晶内部,Al均匀分布在2个区域。
2)冷喷涂辅助原位合成CuNiCoFeCrAl2.3高熵合金涂层的显微硬度为576HV左右,是45#钢基底的3倍。
3)冷喷涂辅助原位合成CuNiCoFeCrAl2.3高熵合金涂层的平均摩擦因数为0.38,约为45#钢基底的56%,在干滑动条件下,冷喷涂辅助原位合成CuNiCoFeCrAl2.3高熵合金涂层的磨损率为2.95×10‒5mm3/(N·m),比45#钢基材低59%。
[1] YEH J W, CHEN S K, LIN S J, et al. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes[J]. Advanced Engineering Materials, 2004, 6(5): 299-303.
[2] CANTOR B, CHANG I T H, KNIGHT P, et al. Microstructural Development in Equiatomic Multicomponent Alloys[J]. Materials Science and Engineering: A, 2004, 375-377: 213-218.
[3] OTTO F, YANG Y, BEI H, et al. Relative Effects of Enthalpy and Entropy on the Phase Stability of Equiatomic High-Entropy Alloys[J]. Acta Materialia, 2013, 61(7): 2628-2638.
[4] QIU Yao, THOMAS S, GIBSON M A, et al. Corrosion of High Entropy Alloys[J]. Npj Materials Degradation, 2017, 1: 15.
[5] 赵伊, 曹京宜, 方志刚, 等. 耐蚀高熵合金在岛礁装备中的应用前景[J]. 装备环境工程, 2021, 18(11): 42-50.
ZHAO Yi, CAO Jing-yi, FANG Zhi-gang, et al. Application Prospects of Corrosion-resistant High-entropy Alloys in Island and Reef Equipment[J]. Equipment Environmental Engineering, 2021, 18(11): 42-50.
[6] FU Zhi-qiang, JIANG Lin, WARDINI J L, et al. A High- Entropy Alloy with Hierarchical Nanoprecipitates and Ultrahigh Strength[J]. Science Advances, 2018, 4(10): 8712.
[7] LIU Jing-da, GUAN Yu-xin, XIA Xue-chen, et al. Laser Cladding of Al0.5CoCrCuFeNiSi High Entropy Alloy Coating without and with Yttria Addition on H13 Steel[J]. Crystals, 2020, 10(4): 320.
[8] 张玉林, 庞雅洁, 海潮, 等. 钛合金表面微弧氧化涂层在模拟海洋环境下摩擦腐蚀规律研究[J]. 装备环境工程, 2021, 18(6): 42-50.
ZHANG Yu-lin, PANG Ya-jie, HAI Chao, et al. Tribo- corrosion Behaviours of Microarc Oxidation Coating on Titanium Alloy Surface in Simulated Marine Environment[J]. Equipment Environmental Engineering, 2021, 18(6): 42-50.
[9] ZENG Qun-feng, XU Ya-ting. A Comparative Study on the Tribocorrosion Behaviors of AlFeCrNiMo High Entropy Alloy Coatings and 304 Stainless Steel[J]. Materials Today Communications, 2020, 24: 101261.
[10] LEHTONEN J, KOIVULUOTO H, GE Yan-ling, et al. Cold Gas Spraying of a High-Entropy CrFeNiMn Equiatomic Alloy[J]. Coatings, 2020, 10(1): 53.
[11] YE Xiao-yang, MA Ming-xing, CAO Y, et al. The Property Research on High-Entropy Alloy AlFeCoNiCuCr Coating by Laser Cladding[J]. Physics Procedia, 2011, 12: 303-312.
[12] SOARE V, BURADA M, CONSTANTIN I, et al. Electrochemical Deposition and Microstructural Characterization of AlCrFeMnNi and AlCrCuFeMnNi High Entropy Alloy Thin Films[J]. Applied Surface Science, 2015, 358: 533-539.
[13] HUANG P K, YEH J W, SHUN T T, et al. Multi-Principal-Element Alloys with Improved Oxidation and Wear Resistance for Thermal Spray Coating[J]. Advanced Engineering Materials, 2004, 6(12): 74-78.
[14] ANG A S M, BERNDT C C, SESSO M L, et al. Plasma- Sprayed High Entropy Alloys: Microstructure and Properties of AlCoCrFeNi and MnCoCrFeNi[J]. Metallurgical and Materials Transactions A, 2015, 46(2): 791-800.
[15] YIN Shuo, LI Wen-ya, SONG Bo, et al. Deposition of FeCoNiCrMn High Entropy Alloy (HEA) Coating via Cold Spraying[J]. Journal of Materials Science & Technology, 2019, 35(6): 1003-1007.
[16] ANUPAM A, KUMAR S, CHAVAN N M, et al. First Report on Cold-Sprayed AlCoCrFeNi High-Entropy Alloy and Its Isothermal Oxidation[J]. Journal of Materials Research, 2019, 34(5): 796-806.
[17] HAN Chao, MA Li, SUI Xu-dong, et al. Influence of Low Energy Density Laser re-Melting on the Properties of Cold Sprayed FeCoCrMoBCY Amorphous Alloy Coatings[J]. Coatings, 2021, 11(6): 695.
[18] 冯力, 王贵平, 安国升, 等. 一种原位合成低压冷喷涂CuNiCoFeCrAl(2.8)高熵合金涂层的制备方法[P]. CN110344047A, 2019-10-18.
FENG Li, WANG Gui-ping, AN Guo-sheng, et al. Preparation Method of In situ Synthetic Low Pressure Cold Spraying CuNiCoFeCrAl(2.8) High Entropy Alloy Coating[P]. CN110344047A, 2019-10-18.
[19] 冯力, 胡昱轩, 李文生, 等. 冷喷涂辅助原位合成CuFeCrAlNiTi高熵合金涂层的组织性能研究[J]. 表面技术, 2021, 50(7): 194-202.
FENG Li, HU Yu-xuan, LI Wen-sheng, et al. Microstructure and Properties of CuFeCrAlNiTi High Entropy Alloy Coating Prepared by Cold Spray Assisted In-Situ Synthesis[J]. Surface Technology, 2021, 50(7): 194-202.
[20] WANG Ying, LU Xiao-xing, YUAN Ning-yi, et al. A Novel Nickel-Copper Alternating-Deposition Coating with Excellent Tribological and Antibacterial Property[J]. Journal of Alloys and Compounds, 2020, 849: 156222.
[21] ZHANG Yong, ZUO Ting-ting, TANG Zhi, et al. Microstructures and Properties of High-Entropy Alloys[J]. Progress in Materials Science, 2014, 61: 1-93.
[22] YANG X, ZHANG Y. Prediction of High-Entropy Stabilized Solid-Solution in Multi-Component Alloys[J]. Materials Chemistry and Physics, 2012, 132(2-3): 233-238.
[23] GUO Sheng, HU Qiang, NG C, et al. More than Entropy in High-Entropy Alloys: Forming Solid Solutions or Amorphous Phase[J]. Intermetallics, 2013, 41: 96-103.
[24] ZHOU Y J, ZHANG Y, WANG F J, et al. Phase Transformation Induced by Lattice Distortion in Multiprincipal Component CoCrFeNiCuAl1–xSolid-Solution Alloys[J]. Applied Physics Letters, 2008, 92(24): 241917.
[25] 余永宁. 金属学原理[M]. 北京: 冶金工业出版社, 2000.
YU Yong-ning. Principle of Metal Science[M]. Beijing: Metallurgical Industry Press, 2000.
[26] GUO Lin, WU Wen-qian, NI Song, et al. Effects of Annealing on the Microstructural Evolution and Phase Transition in an AlCrCuFeNi2High-Entropy Alloy[J]. Micron, 2017, 101: 69-77.
[27] CHUANG Ming-hao, TSAI M H, WANG W R, et al. Microstructure and Wear Behavior of AlCo1.5CrFeNi1.5TiHigh-Entropy Alloys[J]. Acta Materialia, 2011, 59(16): 6308-6317.
[28] [28] WU J M, LIN S J, YEH J W, et al. Adhesive Wear Behavior of AlCoCrCuFeNi High-Entropy Alloys as a Function of Aluminum Content[J]. Wear, 2006, 261(5-6): 513-519.
[29] GEORGE E P, RAABE D, RITCHIE R O. High-Entropy Alloys[J]. Nature Reviews Materials, 2019, 4(8): 515-534.
[30] JOSEPH J, HAGHDADI N, SHAMLAYE K, et al. The Sliding Wear Behaviour of CoCrFeMnNi and AlCoCrFeNi High Entropy Alloys at Elevated Temperatures[J]. Wear, 2019, 428-429: 32-44.
[31] TIAN Fu-yang, VARGA L K, CHEN Nan-xian, et al. Empirical Design of Single Phase High-Entropy Alloys with High Hardness[J]. Intermetallics, 2015, 58: 1-6.
[32] QIU Xing-wu. Microstructure, Hardness and Corrosion Resistance of Al2CoCrCuFeNiTiHigh-Entropy Alloy Coatings Prepared by Rapid Solidification[J]. Journal of Alloys and Compounds, 2018, 735: 359-364.
Microstructure and Properties of CuNiCoFeCrAl2.3High Entropy Alloy Coating Prepared by Cold Spray Assisted in Situ Synthesis
1,2,1,1,1,1,1,2
(1. College of Material Science and Technology, Lanzhou University of Technology, Lanzhou 730050, China; 2. State Key Laboratory of Advanced Processing and Recycling of Nonferrous Metals, Lanzhou University of Technology, Lanzhou 730050, China)
In recent years, the rapid development of material surface treatment technology plays a particularly important role in the progress of modern science and technology, industry and economic development. Surface treatment technology is an important method to improve and repair matrix materials. It can improve the strength, hardness, wear resistance and corrosion resistance of materials. Preparing a layer of alloy coating on the surface of ordinary industrial materials is one of the most effective measures of surface treatment. high entropy alloy coating assisted in situ synthesis by cold spraying was prepared,This method can effectively eliminate the interface between powder particles in cold spraying coating. Improve the adhesion and cohesion of the coating, The adhesion of the coating can reach 120 Mpa. In this method, the high entropy alloy powder is not required to be prefabricated, and the mixed metal powder is used as the spraying material to prepare the prefabricated mixed metal coating on the substrate. High entropy alloy coating was synthesized in situ by induction remelting technique. Therefore, this method has the advantages of low preparation cost, short cycle and easy to adjust the composition of the coating. High entropy alloys have high entropy effect in thermodynamics, lattice distortion effect in structure, hysteresis diffusion effect in dynamics, "cocktail" effect in performance and stability in structure. These effects give the alloys excellent mechanical properties such as high strength, good corrosion resistance and excellent wear resistance. These new metal materials are suitable for many engineering applications, Scholars prepared high entropy alloy as surface coating for wear and corrosion conditions, which can not only improve the service life and performance of metal parts, but also reduce the cost of preparation. In order to improve the surface corrosion and wear resistance of ordinary metal materials, a technology for preparing CuNiCoFeCrAl2.3high entropy alloy coating with good performance on the surface of common metal materials was proposed. Methods The mixed metal coating was prepared on 45#steel by cold spraying at low pressure, and then synthesized into CuNiCoFeCrAl2.3high entropy alloy coating by induction remelting technology. The phase composition, microstructure, hardness and wear resistance of the coating were analyzed by scanning electron microscope (SEM), X-ray diffraction (XRD)、energy dispersive spectrometer (EDS)、microhardness tester and abrasive wear tester. Results The microstructure of CuNiCoFeCrAl2.3high entropy alloy coating was compact, the elements were uniformly distributed, the alloy coating was composed of simple BCC phase, and the microstructure of the coating showed typical dendrite structure. Co、Cr、Fe and Ni are mainly abundant in the inner dendrite region, while Cu and Al are abundant in the inter-dendrite region. The microhardness of CuNiCoFeCrAl2.3high entropy alloy coating is three times that of 45#steel alloy matrix under dry friction condition. The wear rate of CuNiCoFeCrAl2.3high entropy alloy coating under dry sliding condition is 59% lower than that of 45#steel substrate, and the friction coefficient is 0.38. the wear rate of CuNiCoFeCrAl2.3high entropy alloy coating is 2.95×10‒5mm3/(N·m). The CuNiCoFeCrAl2.3high entropy alloy coating assisted by cold spraying has high hardness and excellent wear resistance.
high entropy alloy; cold spray; induction remelting; microstructure; wear resistance
TG174.442
A
1001-3660(2022)10-0344-09
10.16490/j.cnki.issn.1001-3660.2022.10.037
2021–08–17;
2021–11–29
2021-08-17;
2021-11-29
国家重点研发计划(2016YFE0111400);国家自然科学基金(52075234);中国博士后科学基金项目(2018-63-200618-34);甘肃省青年博士基金项目(2021QB-043)
National Key R&D Plan (2016YFE0111400); National Natural Science Foundation of China (52075234); China Postdoctoral Science Foundation Program (2018-63-200618-34); Gansu Young Doctor Fund Project (2021QB-043)
冯力(1981—),男,博士,教授,主要研究方向为冷喷涂增材制造技术、金属陶瓷复合涂层的制备与研究。
FENG Li (1981-), Male, Doctor, Professor, Research focus: manufacturing technology of cold spraying additive, preparation and research of metal ceramic composite coating.
冯力, 马凯, 杨伟杰, 等. 冷喷涂辅助原位合成CuNiCoFeCrAl2.3高熵合金涂层组织与性能研究[J]. 表面技术, 2022, 51(10): 344-352.
FENG Li, MA Kai, YANG Wei-jie, et al. Microstructure and Properties of CuNiCoFeCrAl2.3High Entropy Alloy Coating Prepared by Cold Spray Assisted in Situ Synthesis[J]. Surface Technology, 2022, 51(10): 344-352.
责任编辑:万长清
